Abstract
Objectives
Evidence suggests eosinophils may be acting as antigen-presenting cells (APCs) by presenting antigen to T cells. We investigated the surface proteins of eosinophils and T cells in the esophageal biopsies of patients with eosinophilic esophagitis (EoE), patients with gastroesophageal reflux disease (GERD), and healthy controls (HCs).
Methods
Subjects were categorized as EoE, GERD, or HC. In esophageal tissue, EG2+ eosinophils were stained for the APC markers, CD40 or CD80, via immunohistochemistry. CD3+ T cells were stained for costimulatory markers, CD40L or CD28, and for activation markers, CD69 or CD134, via immunofluorescence or immunohistochemistry.
Results
Eosinophils stained with CD40 and CD80. The number of EG2+ CD40+ cells was increased in EoE (mean 19.1 ± 14.8 cells/high-power field [HPF], n = 11), compared with GERD (mean 0.13 ± 0.19 cells/HPF, n = 5, P < 0.01) and HC (mean 0.3 ± 0.7 cells/HPF, n = 5, P < 0.01). There was an elevation in EG2+CD80+ cells in EoE (mean 18.1 ± 16.2 cells/HPF, n = 10), GERD (mean 1.7 ± 2.8 cells/HPF, n = 6, P < 0.01), or HC (mean 0.8 ± 1.3 cells/HPF, n = 6, P < 0.01). CD3+ T cells stained with CD40L (not quantified). CD3+ T cells stained with CD28 at elevated levels in EoE (mean 14 ± 8.7 cells/HPF, n = 9) versus GERD (mean 3.3 ± 1.2 cells/HPF, n = 6, P < 0.05) or HC (mean 3.0 ± 3.2 cells/HPF, n = 7, P < 0.01). The number of CD3+CD69+ cells was highest in EoE (mean 14.8 ± 7.5 cells/HPF, n = 6) versus GERD (mean 0.8 ± 0.9 cells/HPF, n = 6, P < 0.001) or HC (mean 2.7 ± 2.5 cells/HPF, n = 6, P < 0.001).
Conclusions
We show that esophageal eosinophils express CD40 and CD80, and T cells with CD40L, CD28, and CD69. The number of double-stained cells was higher in EoE in comparison to control groups. These data support the hypothesis that eosinophils in EoE may act as APCs, activating T cells.
Keywords: antigen-presenting cells, eosinophilic esophagitis, eosinophils, T-cell activation
Eosinophilic esophagitis (EoE) is an emerging clinicopathologic disease characterized by the abnormal infiltration of eosinophils into the esophageal mucosa. EoE typically presents with nonspecific upper gastrointestinal symptoms such as emesis, poor feeding, and epigastric pain, and can clinically be indistinguishable from gastroesophageal reflux disease (GERD). As supported by the high association with other atopic conditions such as asthma and food allergies, EoE is believed to be triggered by food and environmental allergens, implying an interaction between adaptive immunity, that is the T cells, and eosinophils. The underlying pathogenesis of EoE is not well characterized; specifically, how eosinophils interact with T cells remains unknown (1,2).
Previous work from our laboratory and other supports T-cell involvement in the disease pathogenesis of EoE (3). Increased levels of CD3+FoxP3+ cells were found in the esophageal biopsies of patients with EoE, compared with healthy controls (HCs) and patients with GERD. Additionally, performing real-time quantitative polymerase chain reaction assays on the RNA purified from esophageal biopsies revealed 1.5-fold higher FoxP3 mRNA expression in EoE in comparison with control groups (4).
Additional studies on esophageal biopsies investigate the role of eosinophils in local inflammation. Recent data suggest that eosinophils in several allergic conditions may be acting as antigen-presenting cells (APCs) by presenting processed antigen to T cells and initiating the inflammatory cascade (Fig. 1) (5–10). Previous work from our laboratory found major histocompatibility complex (MHC) class II molecules or human leukocyte antigen, HLA-DR, expressed on eosinophils. Moreover, the proportion of HLA-DR expression was found to be higher in EoE than in either HC or GERD groups, suggesting eosinophils may be playing a role in antigen presentation (11).
Figure 1.
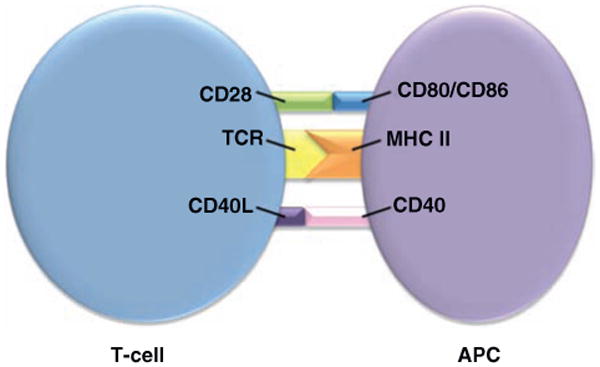
Schematic of T-cell and antigen-presenting cell (APC) interaction. TCR = T-cell receptor, CD28 and CD40L, and MHC II = major histocompatiblity complex, CD80 and CD40 on T cells and APC, respectively.
In the present study, we hypothesized that eosinophils may act at APCs and activate T cells. To test this, we studied whether costimulatory markers CD28 and CD40L (typical of T cells), and CD80 and CD40 (typical of APCs), were present on eosinophils and T cells, respectively, from subjects with EoE. In the same samples, we also investigated several markers of T-cell activation including CD 69, the earliest inducible marker of activation, and CD134, an acute marker that occurs within days. Studies were performed on esophageal biopsies of subjects with EoE and compared with those of patients with GERD and HCs.
Methods
Patient Selection
Pediatric and adult patients undergoing upper endoscopy for upper gastrointestinal symptoms at Lucile Packard Children's Hospital or California Pacific Medical Center were eligible to participate in the present study (Table 1). Patients willing to participate were consented before the endoscopy. Subjects who had no pathological basis for symptoms and who had a negative pH probe were considered HCs. Patients were excluded from the study if they were diagnosed as having eosinophilic gastroenteritis or colitis, inflammatory bowel disease, infection, autoimmune disease, celiac disease, or oncologic process. Systemic steroid use also precluded participation in the study. Patients using topical steroids or on diet elimination were eligible to participate. Information regarding medication use, allergies, and diet restrictions was collected when possible. The study was performed under the Stanford University institutional review board protocols according to the Declaration of Helsinki guidelines.
Table 1. Patient demographics.
| Patient no. | Diagnosis | Age | Sex | Allergies | Therapy | Antibodies |
|---|---|---|---|---|---|---|
| 1 | EoE | 4 y | M | Food allergies | Diet elimination | CD28, CD40 (IHC, IF), CD40L, CD69, CD80 |
| 2 | EoE | 8 y | M | Multiple food allergies | CD28, CD40 (IHC), CD40L, CD69, CD80, CD134 | |
| 3 | EoE | 18 y | M | None | CD28, CD40 (IHC, IF), CD69 | |
| 4 | EoE | 17 y | M | None | CD28, CD40 (IHC), CD40L, CD80 | |
| 5 | EoE | 15 y | M | None | PPI, viscous budesonide | CD28, CD40 (IHC, IF), CD40L, CD80, CD134 |
| 6 | EoE | 9 y | M | None | CD28, CD40 (IHC), CD40L, CD69, CD80, CD134 | |
| 7 | EoE | 19 y | M | Multiple food allergies | PPI, swallowed fluticasone | CD28, CD40 (IHC, IF), CD40L, CD80, CD134 |
| 8 | EoE | 16 y | F | Food allergy | PPI | CD28, CD40 (IHC, IF), CD40L, CD69, CD80 |
| 9 | EoE | 3 y | M | Multiple food allergies | H2-blocker | CD28, CD40 (IHC, IF), CD40L, CD69, CD80 |
| 10 | EoE | 4 y | M | Food allergy | CD40 (IHC, IF), CD40L, CD80, CD134 | |
| 11 | EoE | 11 y | M | Food allergy | CD80, CD134 | |
| 12 | EoE | 2 y | F | None | CD40 (IHC, IF), CD40L | |
| 13 | EoE | 13 y | M | Food and environmental allergies | Diet elimination, swallowed fluticasone | CD134 |
| 14 | EoE | 17 y | M | Multiple food allergies | CD134 | |
| 15 | GERD | 15 y | M | CD28, CD40 (IHC, IF), CD40L, CD69, CD80, | ||
| 16 | GERD | 15 mo | M | Food allergies | CD28, CD40 (IHC), CD69 | |
| 17 | GERD | 18 y | M | PPI | CD28, CD80 | |
| 18 | GERD | 12 y | F | CD28, CD69, CD80 | ||
| 19 | GERD | 10 y | F | CD28, CD40 (IHC), CD40L, CD69, CD80, CD134 | ||
| 20 | GERD | 2 mo | M | CD28, CD40 (IHC, IF), CD69, CD80 | ||
| 21 | GERD | 6 y | F | PPI | CD40 (IHC), CD69 | |
| 22 | GERD | 16 y | M | Food and environmental allergies | CD80, CD134 | |
| 23 | HC | 4 y | M | CD28, CD40 (IHC), CD69, CD80, | ||
| 24 | HC | 2 y | F | CD28 | ||
| 25 | HC | 11 y | F | CD28, CD40L, CD69, CD80 | ||
| 26 | HC | 2 y | M | Food allergy | CD28, CD40 (IHC), CD80 | |
| 27 | HC | 2 y | M | CD28, CD40 (IHC), CD80 | ||
| 28 | HC | 4 y | F | CD28, CD40 (IHC, IF), CD69, CD80 | ||
| 29 | HC | 6 y | F | CD28, CD40 (IHC), CD69, CD80 | ||
| 30 | HC | 4 y | M | Many food allergies, asthma | CD134 | |
| 31 | HC | 10 y | F | Food and environmental allergies | Diet elimination | CD134 |
| 32 | HC | 6 y | F | CD40L, CD69 | ||
| 33 | HC | 18 y | F | CD69 |
EoE = eosinophilic esophagitis; GERD = gastroesophageal reflux disease; H2-blocker = H2-receptor antagonist; HC = healthy control; IF = immunofluorescence; IHC = immunohistochemistry; PPI = proton pump inhibitor.
According to EoE general clinical guidelines, we used the histologic criteria of ≥ 15 eosinophils/high-power field (HPF) in conjunction with the presentation of upper gastrointestinal symptoms to define the EoE group (1). HCs were considered those who had no clinicopathological basis for their upper gastrointestinal symptoms. Patients who presented with upper gastrointestinal symptoms and/or had a positive pH probe study, as well as <15 eosinophils/HPF, were diagnosed as having GERD. Clinical pathologist in the Stanford University's Department of Pathology made the histologic diagnosis using hematoxylin and eosin, according to standard of care.
Sample Collection
Proximal and distal esophageal biopsies were collected from subjects during endoscopy. Samples were immediately placed in formalin after collection and later, were paraffin embedded by the Stanford University's Department of Pathology. Separate sample sets were used for histologic diagnosis by the pathologist, and investigation in our study.
Immunohistochemistry
We used a random combination of both proximal and distal esophageal biopsies throughout our study. Paraffin-embedded esophageal tissues were placed in xylene, then sequentially in 100%, 95%, and 70% ethanol to remove the paraffin wax. To unmask antigen, slides were placed in a pressure cooker to 120°C in Diva Decloaking buffer (Biocare Medical, Concord, CA), and then cooled. To block endogenous peroxidase and non-specific binding, H2O2 and protein blocks were applied. Tissue was exposed to antihuman primary antibodies for 2 hours or overnight at varying concentrations: mouse EG2 (1:150, kind gifts from Dr Reinhard Raggam and Phadia, Uppsala, Sweden), rabbit CD40 (1:150, Abcam, Cambridge, MA), mouse CD28 (1:250, BD Biosciences, San Jose, CA), rabbit CD3 (1:150, Vector Labs, Burlingame, CA), mouse CD134 (1:50, Biolegend, San Diego, CA), and mouse CD69 (1:50, Abcam). Following washes, secondary antibodies or MACH 2 Double Stain (mouse horseradish peroxidase and rabbit alkaline phosphatase, BioCare, Concord, CA) was incubated for 30 minutes. Slides were developed with 3,3′-diaminobenzidine and ferengi blue, and then mounted for visualization. Cells were then counted under HPF (×400) at 3 different areas of highest eosinophil infiltration in the tissue.
Immunohistochemistry With Biotin-Strepavidin
Similar to immunohistochemistry (IHC), slides underwent the same procedure for paraffin wax removal and antigen unmasking. H2O2 and goat serum (5%, Jackson ImmunoResearch, West Grove, PA) were used to block endogenous peroxidase and non-specific binding, respectively. Tissue was exposed to anti-human primary antibody rabbit CD80 (1:500, Epitomics, Burlingame, CA) for 2 hours or overnight. Following washes with phosphate-buffered saline Tween-20 (Abcam), biotin-SP-conjugated goat anti-rabbit was applied for 30 minutes (1:2000, Jackson ImmunoResearch). Slides were washed and then incubated with strepavidin conjugated with alkaline phosphatase (2 μg/mL, Jackson ImmunoResearch) and MACH 2 mouse horseradish peroxidase (BioCare) for 30 minutes. Following another set of washes, samples were then developed and mounted as above.
Immunofluorescence
Similar to IHC, slides underwent the same procedure for paraffin wax removal and antigen unmasking. H2O2 and donkey serum (5%, Abcam) were used to block endogenous peroxidase and nonspecific binding, respectively. Tissue was exposed to anti-human primary antibodies for 2 hours or overnight: mouse EG2 (1:150, kind gifts from Dr Reinhard Raggam and Phadia), rabbit CD40 (1:150, Abcam), mouse CD40L (1:50, Santa Cruz Biotech, Santa Cruz, CA), rat HLA-DR (1:75, Biolegend) and rabbit CD3 (1:150, Vector Labs). Following washes, donkey anti-mouse Alexa Fluor 594, donkey anti-rabbit Alexa Fluor 350, and donkey anti-rat Alexa Fluor 488 (1:500, all Invitrogen, Grand Island, NY) were applied. Slides were then mounted for visualization.
Statistics
We used the GraphPad Prism software (GraphPad Software, La Jolla, CA) for statistical analysis. To determine stastical significance, we used the 1-way analysis of variance nonparametric Kruskal-Wallis test and Dunn posttest with a P value of <0.05 used as the threshold for significance.
Results
Eosinophil Staining
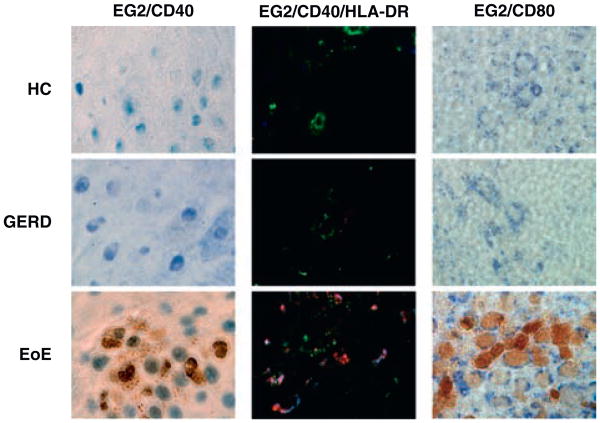
To identify eosinophils, we used a monoclonal EG2 antibody, which has been established to reliably identify eosinophils (12,13). Esophageal tissue was double stained with EG2 and APC cell surface proteins, CD40 receptor or CD80 ligand. Using IHC, we found the majority of all eosinophils (78%) in the patients with EoE were costained with CD40 (n = 11) (Fig. 2) versus (0%–8%) in the HC tissue. The percentage of the few eosinophils that also double stained for CD40 was 66% in GERD (n = 5), and 100% in HC (n = 5). The number of EG2+CD40+ cells was significantly elevated in the EoE group (mean 19.1 ± 14.8 cells/HPF) in comparison with the control groups, GERD (mean 0.13 ± 0.19 cells/HPF, P < 0.01), and HC (mean 0.3 ± 0.7 cells/HPF, P < 0.01) (Fig. 3A). CD40+ staining was observed in cells other than eosinophils. The number of these unidentified EG2–CD40+ showed great variability among the samples with EoE (mean 99.3 ± 25.1 cells/HPF), GERD (mean 53.7 ± 26.4 cells/HPF, P < 0.05), and HC (mean 63.7 ± 49.2 cells/HPF, P > 0.05). Similar findings occurred in immunofluorescence, in which EG2+ cells costained with CD40, as well as HLA-DR (quantification was not possible given the absence of a nuclear stain to identify individual cells) (Fig. 2). Approximately, 64% of EG2+ eosinophils were CD80+ in the patients with EoE (n = 10). The percentage of eosinophils that were CD80+ in GERD and HC was 87% (n = 6) and 100% (n = 6), respectively (Fig. 2). The number of double-stained EG2+CD80+ cells was highest in EoE (mean 18.1 ± 16.2 cells/HPF) versus GERD (mean 1.7 ± 2.8 cells/HPF, P < 0.01) or HC (mean 0.8 ± 1.3 cells/HPF, P < 0.01) (Fig. 3B). We also observed EG2 – cells that stained with CD80. The number of EG2–CD80+ cells was highest in EoE (mean 158.2 ± 63.1 cells/HPF) in comparison to GERD (mean 96.8 ± 32.1 cells/HPF, P > 0.05) or HC (mean 81.2 ± 24.8 cells/HPF, P < 0.05).
Figure 2.
Representative eosinophil staining, (400×). Left column: EG2+(brown), CD40+(blue), (IHC). Middle column: EG2+(red), HLA-DR+(green), CD40+(blue), (IF). Right column: EG2+(brown), CD80+(blue), (IHC). EG2 = eosinophil cationic protein-clone EG2; EoE = eosinophilic esophagitis; GERD = gastroesophageal reflux disease; HC = healthy control; HLA-DR = human leukocyte antigen-DR; IF = immunofluorescence; IHC = immunohistochemistry.
Figure 3.
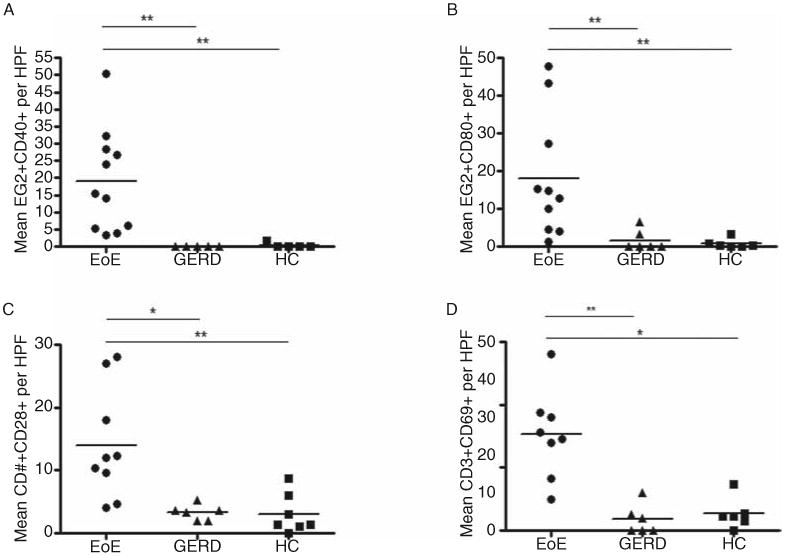
Comparison of double-stained cells. A, Number of tissue EG2+ eosinophils that demonstrated double staining with CD40+ among patients with EoE, GERD, and HC. B, Same for EG2+ eosinophils and CD80+ double staining among all groups. C, Number of CD3+ T cells that demonstrated double staining for CD28+ among all groups. D, Same for CD3+ T cells and CD69+ among all groups. EoE = eosinophilic esophagitis; GERD = gastroesophageal reflux; HC = healthy control.
T-Cell Staining
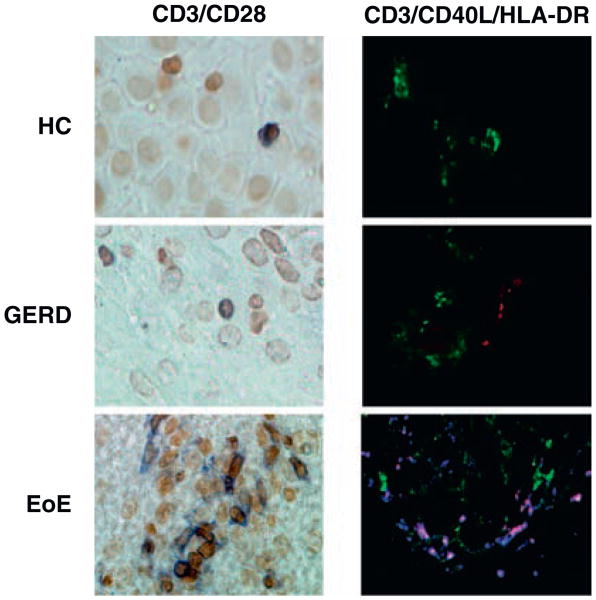
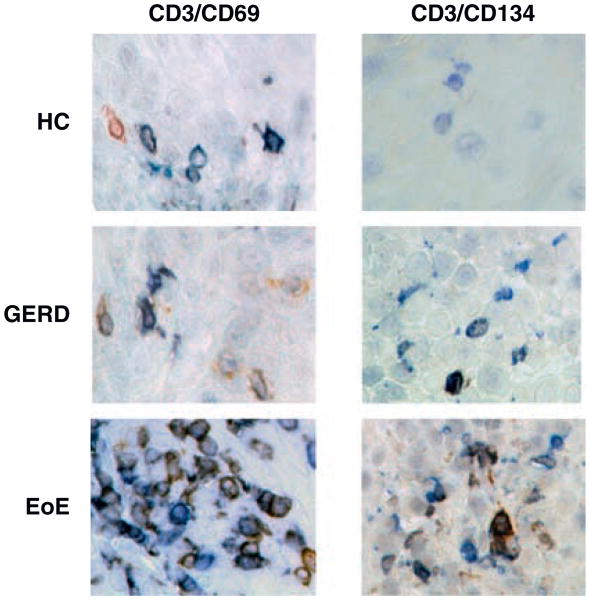
T cells were identified by activation marker CD3. In IHC, the number of CD3+ T cells in patients with EoE (mean 19.6 ± 20.3 cells/HPF, n = 9) was compared with HC (mean 5.2 ± 4.0 cells/HPF, n = 10, P < 0.05) and GERD (mean 6.8 ± 3.5 cells/HPF, n = 8, P > 0.05). We also found CD3+ T cells costained with CD40L using immunofluorescence (cells not quantified). We did not observe any CD3+CD40L+ cells staining for HLA-DR (Fig. 4). T-cell receptor, CD28, was present in 64% of T cells in EoE (n = 9), 67% GERD (n = 6), and 69% HC (n = 7) (Fig. 4). The number of CD3+CD28+ cells was significantly elevated in EoE (mean 14 ± 8.7 cells/HPF, n = 9) patients versus GERD (mean 3.3 ± 1.2 cells/HPF, n = 6, P < 0.05) or HC (mean 3.0 ± 3.2 cells/HPF, n = 7, P < 0.01) (Fig. 3C). Lastly, in IHC, we found CD3+ T cells costained with CD69 and CD134 (Fig. 5). The proportion of CD3+ cells that were also CD69+ was 75%, 12%, and 33% in EoE (n = 6), GERD (n = 6), and HC (n = 6), respectively. Also, the mean number of CD3+CD69+ cells was highest in EoE (mean 14.8 ± 7.5 cells/HPF) versus either GERD (mean 0.8 ± 0.9 cells/HPF, P < 0.001) or HC (mean 2.7 ± 2.5 cells/HPF, P < 0.001) (Fig. 3D). Preliminary data on CD134 showed that some patients with EoE had elevated CD3+CD134+ cells (Fig. 5).
Figure 4.
Representative T-cell staining (original magnification ×400). Left column: CD3+(blue), CD28+(brown), (IHC). Right column: CD3+(blue), CD40L+(red), HLA-DR+(green), (IF). EoE = eosinophilic esophagitis; GERD = gastroesophageal reflux disease; HC = healthy control; IF = immunofluorescence; IHC = immunohistochemistry.
Figure 5.
Representative T-cell activation staining, (original magnification ×400). Left column: CD3+ (blue), CD69+ (brown), (IHC). Right column: CD3+ (blue), CD134/OX40+ (brown) (IHC). EoE = eosinophilic esophagitis; GERD = gastroesophageal reflux disease; HC = healthy control; IHC = immunohistochemistry.
Discussion
The hallmark of EoE is marked eosinophilic infiltration in the esophageal mucosa; however, the role these eosinophils play in the underlying pathogenesis is not well characterized. It is unclear whether eosinophils are simple bystanders of disease or active participants driving the inflammatory process.
Recent evidence in mice models and in vitro suggests eosinophils may play a central role as APC, processing and presenting antigen to T cells (5,6,9). In asthmatics, airway eosinophils have been shown to express MHC class II protein, HLA-DR (8). Additionally, following exposure to allergen, eosinophils in the airway of allergic patients were found to process antigen and promote lymphocytic proliferation (7). Previous work from our laboratory was the first to suggest that eosinophils in EoE may also be acting as APC by demonstrating esophageal eosinophils express HLA-DR (11).
The present study investigates several costimulatory proteins that are integral for APC and T-cell communication. Following TCR and MHC class II binding, costimulatory proteins CD40, CD80 (B7.1), and CD86 (B7.2) are upregulated by the APC. T-cell activation occurs following binding of CD40 with CD40L (CD154), and CD80 or CD86 with CD28, on APC and T cells, respectively. In esophageal biopsies, we found that EG2+ eosinophils costain with CD40 and CD80, and that the number of double-stained cells was significantly elevated in EoE in comparison to either GERD or HC tissue. Such findings support the hypothesis that eosinophils in EoE may play an active role as APC, driving inflammation and tissue remodeling in esophageal mucosa. Of note, we found cells other than eosinophils expressed both CD40 and CD80—not surprising findings as both proteins are known to be present on B cells, dendritic cells, and macrophages. Additionally, CD80 has been found on epithelial cells (14). The role these cells play in the disease pathways of EoE is under investigation (15).
Moreover, studies support Th2-cell signaling in EoE pathogenesis, which is crucial in mediating allergic responses. T cells, unlike B cells, are required for trafficking of eosinophils into the esophagus (16). We found elevated levels of CD3+ T cells in esophageal tissue of EoE when compared with HC (17,18). Interleukins (IL) produced by T cells such as IL-13 and IL-5 are critical to eosinophil recruitment and activity (19–21). Upon allergen stimulation, T cells produce IL-13, which in turn leads to over-expression of eotaxin-3 and eosinophil attraction (22,23). IL-5 mediates eosinophilic-induced collagen deposition and tissue remodeling. Clinical trials show IL-5 antibodies decrease esophageal eosinophilic infiltration in patients with EoE (24).
The results from our study presented here provide insights into the role of T cells in EoE. We found that CD3+ T cells stain positive for both CD28 and CD40L. The number of CD3+CD28+ and CD3+CD40L cells was elevated in EoE in comparison with controls providing additional support that antigen presentation may play an important role in disease. We also investigated nonconstitutive markers (unlike CD28) involved in T-cell activation, CD69 and CD134. CD69, the earliest inducible protein following lymphocyte activation, has been found to be upregulated in allergic airway in chronic inflammatory conditions (25). CD134 (OX40), a tumor necrosis factor receptor, has been shown to have a role in allergic conditions of the esophagus and airway. CD134 is also believed to be a marker of acute not chronic inflammation, appearing within several days of activation (26,27). We found CD3+ T cells stain for CD69, and elevated levels in CD3+CD69+ cells in EoE versus HC or GERD. For some but not all patients with EoE, we found elevated levels of CD3+CD134+ cells. This subset of patients was observed to have other untreated atopic conditions (eg, food allergies, asthma). Because this was a cross-sectional study, we have not been able to investigate the initial steps of immune activation during EOE. We hypothesize that eosinophils and T cells interact in a circular pathway to promote the pathology of EOE; however, to date we have not yet determined whether eosinophils are first recruited to the site of inflammation and then stimulate antigen-specific T cells. Alternatively, T cells could be activated by other resident or infiltrating professional APCs (eg, macrophages, dendritic cells).
In summary, the present study provides initial data to help determine how eosinophils and T cells may interact during EoE pathogenesis. We demonstrate that proteins involved in antigen presentation are present on both eosinophils (CD40 and CD80) and T cells (CD40L and CD28). Moreover, we found acute markers of T-cell activation elevated in EoE. These data add additional support to the hypothesis that eosinophils in EoE are processing and presenting allergen to T cells, and could be involved in T-cell activation, inflammation, and tissue remodeling.
Acknowledgments
The authors thank the patients and families involved in the present study for their generous support. The authors additionally thank Dr Eric Sibley, Dr K.T. Park, Dr John Kerner, Dr Dorsey Bass, and Amanda Jacobson for their kind assistance with this project.
This study was supported by the Transplant and Tissue Engineering Center for Excellence Endowment Fund (Le-Carlson, Seki) and the Harry Lyon Machen Fellowship Award (Le-Carlson).
Footnotes
The authors report no conflicts of interest.
See “Antigen Presentation by Eosinophils in Eosinophilic Esophagitis?” by Davis and Rothenberg on page 242.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3e6–20e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Tantibhaedhyangkul U, Tatevian N, Gilger MA, et al. Increased esophageal regulatory T cells and eosinophil characteristics in children with eosinophilic esophagitis and gastroesophageal reflux disease. Ann Clin Lab Sci. 2009;39:99–107. [PubMed] [Google Scholar]
- 4.Fuentebella J, Patel A, Nguyen T, et al. Increased number of regulatory T cells in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:283–9. doi: 10.1097/MPG.0b013e3181e0817b. [DOI] [PubMed] [Google Scholar]
- 5.Hansel TT, Braunstein JB, Walker C, et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–7. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung YJ, Woo SY, Jang MH, et al. Human eosinophils show chemo-taxis to lymphoid chemokines and exhibit antigen-presenting-cell-like properties upon stimulation with IFN-gamma, IL-3 and GM-CSF. Int Arch Allergy Immunol. 2008;146:227–34. doi: 10.1159/000115891. [DOI] [PubMed] [Google Scholar]
- 7.Shi HZ, Humbles A, Gerard C, et al. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–53. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi HZ. Eosinophils function as antigen-presenting cells. J Leukoc Biol. 2004;76:520–7. doi: 10.1189/jlb.0404228. [DOI] [PubMed] [Google Scholar]
- 9.Wang HB, Ghiran I, Matthaei K, et al. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–92. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padigel UM, Hess JA, Lee JJ, et al. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–51. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel AJ, Fuentebella J, Gernez Y, et al. Increased HLA-DR expression on tissue eosinophils in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:290–4. doi: 10.1097/MPG.0b013e3181e083e7. [DOI] [PubMed] [Google Scholar]
- 12.Lackner A, Raggam RB, Stammberger H, et al. The role of interleukin-16 in eosinophilic chronic rhinosinusitis. Eur Arch Otorhinolaryngol. 2007;264:887–93. doi: 10.1007/s00405-007-0300-6. [DOI] [PubMed] [Google Scholar]
- 13.Fan GK, Wang H, Takenaka H. Eosinophil infiltration and activation in nasal polyposis. Acta Otolaryngol. 2007;127:521–6. doi: 10.1080/00016480600951368. [DOI] [PubMed] [Google Scholar]
- 14.Kindt TJ, Goldsby A, Osborne BA, et al. Kuby Immunology. New York: W.H. Freeman; 2007. [Google Scholar]
- 15.Mulder DJ, Pooni A, Mak N, et al. Antigen presentation and MHC class II expression by human esophageal epithelial cells: role in eosinophilic esophagitis. Am J Pathol. 2011;178:744–53. doi: 10.1016/j.ajpath.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra A, Schlotman J, Wang M, et al. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81:916–24. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 17.Lucendo AJ, Navarro M, Comas C, et al. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31:598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 18.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–61. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 19.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–27. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Akei HS, Mishra A, Blanchard C, et al. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–94. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Mishra A, Hogan SP, Brandt EB, et al. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–9. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Kitaura M, Suzuki N, Imai T, et al. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. 1999;274:27975–80. doi: 10.1074/jbc.274.39.27975. [DOI] [PubMed] [Google Scholar]
- 24.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–63. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Miki-Hosokawa T, Hasegawa A, Iwamura C, et al. CD69 controls the pathogenesis of allergic airway inflammation. J Immunol. 2009;183:8203–8215. doi: 10.4049/jimmunol.0900646. [DOI] [PubMed] [Google Scholar]
- 26.Salek-Ardakani S, Song J, Halteman BS, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur D, Brightling C. OX40/OX40 ligand interactions in T-cell regulation and asthma. Chest. 2012;141:494. doi: 10.1378/chest.11-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]