Abstract
Loss-of-function mutations in DJ-1 (PARK7) gene account for about 1% of all familial Parkinson's disease (PD). While its physiological function(s) are not completely clear, DJ-1 protects neurons against oxidative stress in both in vitro and in vivo models of PD. The molecular mechanism(s) through which DJ-1 alleviates oxidative stress-mediated damage remains elusive. In this study, we identified Paraoxonase-2 (PON2) as an interacting target of DJ-1. PON2 activity is elevated in response to oxidative stress and DJ-1 is crucial for this response. Importantly, we showed that PON2 deficiency hypersensitizes neurons to oxidative stress induced by MPP+ (1-methyl-4-phenylpyridinium). Conversely, over-expression of PON2 protects neurons in this death paradigm. Interestingly, PON2 effectively rescues DJ-1 deficiency-mediated hypersensitivity to oxidative stress. Taken together, our data suggest a model by which DJ-1 exerts its antioxidant activities, at least partly through regulation of PON2.
Introduction
PD is a progressive neurodegenerative disorder characterized by selective loss of the pigmented dopaminergic neurons of the Substantia nigra pars compacta (SNc) [1], and reduction in striatal dopamine level. The majority of PD cases do not follow a genetic inheritance pattern [2]. However, rare familial forms of this disease with their causative genes have been identified [3], [4], [5], [6].
DJ-1 was identified as one of these PD-related genes [7]. It was first identified as an oncogene and associated with fertility factors [8], [9]. However, recent evidence in several families showed linkage of homozygous loss of function mutations in DJ-1 to early onset PD [7], [10]. The mechanisms by which loss of DJ-1 function promotes PD are unclear. However, it has been most associated with management of reactive oxygen species (ROS). For example, our previous data demonstrated that DJ-1 null mice are hypersensitive to dopaminergic toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [11]. Consistent with this, numerous reports utilizing in vitro and in vivo models in both mammalian and drosophila systems support the idea that DJ-1 plays a neuroprotective role under pathological conditions where oxidative stress predominates [12], [13], [14], [15], [16], [17], [18], [19]. How DJ-1 may regulate ROS is not completely clear. DJ-1 is oxidized on its cysteine residues which are also critical for the ability of DJ-1 to manage ROS [20]. DJ-1 also possesses atypical peroxiredoxin activity, although this activity is weak compared to other antioxidant enzymes [21]. Others have demonstrated that DJ-1 somehow regulates Nrf2, a master transcription factor for a variety of antioxidant enzymes [22]. However, whether this is true in neurons is controversial [23].
Recently, to further examine the underlying mechanism(s) by which DJ-1 exerts protection, we performed a proteomics interaction screen for DJ-1 interacting partners. By mass spectrometric analyses, we identified Paraoxonase-2 (PON2) as a novel interacting candidate for DJ-1 [24]. PON2 is a member of Paraoxonase family of genes (Paraoxonase-1, 2, 3), which are located as a cluster on chromosome 7 in human and chromosome 6 in mouse. PON2 is ubiquitously expressed in a wide variety of tissues and localized in cytoplasm and membranous structures, such as plasma membrane [25], endoplasmic reticulum [26], and mitochondria [27]. Several in vitro and in vivo studies indicate a role for PON2 in diminishing oxidative stress [25], [28], [29], [30], [31]. For example, PON2 deficient HeLa cells exhibit elevated intracellular oxidative level which can be reversed by over-expression of PON2 [25]. PON2 deficiency in mice increases the risk of oxidative stress-related pathophysiological conditions such as development of atherosclerotic lesions [27], [28]. Furthermore, numerous studies on several human populations reported the association of PON2 polymorphisms with severe ischemic stroke [32], sporadic amyotrophic lateral sclerosis (SALS) [33], [34], [35], asthma [36] and Alzheimer's disease (AD) [37], [38]. Polymorphisms in another PON member, PON1, have also been associated with susceptibility to PD [39], [40]. However, the role of PON2 in the context of neuronal loss induced by oxidative stress is unknown.
Given the initial interaction data from our proteomics screen, we examined whether DJ-1 may modulate susceptibility to oxidative stress through regulation of the PON2 enzyme. We provide evidence that DJ-1 interacts with and promotes PON2 activity in the presence of oxidative stress and that this mechanism is one central mechanism by which DJ-1 promotes survival.
Methods
Ethics statement
All animal-related experiments were performed based on the protocols provided by the Canadian Council on Animal Care (CCAC), the Canadian Institutes of Health Research, and the University of Ottawa Animal Care and Veterinary Services (ACVS). This study was approved by University of Ottawa Animal Care Committee (ACC). All steps of animal welfare, maintenance, and medical care were also performed by University of Ottawa ACVS.
In the present study, mice were not subjected to any experiment while alive and we ensure that they did not suffer during the process of sacrifice. In order to sacrifice the mice to extract cells or tissues for in vitro experiments, they were first injected intraperitoneally with Euthanyl, then, after confirming they are not awake, they were subjected to cervical dislocation.
Proteomic screen
The original proteomic screen, utilized to obtain DJ-1 interacting proteins, was published previously [24]. Briefly, approximately 1×107 of human embryonic kidney 293 (HEK293) cells (approximately 40% confluent) were transiently transfected by calcium phosphate/DNA co-precipitation method, where calcium chloride were mixed with the target gene-expressing plasmid and then diluted with an inorganic phosphate buffer. The calcium phosphate/DNA precipitate was then incubated with the cells at 37°C for 12–16 hours. Cells were then cultured in fresh medium (Dulbecco's modified Eagle's medium (DMEM) +10% fetal bovine serum (FBS)) for further 24 hours. Cells were then scraped and lysed by lysis buffer (20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 10 µg/ml aprotinin, 0.2 mM AEBSF (Calbiochem)), and cleared from cell debris by centrifugation at 20000 g for 30 min. Cleared cell lysate, containing FLAG-tagged target protein was exposed to M2-Agarose resin (Sigma-Aldrich)(the monoclonal anti-Flag M2 antibody covalently bound to agarose resin) for 1 hour, and the precipitated immune complexes (target protein and its interacting proteins) were eluted by 50 mM ammonium bicarbonate, containing 400 µM Flag peptide. The purified protein complexes were subjected to SDS-PAGE, detected by colloidal Coomassie staining, and protein bands from qualified lanes were excised from the gel. These proteins were treated with DTT, iodoacetamide (to alkylate the free sulfhydryl groups) and trypsin, and the produced peptides were then purified from the gel and concentraded and analyzed by mass spectrometry. As reported earlier [24], the data was generated using an LCQ Deca mass spectrometer (Thermo Finnigan). Mascot version 1.9 (Matrix Sciences, www.matrixscience.com) was used to analyze the obtained spectra by searching against a human protein sequence database with 122989 entries. This database was generated utilizing the main sources of human protein sequences including GenBank, TrEMBL, SwissProt, IPI, Ensembl. The settings to run the Mascot were as follows: search mode: MS/MS Ion, fixed modification: carbamidomethyl on cysteine, variable modification: oxidation on methionine, peptide mass tolerance: 2 Da, fragment mass tolerance: 0.4 Da, maximum missed cleavages: 2, enzyme: trypsin. The Mascot score is the probability of randomness of the match, and is reported as -10LOG10(P), where P is the absolute probability. In other word, the score of 30 means the absolute probability of 10−3.
Cortical neuron culture
Cortical neuron cultures were prepared as described before [41], [42]. Briefly, embryos were extracted at 14.5–15.5 days gestation. Their cortices were dissected and incubated with 0.50 mg/ml trypsin with shaking for 20 minutes at 37°C in Hank's balanced salt solution. Trypsinization was stopped with 0.2 mg/ml trypsin inhibitor and 0.2 mg/ml DNaseI at room temperature. Cells were spun down at 150xg and triturated in Neurobasal medium containing 0.2 mg/ml trypsin inhibitor and 0.25 mg/ml DNaseI. Cells were pelleted and resuspended in Neurobasal medium containing B-27 and N-2 supplements and 0.5 mM glutamine. Cells were then plated in dishes pre-coated with poly-D-lysine.
PON2 deficient and DJ-1 KO mouse embryonic fibroblasts (MEFs) culture
To culture MEFs, mouse embryos were extracted at 14.5–15.5 days of gestation, their skin was dissected and cut into smaller pieces in Hank's balanced salt solution, and incubated in 0.5 mg/ml trypsin for 60 minutes at 37°C. Trypsinization was stopped with 0.2 mg/ml trypsin inhibitor and 0.2 mg/ml DNaseI. Cells were spun down at 150xg, triturated, resuspended, and cultured in DMEM medium with 10% FBS.
GST pull down assay, immunoprecipitation (IP) and immunoblotting
Samples (HEK293 cells for IP of over-expressed proteins and primary cortical neurons for IP of endogenous proteins) were washed with phosphate buffered saline (PBS) and harvested and lysed in lysis buffer (50 mM Tris HCl pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.2% NP-40 and protease inhibitor). Lysate was cleared of cell debris with centrifugation at 17000xg for 20 minutes and supernatant was used for IP. In the case of GST pull down assay in cells expressing GST-DJ-1, cleared cell lysate was incubated with 50 µl glutathione sepharose for 2–4 hours. In other cases, cell lysate was incubated with 4 µg of Myc antibody (Santa Cruz Biotechnology) or DJ-1 antibody (Abcam) overnight and with TrueBlot IgG beads (eBiosciences) for 2 hours. Precipitated complexes were washed 3 times with lysis buffer and eluted by boiling in 2x SDS-loading buffer. Proteins were separated on 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The membrane was blocked with 1% milk for 1 hour at room temperature and treated with primary antibody overnight to probe the target protein. Membrane was washed 3 times and treated with TrueBlot secondary antibody (to avoid IgG signal) for 1 hour. Primary antibodies used for Western blot analyses are: DJ-1 (Abcam), Myc (Santa Cruz Biotechnology), PON2 (GenScript), β-actin (Sigma).
Membrane extraction and paraoxonase-2 activity using 3-oxo-C12-homoserine lacton
Cells were homogenized in homogenization buffer (5 mM Tris/HCl pH 7.4, 1 mM CaCl2 and EDTA-free protease inhibitor). Homogenized cells were pelleted at 17000xg for 30 minutes, resuspended in extraction buffer (25 mM Tris/HCl pH 7.4, 1 mM CaCl2, 10% glycerol, 1% w/v dodecyl-β-d-maltoside (DDM) (Sigma-Aldrich Chemicals) and EDTA-free protease inhibitor (Roche)) and incubated at 4°C with agitation overnight for complete resuspension. Cell debris was extracted with centrifuging at 2000xg for 5 min. For PON2 activity, 4 µg of crude membrane extracts prepared from cultured cortical neurons or murine embryonic fibroblasts (MEFs) was incubated with 10 µM 3-oxo-C12-homoserine lactone (C12) (Vertex Pharmaceuticals) in a 50 µl volume of 25 mM Tris-HCl, pH 7.4, and 1 mM CaCl2 at room temperature. Reactions were stopped with an equal volume of acetonitrile, and 5 µl was used to measure C12 by quantitative autoinducer bioassay using E.coli MG4 containing pKDT17 (provided by E. Greenberg, University of Iowa), [22]. The P. aeruginosa lasB gene is activated with 3-oxo-C12-homoserine lacton (C12). E.coli MG4 containing a plasmid with lasB::lacZ transcriptional fusion (pKDT17), can be induced by C12 to activate Beta-galactosidase gene. Beta-galactosidase will then hydrolyze ortho-Nitrophenyl-β-galactoside (ONPG) to ortho-nitrophenol with yellow color. The more C12 remaining in the buffer, the more signal will be produced by beta-galactosidase activity. For this assay, E.coli MG4 (pKDT17) was divided to 1 ml aliquots. 0.01 ml of membrane samples (already treated with C12) was added to each aliquot and incubated for 4 hours at 37°C. 0.1 ml of the culture was added to 1 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 50 mM beta-mercaptoethanol) and vortexed for 10 seconds. 0.1 ml of the mixture was transferred to a 96 well plate in triplicates and Z buffer only was used as blank. 0.02 ml of ONPG was added to each well and incubated for 10 minutes at room temperature. Reaction was stopped with 0.05 ml of 1 M Na2CO3 and ONPG signal was read at 420 nm. [43], [44], [45], [46].
PON2 activity using Dihydrocoumarin as a substrate
Intact cells were washed with PBS and incubated with activity buffer (50 mM Tris-HCl pH 7.4, 1 mM CaCl2 and 1 mM Dihydrocumarin (DHC) (Sigma-Aldrich Chemicals) as substrate) at room temperature. UV absorbance at 270 nm was measured after 10 minutes incubation. One unit of PON2 activity is equal to 1 µmol DHC hydrolyzed/ml/min [47].
In vitro Adenoviral gene delivery, MPP+ treatment and survival assessment
Adenovirus vector expressing DJ-1 was produced in house by subcloning the cDNA of WT DJ-1 into pAdTRACK vector, where the expression of GFP and DJ-1 is controlled by independent cytomegalovirus promoters. Adenovirus was produced and titered as described before [48]. Adenovirus vector expressing PON2 was kindly provided by Dr. Srinivasa Reddy (UCLA), where it was also generated by subcloning WT human PON2 cDNA into pAdTRACK vector [49]. Adenoviral infection was performed at the time of plating, at a multiplicity of infection (MOI) of 30 for survival experiments and MOI of 100 for biochemical analyses. For survival assays, 48 hours after plating, the cultures were treated with 20 µM of MPP+ (Sigma-Aldrich Chemicals) for 48 hours as previously described [50], [51]. Cultures were then fixed with 4% Paraformaldehyde (PFA), washed 2 times with PBS and stained with Hoechst 33258 (0.5 ng/ml). The percentage of surviving neurons was calculated as the number of GFP-positive neurons with intact nucleus over the total number of GFP-positive neurons [52]. For survival assays with no adenoviral infection, primary cortical neurons obtained from PON2 deficient or wild type mice were subjected to 10, 20 and 40 µM MPP+ treatment for 48 hours. Cells were lysed and the survival rate was assessed by direct microscopy and counting intact nuclei.
Statistical analysis
Statistical significance was assessed by Anova and post-hoc test Tukey on data obtained from three independent experiments. All data are presented as mean ± SEM, and significance is marked by * in case of p<0.05, ** in case of p<0.01 and *** in case of p<0.001.
Results
DJ-1 interacts with PON2
We previously reported a systems biological approach to generation of a large scale human protein-protein interaction map as a tool for understanding proteins functions and the mechanisms of disease [24]. This map was generated based upon a screen utilizing a large number of human bait proteins (407 unique bait proteins) mostly known for their role in diseases such as breast cancer, colon cancer, diabetes and obesity. These bait proteins were used to immunoprecipitate potential interacting partners subsequently identified through mass spectrometric analyses.
Our original data set was filtered with a number of criteria designed to eliminate false positive and non specific interactions which eliminated a large number of valid potential interactors. These exclusion criteria included targets which appeared to interact with more than 5% of bait proteins. Accordingly, we reanalyzed our data sets with focus on DJ-1 eliminating these exclusion criteria. We further analyzed DJ-1 interacting candidates with proper biochemical interaction studies to further validate any potential hits obtained through our systems biology directed screen.
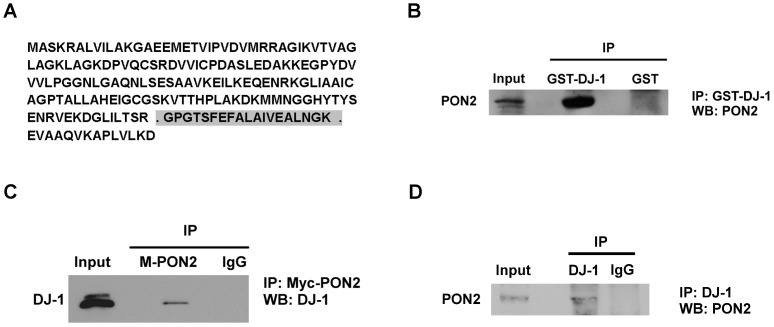
In this study we report the identification and characterization of a new DJ-1 interacting partner, Paraoxonase-2 (PON2). We initially identified DJ-1 through peptide analyses using PON2 as bait (mascot score 30.2, Figure 1A). We next confirmed the interaction of DJ-1 and PON2 in HEK293 cells. The initial experiments were performed utilizing expressed DJ-1. Plasmids expressing GST-DJ-1 were transfected into HEK293 cells and analyses performed by affinity precipitating with glutathione sepharose beads and Western blot analyses for endogenous PON2, utilizing a PON2 antibody. In figure 1B, we show that expression of GST-DJ-1 but not a GST control plasmid immunoprecipitates PON2. The reciprocal experiment was also performed, HEK293 cells were transfected with a vector expressing Myc-PON2 (M-PON2). PON2 was immunoprecipitated with a Myc antibody and immunoblotted for endogenous DJ-1 utilizing a DJ-1 antibody (Figure 1C). In figure 1C, we show that immunoprecipitation with Myc antibody but not IgG control antibody reveals interaction of PON2 with DJ-1. Finally, we tested whether both endogenous PON2 and DJ-1 interact in neurons. We carried out co-immunoprecipitation-Western blot assay using cultured murine cortical neurons. Endogenous DJ-1 was immunoprecipitated with DJ-1 antibody and immunoblotted with PON2 antibody. As shown in figure 1D, PON2 was co-immunoprecipitated with DJ-1 antibody but not with IgG control antibody. Taken together, this indicates that PON2 associates with DJ-1 in vivo.
Figure 1. DJ-1 and PON2 interact.
(A) DJ-1 full length protein sequence. Peptide observed from DJ-1 after using PON2 as bait is highlighted. Mascot peptide score is 30.2. (B) HEK293 cells expressing GST-DJ-1 or GST as control were lysed and GST-DJ-1 was precipitated by glutathione sepharose beads and analyzed with Western blotting using PON2 antibody. (C) HEK293 cells were transfected with plasmid expressing Myc-PON2 (M-PON2). Cells were lysed and Myc-PON2 was precipitated with Myc antibody. Isolated complexes were analyzed with Western blotting using DJ-1 antibody. (D) DJ-1 was pulled down by DJ-1 antibody from cell lysate extracted from cultured cortical neurons. Immune complexes were analyzed with Western blotting using PON2 antibody.
Effects of DJ-1 and oxidative stress on PON2 activity
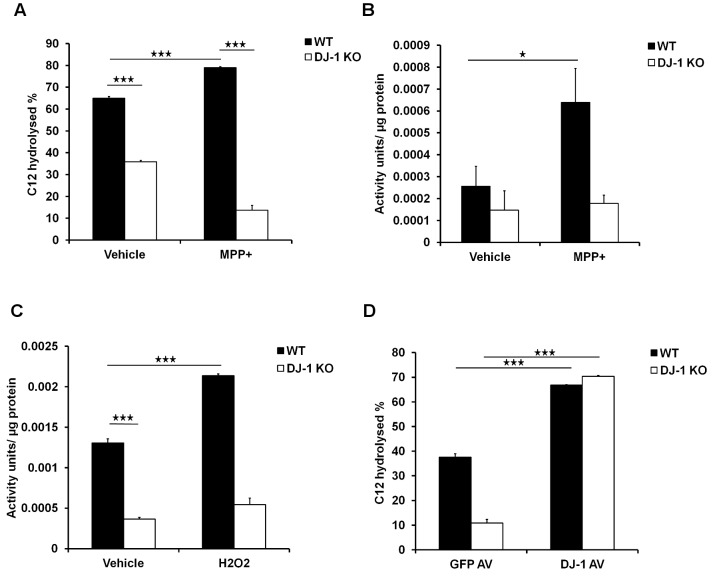
Previous reports have shown that PON2 lactonase activity increases in response to oxidative stress [53]. Given that DJ-1 interacts with PON2, we hypothesized that DJ-1 modulates PON2 activity in this paradigm. To test this hypothesis, we measured PON2 lactonase activity in cortical neurons derived from DJ-1 wild-type (WT) or knockout (KO) embryos treated with MPP+ (20 µM), for 12 hours. MPP+ is a complex I inhibitor which leads to oxidative stress and death of a number of different neurons [54], [55], [56], [57], [58]. PON2 lactonase activity was first measured by assessing the percentage of hydrolysis of PON2 specific substrate, 3-oxo-C12-homoserine lactone (C12) by PON2 [28]. As shown in figure 2A, PON2 activity is significantly elevated after MPP+ treatment in wild-type neurons. Remarkably, DJ-1 deficiency not only blocked PON2 basal lactonase activity, but also blocked MPP+-induced enzymatic activity. We then confirmed this result using a second assay protocol which involves hydrolysis of dihydrocoumarin (DHC), a lactone which can be hydrolyzed by PON2 [47], [53], [59]. Similarly, with this assay, oxidative stress induced PON2 activity only in WT neurons and not in DJ-1 deficient neurons (Figure 2B). To further confirm this observation, we measured hydrolysis of DHC in another cell type challenged with a different oxidative reagent. Indeed, PON2 activity was also elevated in response to oxidative stress induced by hydrogen peroxide (100 µM for 24 hours) in WT murine embryonic fibroblasts (MEFs) but not in DJ-1 KO MEFs (Figure 2C). This supports the idea that DJ-1 regulates PON2 activity in multiple cellular contexts and ROS conditions. Finally, we determined whether low PON2 activity observed under conditions of DJ-1 deficiency could be rescued by DJ-1 expression. Accordingly, we expressed DJ-1 or GFP in DJ-1 WT or KO MEFs (Figure 2D). DJ-1 KO MEFs expressing GFP have less PON2 activity measured by C12. This activity in DJ-1 KO MEFS expressing DJ-1 increases by almost 59%. Taken together, these results indicate that loss of DJ-1 impairs PON2 activity and that this loss can be rescued by DJ-1 re-expression.
Figure 2. DJ-1 and oxidative stress modulate PON2 activity.
(A) Cultured WT and DJ-1 KO cortical neurons were treated with MPP+ (20 µM) for 12 hours and cells were washed and membrane was extracted. Crude membrane was exposed to the substrate C12 for 60 minutes and the percentage of remaining C12 was measured. (B) Cultured WT and DJ-1 KO cortical neurons were treated with MPP+ (20 µM) for 24 hours. Neurons were then exposed to DHC for 10 minutes and the amount of hydrolysis of DHC was assessed with measuring UV absorbance. One unit of PON2 activity is equal to 1 µmol DHC hydrolyzed/ml/min. (C) WT and DJ-1 KO MEFs were treated with hydrogen peroxide (100 µM) for 24 hours and PON2 activity was measured as described in B. (D) WT and DJ-1 KO MEFs were infected with adenovirus expressing DJ-1 or GFP alone as control. After 48 hours of expression, cells were lysed and exposed to C12 as the substrate for 60 minutes. Percentage of C12 remaining in activity buffer was measured. Statistical significance was assessed by Anova and post-hoc test Tukey on data obtained from three independent experiments (n = 3). * denotes p<0.05, ** denotes p<0.01, and *** denotes p<0.001.
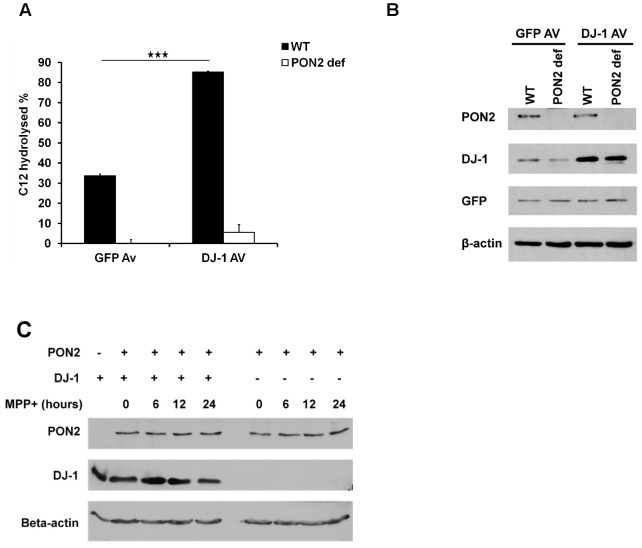
Importantly, we also determined the effects of DJ-1 expression in PON2 deficient (PON2 def) cells. We expressed DJ-1 or GFP as control in PON2 WT or deficient MEFs (Figure 3B), and PON2 activity was measured as described above. As shown in figure 3A, DJ-1 expression in PON2 WT MEFs induced PON2 activity by almost 51% compared to GFP control group. However, the induced activity observed in PON2 deficient MEFs was dramatically lower (less than 5%). The small amount of background lactonase activity observed in PON2 deficient cells may be the contribution of other PON members [60], [61], [62], [63], [64], [65], or the fact that PON2-def mice are reported to having up to 5% of leakiness based on the mouse construction method [66], although this is unclear at the moment. These results indicate that the lactonase activity induced by DJ-1 is almost exclusively through PON2.
Figure 3. DJ-1 has no lactonase activity and no effects on PON2 protein level.
(A) WT and PON2 deficient MEFs were infected with adenovirus expressing DJ-1 or GFP. PON2 activity was then measured using C12 as described before. (B) Samples used in panel A was exposed to SDS-PAGE analysis to assess their levels of DJ-1, PON2 and GFP. (C) Cultured cortical neurons extracted from DJ-1 WT and DJ-1 KO were treated with MPP+ (20 µM) for different durations. Cells were lysed and PON2 protein level was assessed by western blotting. Statistical significance was assessed by Anova and post-hoc test Tukey on data obtained from three independent experiments (n = 3). * denotes p<0.05, **denotes p<0.01 and *** denotes p<0.001.
DJ-1 does not affect PON2 protein level in neurons
DJ-1 is reported to interact with RNA and/or localize to the nucleus [67], [68]. Accordingly, it is possible that DJ-1 acts through regulation of transcription/translation/stabilization of PON2 and that direct interaction demonstrated above, is not necessary for the modulation of PON2 by DJ-1. To examine this possibility, we treated cortical neurons obtained from DJ-1 WT or KO embryos with MPP+ (20 µM) for 0, 6, 12 and 24 hours and compared their PON2 protein levels using western blot analysis. Our data demonstrates that there is no significant difference in PON2 protein level between DJ-1 WT and KO neurons. In addition, PON2 protein level does not change in response to MPP+ induced oxidative stress (Figure 3C). This observation rules out the possibility that DJ-1 increases PON2 activity through increasing PON2 protein levels.
PON2 protects against MPP+-induced neuronal death
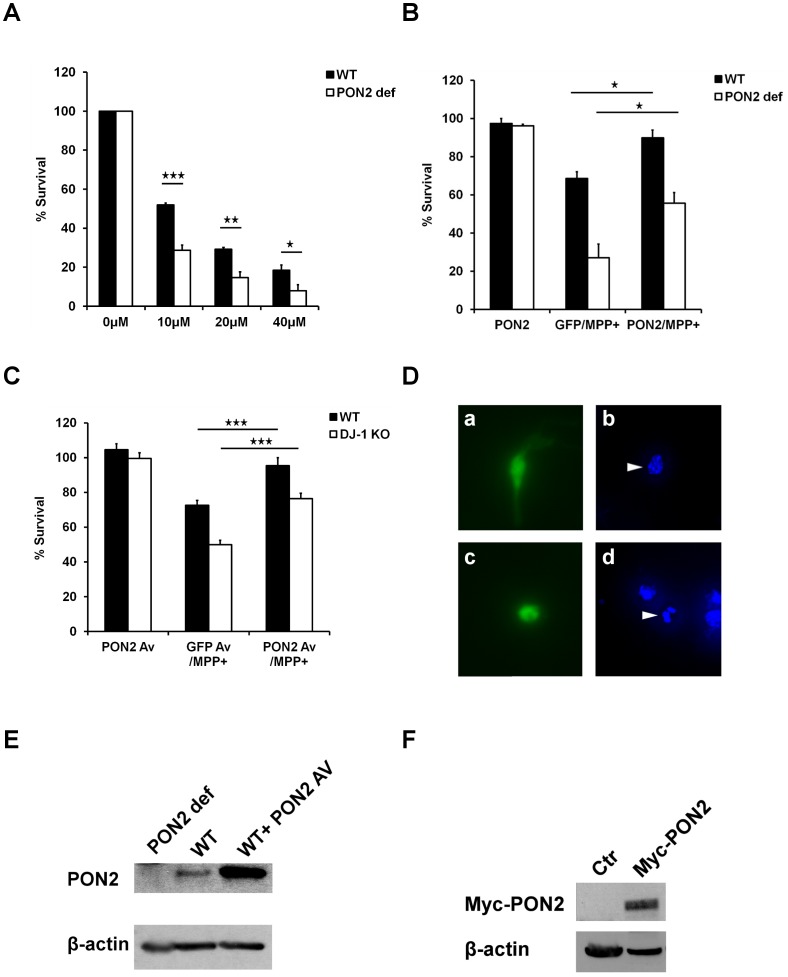
Loss of DJ-1 results in hypersensitization to a number of death-inducing oxidative stress stimuli. If the regulation of PON2 by DJ-1 is biologically significant we would anticipate that a) PON2 loss would also sensitize neurons to oxidative stress and b) PON2 expression would rescue the sensitization to stress induced by loss of DJ-1. This would also suggest PON2 as a downstream target of DJ-1. To test this hypothesis, we first treated PON2 WT or deficient cortical neurons with 0, 10, 20 and 40 µM MPP+ for 48 hours and assessed the neuronal cell survival by nuclear integrity. Our data shows that PON2 deficient neurons are significantly hypersensitive to MPP+ treatment when compared to neurons from WT littermate controls (Figure 4A). To confirm the protective function of PON2, we expressed Myc-PON2 along with GFP, or GFP alone as control in WT or PON2 def cortical neurons. The cells were exposed to 20 µM MPP+ for 48 hours and their survival was assessed by counting proportion of GFP positive cells with intact nuclei to total GFP positive cells, as described previously [11]. Our data demonstrate that PON2 expression rescues PON2 deficiency-mediated hypersensitivity to MPP+ (Figure 4B). Finally, we examined whether PON2 expression can also rescue DJ-1 loss-mediated hypersensitivity to MPP+. To test this, we expressed PON2 and GFP, or GFP alone as control by adenoviral infection in DJ-1 WT or KO cortical neurons. After treatment with MPP+ (20 µM) for 48 hours, the cell survival was assessed as above. Consistent with our hypothesis, PON2 expression protects neurons against MPP+ and can also reverse the hypersensitivity observed with DJ-1 loss (Figure 4C).
Figure 4. PON2 protects neurons against MPP+.
(A) Primary cortical neurons obtained from PON2 deficient or wild type mice were subjected to 10, 20 and 40 µM MPP+ treatment for 48 hours. Cells were lysed and viability was assessed by direct microscopy and counting intact nuclei. (B) WT and PON2 def cortical neurons were transfected with plasmid expressing Myc-PON2 and GFP (under independent promoters), or GFP as control, and subjected to 20 µM MPP+ for 48 hours. Cells were fixed and the nuclei were stained with Hoechst. Survival percentage represents the ratio of GFP-expressing cells with morphologically intact nuclei (D, a and b) to the total number of GFP positive cells. (C) WT and DJ-1 KO cortical neurons over-expressing PON2 and GFP or GFP alone as control (using adenovirus expressing PON2 or GFP) were subjected to 20 µM MPP+ for 48 hours. The survival assay was performed as described in part B. (D) Representative image of GFP positive neurons (a and c), and Hoechst-stained surviving (b) and dead (d) nuclei. (E) Western blot analysis of PON2 levels in PON2 deficient (PON2 def) and WT MEFs and also in WT MEFs infected with PON2-expressing adenovirus (WT+PON2 AV). The membrane was probed with PON2 antibody. (F) Western blot analysis for Myc in WT MEFs expressing control (Ctr) or Myc-PON2 plasmids. The Western blot was analyzed by Myc antibody. Statistical significance was assessed by Anova and post-hoc test Tukey on data obtained from three independent experiments (n = 3). * denotes p<0.05, **denotes p<0.01 and *** denotes p<0.001.
Discussion
Several studies have demonstrated the link between DJ-1 and oxidative damage in neurodegeneration [11], [12], [13], [18], [19], [69]. The purpose of the present study was to investigate the mechanism(s) underlying the capacity of DJ-1 to mediate survival. In an initial mass spectrometry screen for DJ-1 interacting protein, we identified PON2 as a candidate interacting partner. We confirmed this interaction, particularly under endogenous conditions in primary neurons. The model by which DJ-1 is a critical factor in regulating PON2 activity is supported by several observations. First, elevated PON2 activity which occurs in response to MPP+ mediated oxidative stress is dependent upon DJ-1. Multiple cell types including neurons and MEFs have lowered PON2 activity in the absence of DJ-1 in response to oxidative stress. This deficiency can be rescued by DJ-1 expression. Importantly, our results also suggest that manner by which DJ-1 regulates PON2 is not through more potentially indirect effects on PON2 stability since DJ-1 deficiency has no effect on PON2 levels. The manner by which DJ-1 regulates PON2 activity is unclear. Our interaction data between DJ-1 and PON2 suggest that direct or indirect binding of the two proteins may be important. However, this must be confirmed by additional studies which rely on identifying the interaction domains between DJ-1 and PON2. Whatever the mechanism, our data clearly shows the importance of DJ-1 in regulating PON2 lactonase activity.
Second, we show that PON2 itself is critical for regulating survival in response to conditions of oxidative stress (in particular induced by MPP+). Neurons deficient in PON2 are more sensitive to MPP+ treatment which can be rescued by re-introduction of PON2. These results are consistent with the notion that PON2 is known to lower ROS [25], [28], [29], [70]. Interestingly, DJ-1 deficient neurons are also similarly hypersensitive to oxidative stress and this hypersensitivity can be reversed by PON2 expression. This observation is consistent with the model by which DJ-1 acts to increase the activity of PON2. Note that while these observations imply that DJ-1 is a critical regulator of PON2, it is not an absolute requirement for PON2 activity. Finally, even though our data suggest that PON2 is one important factor in the protective effects of DJ-1, we do not imply that it is the only factor. In this regard, we recently also identified VHL as an additional DJ-1 interacting factor[71]. Accordingly, DJ-1 may work through multiple proteins for its survival functions.
The mechanism by which PON2 lactonase activity relates to reduced oxidative stress is unclear. One possibility is that the lactonase activity per se is essential for regulation of death and oxidative stress. Multiple lines of evidence have shown that environmental factors such as pesticide exposure can increase the risk of early onset of Parkinson's disease [72], [73], [74]. Paraoxon is an organophosphorus compounds, active metabolite of the insecticide parathion, whose toxicity is due to their strong anticholinesterase action. Evidence has shown that paraoxon can cause apoptotic cell death in proliferating cells through activation of mitochondrial pathways [75]. Paraoxon-induced AChE inhibition can aggravate experimental Parkinsonism triggered by MPTP in mice [76], suggesting paraoxonase may play a role in defending against Parkinson etiologic factors. Therefore, in this scenario, the defined lactonase activity of PON2 may somehow indirectly lead to reduced oxidative stress, at least under certain conditions. A second possibility is that the lactonase activity is somehow separate from the oxidative capacity of PON2. In support of this hypothesis, it was reported that the antioxidant capacity could be dissociated from the lactonase activity [77]. It is interesting to speculate that perhaps PON2 might modify the antioxidant capacity of DJ-1 directly. However, our studies indicate that expression of PON2 by itself in the absence of DJ-1 is protective, suggesting that this is not the case. Resolution of these questions will be of critical importance in future studies.
A final interesting point is that while both PON2 and DJ-1 have been localized to numerous subcellular compartments, both have been associated with mitochondrial functions. For example, DJ-1 accumulates in mitochondria (presumably outer mitochondrial membrane) in response to oxidant stress [20], [78]. DJ-1 may also be present in more interior mitochondrial compartments [79]. The role of DJ-1 in mitochondrial functions has not been fully understood although it has been suggested to be essential for the survival promoting capacity of DJ-1 [69]. Similarly, PON2 has also been reported in the mitochondria where it binds to coenzyme Q10 [27]. In this regard, it has been shown that PON2 deficient mice have less complex I and III activity and less ATP production and also elevated mitochondrial ROS generation [27]. It is therefore interesting to speculate that perhaps DJ-1 may interact with PON2 in the mitochondria to regulate antioxidant stress responses. This is an exciting possibility given the increasing association of mitochondrial defects with the mechanisms underlying PD and the number of PD linked genes including DJ-1 associated with mitochondrial quality control [20], [78], [80]. In support of this, we have shown that DJ-1 loss leads to increased ROS production from isolated mitochondria [80]. Whether this relates to the function of DJ-1 on PON2 will also be of interest in future studies.
In summary, we demonstrate that DJ-1, a Parkinson's disease related gene, interacts with PON2 in neurons and cell lines. This interaction appear to modulate PON2 activity as DJ-1 KO cells have less basal PON2 activity and do not respond to oxidative stress as DJ-1 WT cells do. This effect can be reversed by expression of DJ-1. In addition, expression of PON2 in DJ-1 KO neurons is more protective against Parkinson's model of neuronal death than expression of DJ-1 in PON2 deficient background.
Acknowledgments
We would like to thank Dr. Sven Horke (Johannes Gutenberg University, Mainz, Germany) and Dr. John Teiber (UT Southwestern Medical Center, Dallas, Texas, U.S.A) for helpful discussions, and Vahid Zandi and Alexander Revelo for technical assistance.
Funding Statement
This work was supported by grants from Parkinson's Society Canada (PSC); Ontario Graduate Scholarships in Science and Technology (OGSST); the Canadian Institutes of Health Research (CIHR); National Heart, Lung, and Blood Institute (NHLBI) [1RO1HL71776 to S.T.R]; Heart and Stroke Foundation of Ontario (HSFO); Neuroscience Canada (Brain Repair Grant); Parkinson's Disease Foundation (PDF); The Michael J. Fox foundation for Parkinson’s research (MJFOX); the Centre for Stroke Recovery (CSR) and World Class University program (WCU) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, South Korea [R31-2008-000-20004-0 to D.S.P]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature 334: 345–348. [DOI] [PubMed] [Google Scholar]
- 2. Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, et al. (1999) Parkinson disease in twins: an etiologic study. JAMA 281: 341–346. [DOI] [PubMed] [Google Scholar]
- 3. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- 4. Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608. [DOI] [PubMed] [Google Scholar]
- 5. Leroy E, Boyer R, Auburger G, Leube B, Ulm G, et al. (1998) The ubiquitin pathway in Parkinson's disease. Nature 395: 451–452. [DOI] [PubMed] [Google Scholar]
- 6. Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet 18: 106–108. [DOI] [PubMed] [Google Scholar]
- 7. Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259. [DOI] [PubMed] [Google Scholar]
- 8. Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, et al. (1997) DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun 231: 509–513. [DOI] [PubMed] [Google Scholar]
- 9. Klinefelter GR, Laskey JW, Ferrell J, Suarez JD, Roberts NL (1997) Discriminant analysis indicates a single sperm protein (SP22) is predictive of fertility following exposure to epididymal toxicants. J Androl 18: 139–150. [PubMed] [Google Scholar]
- 10. van Duijn CM, Dekker MC, Bonifati V, Galjaard RJ, Houwing-Duistermaat JJ, et al. (2001) Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet 69: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, et al. (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A 102: 5215–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu L, Cui T, Fan C, Zhao H, Zhao C, et al. (2009) Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem Biophys Res Commun 383: 469–474. [DOI] [PubMed] [Google Scholar]
- 13. Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, et al. (2006) PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis 24: 144–158. [DOI] [PubMed] [Google Scholar]
- 14. Lev N, Ickowicz D, Barhum Y, Lev S, Melamed E, et al. (2009) DJ-1 protects against dopamine toxicity. J Neural Transm 116: 151–160. [DOI] [PubMed] [Google Scholar]
- 15. Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, et al. (2004) DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 5: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavara-Culebras E, Paricio N (2007) Drosophila DJ-1 mutants are sensitive to oxidative stress and show reduced lifespan and motor deficits. Gene 400: 158–165. [DOI] [PubMed] [Google Scholar]
- 17. Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, et al. (2004) Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol 2: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, et al. (2010) DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A 107: 3186–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aleyasin H, Rousseaux MW, Phillips M, Kim RH, Bland RJ, et al. (2007) The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci U S A 104: 18748–18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, et al. (2004) The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A 101: 9103–9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, et al. (2007) DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A 104: 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP (2006) DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A 103: 15091–15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gan L, Johnson DA, Johnson JA (2010) Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci 31: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ewing RM, Chu P, Elisma F, Li H, Taylor P, et al. (2007) Large-scale mapping of human protein-protein interactions by mass spectrometry. Mol Syst Biol 3: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, et al. (2001) Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem 276: 44444–44449. [DOI] [PubMed] [Google Scholar]
- 26. Horke S, Witte I, Wilgenbus P, Altenhofer S, Kruger M, et al. (2008) Protective effect of paraoxonase-2 against endoplasmic reticulum stress-induced apoptosis is lost upon disturbance of calcium homoeostasis. Biochem J 416: 395–405. [DOI] [PubMed] [Google Scholar]
- 27. Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, et al. (2011) Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid Redox Signal 14: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, et al. (2006) Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem 281: 29491–29500. [DOI] [PubMed] [Google Scholar]
- 29. Mackness MI, Arrol S, Durrington PN (1991) Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett 286: 152–154. [DOI] [PubMed] [Google Scholar]
- 30. Jasna JM, Anandbabu K, Bharathi SR, Angayarkanni N (2014) Paraoxonase enzyme protects retinal pigment epithelium from chlorpyrifos insult. PLoS One 9: e101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagmann H, Kuczkowski A, Ruehl M, Lamkemeyer T, Brodesser S, et al. (2014) Breaking the chain at the membrane: paraoxonase 2 counteracts lipid peroxidation at the plasma membrane. FASEB J 28: 1769–1779. [DOI] [PubMed] [Google Scholar]
- 32. Lazaros L, Markoula S, Kyritsis A, Georgiou I (2010) Paraoxonase gene polymorphisms and stroke severity. Eur J Neurol 17: 757–759. [DOI] [PubMed] [Google Scholar]
- 33. Slowik A, Tomik B, Wolkow PP, Partyka D, Turaj W, et al. (2006) Paraoxonase gene polymorphisms and sporadic ALS. Neurology 67: 766–770. [DOI] [PubMed] [Google Scholar]
- 34. Saeed M, Siddique N, Hung WY, Usacheva E, Liu E, et al. (2006) Paraoxonase cluster polymorphisms are associated with sporadic ALS. Neurology 67: 771–776. [DOI] [PubMed] [Google Scholar]
- 35. Valdmanis PN, Kabashi E, Dyck A, Hince P, Lee J, et al. (2008) Association of paraoxonase gene cluster polymorphisms with ALS in France, Quebec, and Sweden. Neurology 71: 514–520. [DOI] [PubMed] [Google Scholar]
- 36. Polonikov AV, Ivanov VP, Bogomazov AD, Freidin MB, Illig T, et al. (2014) Antioxidant defense enzyme genes and asthma susceptibility: gender-specific effects and heterogeneity in gene-gene interactions between pathogenetic variants of the disease. Biomed Res Int 2014: 708903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi J, Zhang S, Tang M, Liu X, Li T, et al. (2004) Possible association between Cys311Ser polymorphism of paraoxonase 2 gene and late-onset Alzheimer's disease in Chinese. Brain Res Mol Brain Res 120: 201–204. [DOI] [PubMed] [Google Scholar]
- 38. Janka Z, Juhasz A, Rimanoczy AA, Boda K, Marki-Zay J, et al. (2002) Codon 311 (Cys –> Ser) polymorphism of paraoxonase-2 gene is associated with apolipoprotein E4 allele in both Alzheimer's and vascular dementias. Mol Psychiatry 7: 110–112. [DOI] [PubMed] [Google Scholar]
- 39. Akhmedova SN, Yakimovsky AK, Schwartz EI (2001) Paraoxonase 1 Met–Leu 54 polymorphism is associated with Parkinson's disease. J Neurol Sci 184: 179–182. [DOI] [PubMed] [Google Scholar]
- 40. Carmine A, Buervenich S, Sydow O, Anvret M, Olson L (2002) Further evidence for an association of the paraoxonase 1 (PON1) Met-54 allele with Parkinson's disease. Mov Disord 17: 764–766. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Parsanejad M, Huang E, Qu D, Aleyasin H, et al. (2010) Pim-1 kinase as activator of the cell cycle pathway in neuronal death induced by DNA damage. J Neurochem 112: 497–510. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Qu D, Morris EJ, O′Hare MJ, Callaghan SM, et al. (2006) The Chk1/Cdc25A pathway as activators of the cell cycle in neuronal death induced by camptothecin. J Neurosci 26: 8819–8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, et al. (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 91: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pearson JP, Passador L, Iglewski BH, Greenberg EP (1995) A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 92: 1490–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cooley MA, Whittall C, Rolph MS (2010) Pseudomonas signal molecule 3-oxo-C12-homoserine lactone interferes with binding of rosiglitazone to human PPARgamma. Microbes Infect 12: 231–237. [DOI] [PubMed] [Google Scholar]
- 46. Gray KM, Passador L, Iglewski BH, Greenberg EP (1994) Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J Bacteriol 176: 3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shiner M, Fuhrman B, Aviram M (2007) Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis 195: 313–321. [DOI] [PubMed] [Google Scholar]
- 48. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, et al. (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95: 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ng CJ, Hama SY, Bourquard N, Navab M, Reddy ST (2006) Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol Genet Metab 89: 368–373. [DOI] [PubMed] [Google Scholar]
- 50. Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, et al. (2007) Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron 55: 37–52. [DOI] [PubMed] [Google Scholar]
- 51. Huang E, Qu D, Zhang Y, Venderova K, Haque ME, et al. (2010) The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat Cell Biol 12: 563–571. [DOI] [PubMed] [Google Scholar]
- 52. Aleyasin H, Cregan SP, Iyirhiaro G, O′Hare MJ, Callaghan SM, et al. (2004) Nuclear factor-(kappa)B modulates the p53 response in neurons exposed to DNA damage. J Neurosci 24: 2963–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, et al. (2003) Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol 23: 468–474. [DOI] [PubMed] [Google Scholar]
- 54. Desai VG, Feuers RJ, Hart RW, Ali SF (1996) MPP(+)-induced neurotoxicity in mouse is age-dependent: evidenced by the selective inhibition of complexes of electron transport. Brain Res 715: 1–8. [DOI] [PubMed] [Google Scholar]
- 55. Ramsay RR, Singer TP (1986) Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem 261: 7585–7587. [PubMed] [Google Scholar]
- 56. Ramsay RR, Kowal AT, Johnson MK, Salach JI, Singer TP (1987) The inhibition site of MPP+, the neurotoxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine is near the Q-binding site of NADH dehydrogenase. Arch Biochem Biophys 259: 645–649. [DOI] [PubMed] [Google Scholar]
- 57. Poirier J, Barbeau A (1985) A catalyst function for MPTP in superoxide formation. Biochem Biophys Res Commun 131: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 58. Sinha BK, Singh Y, Krishna G (1986) Formation of superoxide and hydroxyl radicals from 1-methyl-4-phenylpyridinium ion (MPP+): reductive activation by NADPH cytochrome P-450 reductase. Biochem Biophys Res Commun 135: 583–588. [DOI] [PubMed] [Google Scholar]
- 59. Shiner M, Fuhrman B, Aviram M (2004) Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med 37: 2052–2063. [DOI] [PubMed] [Google Scholar]
- 60. Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, et al. (2008) Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun 76: 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, et al. (2005) Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol Lett 253: 29–37. [DOI] [PubMed] [Google Scholar]
- 62. Yang F, Wang LH, Wang J, Dong YH, Hu JY, et al. (2005) Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett 579: 3713–3717. [DOI] [PubMed] [Google Scholar]
- 63. Draganov DI (2010) Lactonases with organophosphatase activity: structural and evolutionary perspectives. Chem Biol Interact 187: 370–372. [DOI] [PubMed] [Google Scholar]
- 64. Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, et al. (2005) Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 46: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 65. Precourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, et al. (2011) The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis 214: 20–36. [DOI] [PubMed] [Google Scholar]
- 66. Roshon M, DeGregori JV, Ruley HE (2003) Gene trap mutagenesis of hnRNP A2/B1: a cryptic 3′ splice site in the neomycin resistance gene allows continued expression of the disrupted cellular gene. BMC Genomics 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, et al. (2008) RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A 105: 10244–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hod Y, Pentyala SN, Whyard TC, El-Maghrabi MR (1999) Identification and characterization of a novel protein that regulates RNA-protein interaction. J Cell Biochem 72: 435–444. [PubMed] [Google Scholar]
- 69. Kaneko Y, Tajiri N, Shojo H, Borlongan CV (2014) Oxygen-glucose-deprived rat primary neural cells exhibit DJ-1 translocation into healthy mitochondria: a potent stroke therapeutic target. CNS Neurosci Ther 20: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ng CJ, Shih DM, Hama SY, Villa N, Navab M, et al. (2005) The paraoxonase gene family and atherosclerosis. Free Radic Biol Med 38: 153–163. [DOI] [PubMed] [Google Scholar]
- 71. Parsanejad M, Zhang Y, Qu D, Irrcher I, Rousseaux MW, et al. (2014) Regulation of the VHL/HIF-1 pathway by DJ-1. J Neurosci 34: 8043–8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Godeiro C Jr, Aguiar PM, Felicio AC, Barsottini OG, Silva SM, et al. (2010) PINK1 polymorphism IVS1-7 A–>G, exposure to environmental risk factors and anticipation of disease onset in Brazilian patients with early-onset Parkinson's Disease. Neurosci Lett 469: 155–158. [DOI] [PubMed] [Google Scholar]
- 73. Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, et al. (2011) Rotenone, Paraquat and Parkinson's Disease. Environ Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sanyal J, Chakraborty DP, Sarkar B, Banerjee TK, Mukherjee SC, et al. (2010) Environmental and familial risk factors of Parkinsons disease: case-control study. Can J Neurol Sci 37: 637–642. [DOI] [PubMed] [Google Scholar]
- 75. Saleh AM, Vijayasarathy C, Masoud L, Kumar L, Shahin A, et al. (2003) Paraoxon induces apoptosis in EL4 cells via activation of mitochondrial pathways. Toxicol Appl Pharmacol 190: 47–57. [DOI] [PubMed] [Google Scholar]
- 76. Ben-Shaul Y, Benmoyal-Segal L, Ben-Ari S, Bergman H, Soreq H (2006) Adaptive acetylcholinesterase splicing patterns attenuate 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Eur J Neurosci 23: 2915–2922. [DOI] [PubMed] [Google Scholar]
- 77. Altenhofer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, et al. (2010) One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem 285: 24398–24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM (2009) Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res 87: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, et al. (2005) Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 14: 2063–2073. [DOI] [PubMed] [Google Scholar]
- 80. Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, et al. (2010) Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet 19: 3734–3746. [DOI] [PubMed] [Google Scholar]