Abstract
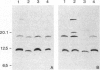
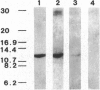
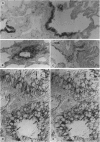
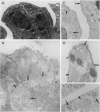
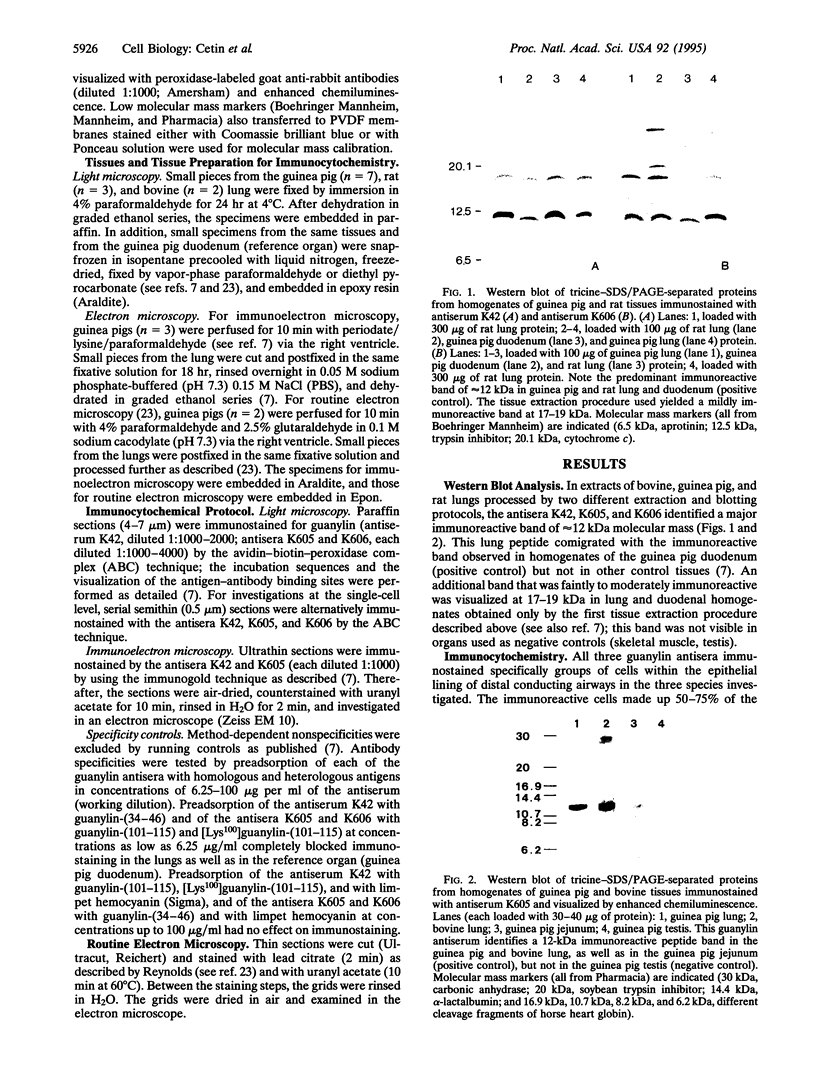
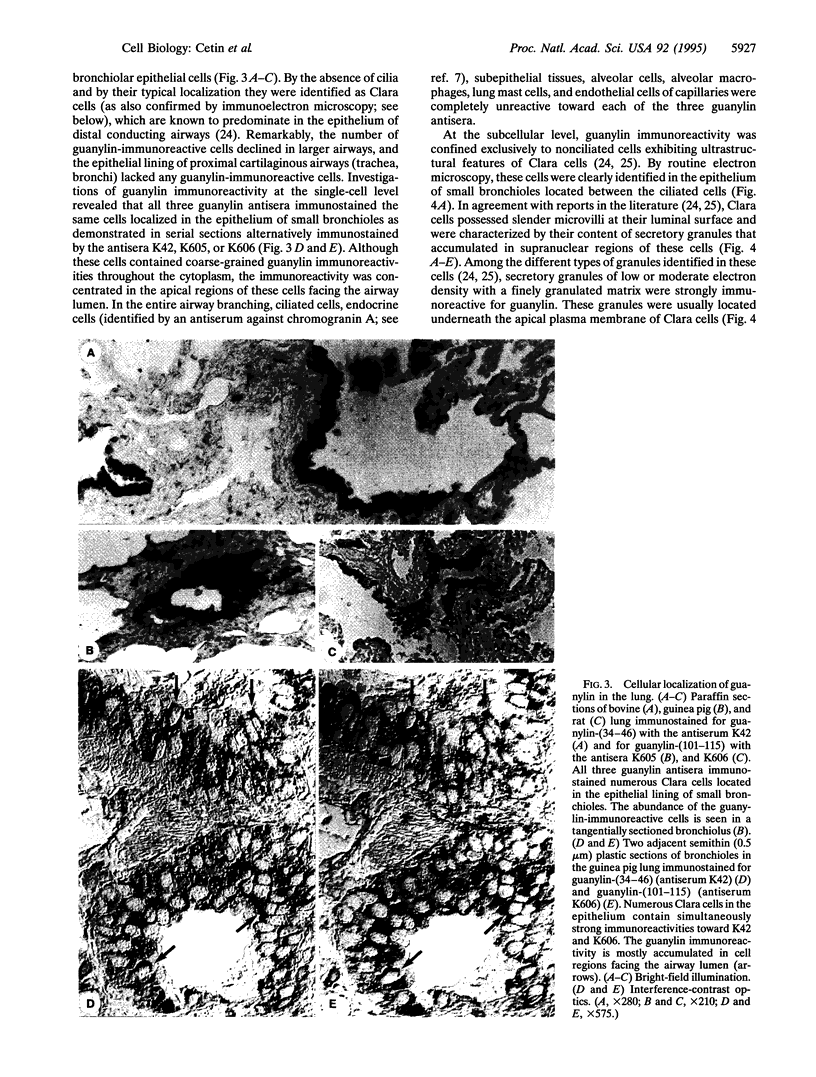
The peptide guanylin, which has recently been isolated from the intestine, is involved in the regulation of fluid secretion in the intestinal epithelium by activation of guanylate cyclase C, the putative guanylin receptor. Since the latter protein is also expressed in airway epithelia, we investigated the lung of three mammalian species for the presence and cellular localization of guanylin by immunoblot (Western blot) analyses and light and electron microscopical immunocytochemistry. In Western blots of bovine, guinea pig, and rat lung extracts, three different guanylin antisera directed against the midportion and against the C terminus of the precursor molecule identified a peptide band corresponding to the apparent molecular mass of guanylin. Localization studies in the lung revealed that guanylin is exclusively confined to nonciliated secretory (Clara) cells in the lining of distal conducting airways. The presence of guanylin in the lung and particularly its specific localization to Clara cells indicate that these cells may play a pivotal role in the local (paracrine) regulation of electrolyte/water transport in airway epithelia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Duguay F., Naismith A. L., Kartner N., Hanrahan J. W., Riordan J. R. Cl- channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J Biol Chem. 1991 Oct 15;266(29):19142–19145. [PubMed] [Google Scholar]
- Bernard A., Dumont X., Roels H., Lauwerys R., Dierynck I., De Ley M., Stroobant V., de Hoffmann E. The molecular mass and concentrations of protein 1 or Clara cell protein in biological fluids: a reappraisal. Clin Chim Acta. 1993 Dec 31;223(1-2):189–191. doi: 10.1016/0009-8981(93)90077-h. [DOI] [PubMed] [Google Scholar]
- Cetin Y. A novel endocrine cell type in the guinea-pig gastric mucosa: cellular source of pro-enkephalin-derived peptides. A histochemical, immunohistochemical, and electron microscopical characterization. Histochemistry. 1990;94(1):31–44. doi: 10.1007/BF00266787. [DOI] [PubMed] [Google Scholar]
- Cetin Y., Kuhn M., Kulaksiz H., Adermann K., Bargsten G., Grube D., Forssmann W. G. Enterochromaffin cells of the digestive system: cellular source of guanylin, a guanylate cyclase-activating peptide. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2935–2939. doi: 10.1073/pnas.91.8.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. C., de Sauvage F. J., Dong Y. J., Wagner J. A., Goeddel D. V., Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994 Mar 1;13(5):1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Hickman M. E., MacVinish L. J., Evans M. J., Colledge W. H., Ratcliff R., Seale P. W., Humphrey P. P. Chloride secretion in response to guanylin in colonic epithelial from normal and transgenic cystic fibrosis mice. Br J Pharmacol. 1994 May;112(1):31–36. doi: 10.1111/j.1476-5381.1994.tb13024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt J. F., Zepeda M., Cohn J. A., Yankaskas J. R., Wilson J. M. Expression of the cystic fibrosis gene in adult human lung. J Clin Invest. 1994 Feb;93(2):737–749. doi: 10.1172/JCI117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte L. R., Eber S. L., Turner J. T., Freeman R. H., Fok K. F., Currie M. G. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest. 1993 Jun;91(6):2423–2428. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Hill O., Kuhn M., Zucht H. D., Cetin Y., Kulaksiz H., Adermann K., Klock G., Rechkemmer G., Forssmann W. G., Mägert H. J. Analysis of the human guanylin gene and the processing and cellular localization of the peptide. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2046–2050. doi: 10.1073/pnas.92.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn K. H. Functional morphology of the distal lung. Int Rev Cytol. 1974;37(0):153–270. doi: 10.1016/s0074-7696(08)61359-5. [DOI] [PubMed] [Google Scholar]
- Kita T., Smith C. E., Fok K. F., Duffin K. L., Moore W. M., Karabatsos P. J., Kachur J. F., Hamra F. K., Pidhorodeckyj N. V., Forte L. R. Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol. 1994 Feb;266(2 Pt 2):F342–F348. doi: 10.1152/ajprenal.1994.266.2.F342. [DOI] [PubMed] [Google Scholar]
- Li Z., Goy M. F. Peptide-regulated guanylate cyclase pathways in rat colon: in situ localization of GCA, GCC, and guanylin mRNA. Am J Physiol. 1993 Aug;265(2 Pt 1):G394–G402. doi: 10.1152/ajpgi.1993.265.2.G394. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Singh G., Mason R., Plopper C. G., Malkinson A. M., Gail D. B. Biology of the Clara cell. Am J Physiol. 1994 Jan;266(1 Pt 1):L101–L106. doi: 10.1152/ajplung.1994.266.1.L101. [DOI] [PubMed] [Google Scholar]
- McCray P. B., Jr, Bettencourt J. D., Bastacky J. Developing bronchopulmonary epithelium of the human fetus secretes fluid. Am J Physiol. 1992 Mar;262(3 Pt 1):L270–L279. doi: 10.1152/ajplung.1992.262.3.L270. [DOI] [PubMed] [Google Scholar]
- McCray P. B., Jr, Welsh M. J. Developing fetal alveolar epithelial cells secrete fluid in primary culture. Am J Physiol. 1991 Jun;260(6 Pt 1):L494–L500. doi: 10.1152/ajplung.1991.260.6.L494. [DOI] [PubMed] [Google Scholar]
- Plopper C. G. Comparative morphologic features of bronchiolar epithelial cells. The Clara cell. Am Rev Respir Dis. 1983 Aug;128(2 Pt 2):S37–S41. doi: 10.1164/arrd.1983.128.2P2.S37. [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Hill L. H., Mariassy A. T. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res. 1980 Jun;1(2):171–180. doi: 10.3109/01902148009069646. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Schulz S., Chrisman T. D., Garbers D. L. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992 Aug 15;267(23):16019–16021. [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Singh G., Katyal S. L., Brown W. E., Phillips S., Kennedy A. L., Anthony J., Squeglia N. Amino-acid and cDNA nucleotide sequences of human Clara cell 10 kDa protein. Biochim Biophys Acta. 1988 Sep 7;950(3):329–337. doi: 10.1016/0167-4781(88)90129-7. [DOI] [PubMed] [Google Scholar]
- Singh G., Singh J., Katyal S. L., Brown W. E., Kramps J. A., Paradis I. L., Dauber J. H., Macpherson T. A., Squeglia N. Identification, cellular localization, isolation, and characterization of human Clara cell-specific 10 KD protein. J Histochem Cytochem. 1988 Jan;36(1):73–80. doi: 10.1177/36.1.3275712. [DOI] [PubMed] [Google Scholar]
- Stinson S. F., Loosli C. G. Ultrastructural evidence concerning the mode of secretion of electron-dense granules by Clara cells. J Anat. 1978 Oct;127(Pt 2):291–298. [PMC free article] [PubMed] [Google Scholar]
- Tien X. Y., Brasitus T. A., Kaetzel M. A., Dedman J. R., Nelson D. J. Activation of the cystic fibrosis transmembrane conductance regulator by cGMP in the human colonic cancer cell line, Caco-2. J Biol Chem. 1994 Jan 7;269(1):51–54. [PubMed] [Google Scholar]
- Trezise A. E., Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991 Oct 3;353(6343):434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Kato J., Currie M. G. Rat guanylin cDNA: characterization of the precursor of an endogenous activator of intestinal guanylate cyclase. Biochem Biophys Res Commun. 1992 Jun 30;185(3):812–817. doi: 10.1016/0006-291x(92)91699-q. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Kato J., Huang M. D., Fok K. F., Kachur J. F., Currie M. G. Human guanylin: cDNA isolation, structure, and activity. FEBS Lett. 1992 Oct 19;311(2):150–154. doi: 10.1016/0014-5793(92)81387-2. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]