Abstract
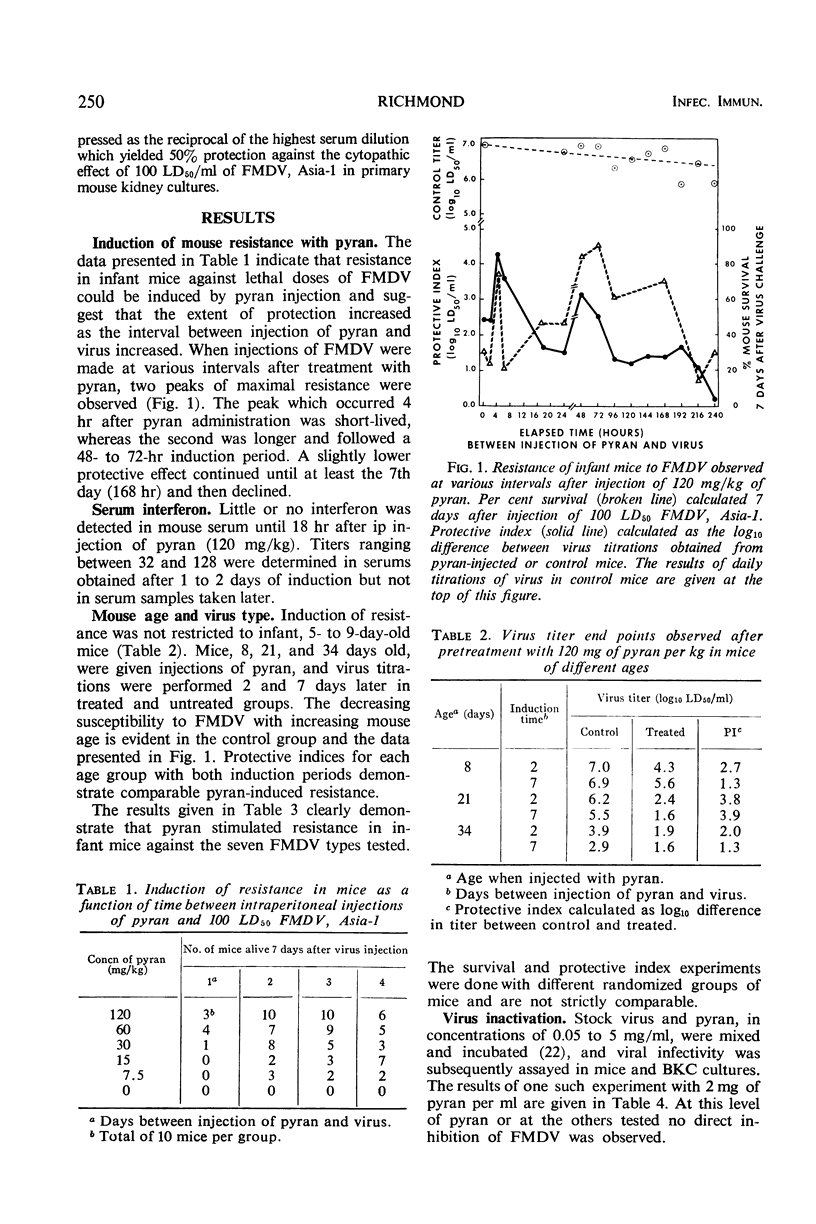
Mouse resistance to foot-and-mouth disease virus (FMDV) was induced by intraperitoneal injections of pyran copolymer. A biphasic pattern of protection occurred with greatest resistance 4 and 48 hr after injection of this polyanion. Viremia was not detectable in pretreated mice challenge-exposed with FMDV. Incubation of virus with pyran did not alter viral infectivity in mice or tissue culture. Serum interferon was demonstrated 1 and 2 days after pyran administration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun W., Nakano M. Antibody formation: stimulation by polyadenylic and polycytidylic acids. Science. 1967 Aug 18;157(3790):819–821. doi: 10.1126/science.157.3790.819. [DOI] [PubMed] [Google Scholar]
- Braun W., Regelson W., Yajima Y., Ishizuka M. Stimulation of antibody formation by pyran copolymer. Proc Soc Exp Biol Med. 1970 Jan;133(1):171–175. doi: 10.3181/00379727-133-34433. [DOI] [PubMed] [Google Scholar]
- Braun W. Relationships between the effects of poly I. poly C and endotoxin. Nature. 1969 Dec 6;224(5223):1024–1025. doi: 10.1038/2241024a0. [DOI] [PubMed] [Google Scholar]
- CHOAY J., DHENNIN L., THELY M., DHENNIN L. [Disorders produced in vivo in the multiplication of viral ribonucleotides]. C R Hebd Seances Acad Sci. 1960 Mar 21;250:2296–2298. [PubMed] [Google Scholar]
- Campbell C. H. Influence of litter size, age, variation, and selective breeding of mice on susceptibility to foot-and-mouth disease virus. Am J Vet Res. 1968 Mar;29(3):685–691. [PubMed] [Google Scholar]
- Campbell C. H. Relationship of donor age to adsorption of foot-and-mouth disease virus by mouse tissues. Am J Vet Res. 1968 Nov;29(11):2205–2212. [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Prolonged antiviral protection by interferon inducers. Proc Soc Exp Biol Med. 1969 Nov;132(2):699–703. doi: 10.3181/00379727-132-34291. [DOI] [PubMed] [Google Scholar]
- De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J Virol. 1968 Sep;2(9):886–893. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES I. J. INDUCTION OF MITOSIS IN MACROPHAGES BY ENDOTOXIN. J Immunol. 1965 Jan;94:37–39. [PubMed] [Google Scholar]
- Freedman H. H., Braun W. Influence of oligodeoxyribonucleotides on phagocytic activity of the reticuloendothelial system. Proc Soc Exp Biol Med. 1965 Oct;120(1):222–225. doi: 10.3181/00379727-120-30492. [DOI] [PubMed] [Google Scholar]
- Gorhe D. S. Inhibition of multiplication of foot and mouth disease virus in adult mice pretreated with Freund's complete adjuvant. Nature. 1967 Dec 23;216(5121):1242–1244. doi: 10.1038/2161242a0. [DOI] [PubMed] [Google Scholar]
- Ho M., Ke Y. H. The mechanism of stimution of interferon production by a complexed polyribonucleotide. Virology. 1970 Mar;40(3):693–702. doi: 10.1016/0042-6822(70)90214-x. [DOI] [PubMed] [Google Scholar]
- Levy H. B., Law L. W., Rabson A. S. Inhibition of tumor growth by polyinosinic-polycytidylic acid. Proc Natl Acad Sci U S A. 1969 Feb;62(2):357–361. doi: 10.1073/pnas.62.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh H. F., Lindsay H. L., Mayberry B. R., Forbes M. Polyinosinic-cytidylic acid complex (poly I:C) and viral infections in mice. Proc Soc Exp Biol Med. 1969 Oct;132(1):83–87. doi: 10.3181/00379727-132-34153. [DOI] [PubMed] [Google Scholar]
- Maes R. F., Fernandes M. V. Interaction between DEAE-dextran and nucleic acids. II. Effect on interferon production in vivo. Arch Gesamte Virusforsch. 1970;30(2):147–154. doi: 10.1007/BF01250182. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Finkelstein M. S. Interferon-stimulating and in vivo antiviral effects of various synthetic anionic polymers. Virology. 1968 Jul;35(3):363–374. doi: 10.1016/0042-6822(68)90215-8. [DOI] [PubMed] [Google Scholar]
- Merigan T. C. Induction of circulating interferon by synthetic anionic polymers of known composition. Nature. 1967 Apr 22;214(5086):416–417. doi: 10.1038/214416a0. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Regelson W. Interferon induction in man by a synthetic polyanion of defined composition. N Engl J Med. 1967 Dec 14;277(24):1283–1287. doi: 10.1056/NEJM196712142772403. [DOI] [PubMed] [Google Scholar]
- Neter E. Endotoxins and the immune response. Curr Top Microbiol Immunol. 1969;47:82–124. doi: 10.1007/978-3-642-46160-6_5. [DOI] [PubMed] [Google Scholar]
- ROCKSTEIN M., BRANDT K. Muscle enzyme activity and changes in weight in ageing white rats. Nature. 1962 Oct 13;196:142–143. doi: 10.1038/196142a0. [DOI] [PubMed] [Google Scholar]
- Regelson W. The antimitotic activity of polyanions (antitumor, antiviral, and antibacterial action of heparin, heparinoids, anionic dyes, and synthetic polymers). Adv Chemother. 1968;3:303–370. [PubMed] [Google Scholar]
- Regelson W. The growth-regulating activity of polyanions: a theoretical discussion of their place in the intercellular environment and their role in cell physiology. Adv Cancer Res. 1968;11:223–304. doi: 10.1016/s0065-230x(08)60389-9. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- Richmond J. Y., Hamilton L. D. Foot-and-mouth disease virus inhibition induced in mice by synthetic double-stranded RNA (polyriboinosinic and polyribocytidylic acids). Proc Natl Acad Sci U S A. 1969 Sep;64(1):81–86. doi: 10.1073/pnas.64.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J. Y. Interferons of foot-and-mouth disease virus: a new assay for interferon. Arch Gesamte Virusforsch. 1970;30(1):75–81. doi: 10.1007/BF01262585. [DOI] [PubMed] [Google Scholar]
- Schmidt J. P., Pindak F. F. Duration of prophylactic effect of statolon against mengovirus in mice. Appl Microbiol. 1968 Mar;16(3):468–469. doi: 10.1128/am.16.3.468-469.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. P., Pindak F. F. Statolon-induced resistance of mice to mengovirus. Appl Microbiol. 1967 May;15(3):654–656. doi: 10.1128/am.15.3.654-656.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THELY M., CHOAY J., DHENNIN L., DHENNIN L. [Virostasis induced in vivo by non-infectious ribonucleic acids]. C R Hebd Seances Acad Sci. 1963 Jan 21;256:1048–1050. [PubMed] [Google Scholar]
- Tunis M., Regelson W. The interaction of nucleated cells with anionic polyelectrolytes and inhibition of the interaction by pancreatic deoxyribonuclease. Exp Cell Res. 1965 Nov;40(2):383–395. doi: 10.1016/0014-4827(65)90271-5. [DOI] [PubMed] [Google Scholar]
- Turner W., Chan S. P., Chirigos M. A. Stimulation of humoral and cellular antibody formation in mice by poly Ir:Cr. Proc Soc Exp Biol Med. 1970 Jan;133(1):334–338. doi: 10.3181/00379727-133-34469. [DOI] [PubMed] [Google Scholar]
- Winchurch R., Braun W. Antibody formation: premature initiation by endotoxin or synthetic polynucleotides in newborn mice. Nature. 1969 Aug 23;223(5208):843–844. doi: 10.1038/223843a0. [DOI] [PubMed] [Google Scholar]
- Woodhour A. F., Friedman A., Tytell A. A., Hilleman M. R. Hyperpotentiation by synthetic double-stranded RNA of antibody responses to influenza virus vaccine in adjuvant 65. Proc Soc Exp Biol Med. 1969 Jul;131(3):809–817. doi: 10.3181/00379727-131-33983. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Interferon production in mice by double-stranded synthetic polynucleotides: induction or release? Virology. 1968 May;35(1):177–179. doi: 10.1016/0042-6822(68)90320-6. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R., Taube S. E. Influence of inhibitors of protein synthesis on interferon formation in mice. Virology. 1965 Dec;27(4):541–550. doi: 10.1016/0042-6822(65)90179-0. [DOI] [PubMed] [Google Scholar]


