Abstract
Purpose.
To compare choroidal thickness and retinal macular volume (RMV) among three groups of women: severe preeclampsia postpartum, normotensive postpartum, and normotensive nongravid. While visual disturbances often accompany severe preeclampsia, the underlying choroidal and retinal changes responsible for these symptoms have not been described.
Methods.
This case-control study was based on 15 severe preeclampsia cases and 15 ethnicity- and parity-matched normotensive controls recruited during the postpartum hospital stay. A reference group of 19 age-matched, nongravid, normotensive women was also studied. Choroidal thickness and RMV were measured by using enhanced depth imaging spectral-domain optical coherence tomography. Two retinal specialists, one of whom was masked to the case-control status, reviewed all images.
Results.
Severe preeclampsia cases demonstrated greater mean choroidal thickness (425 ± 90 μm vs. 354 ± 140 μm; P = 0.021) and RMV (9.0 ± 0.4 mm3 vs. 8.7 ± 0.5 mm3; P = 0.006) than controls. In contrast, control and reference groups were similar with respect to choroidal thickness (354 ± 140 μm vs. 363 ± 82 μm; P = 0.764) and RMV (8.7 ± 0.5 mm3 vs. 8.8 ± 0.4 mm3; P = 0.870). Follow-up imaging of two severe preeclampsia cases within 3 months of delivery revealed decreasing choroidal thickness.
Conclusions.
Our results demonstrate subclinical retinal and choroidal thickening in the setting of severe preeclampsia. This is the likely source of its associated visual phenomena and may reflect rising levels of vascular endothelial growth factor. Retinal and choroidal markers could serve as novel predictive markers of severe preeclampsia.
Keywords: severe preeclampsia, visual disturbances, choroidal thickness, retinal macular volume, vascular endothelial growth factor, predictive markers
We utilized enhanced depth imaging spectral-domain optical coherence tomography to demonstrate subclinical retinal and choroidal thickening in the setting of severe preeclampsia, which is the likely source of its associated visual phenomena and may reflect rising levels of vascular endothelial growth factor.
Introduction
Preeclampsia is one of the leading causes of perinatal and maternal mortality and morbidity across the world.1–5 This condition complicates approximately 3% to 7% of pregnancies,6,7 and the severe form affects 0.6% to 1.2% of pregnancies.8 These observations underscore the acute need to develop biomarkers for early prediction of preeclampsia.9
Preeclampsia is an obstetrical complication characterized by poor placental perfusion as well as systemic vascular changes leading to new-onset hypertension as well as at least one systemic condition including proteinuria, hepatic dysfunction, neurological signs, renal insufficiency, pulmonary edema, or thrombocytopenia.10 Patients with severe preeclampsia (sPE) experience more pronounced manifestations of these signs. Approximately 40% of women with preeclampsia report subjective visual disturbances.11 In some patients, conditions such as cortical blindness, serous retinal detachments, Purtscher's-like retinopathy, and retinal and vitreous hemorrhages12 have been documented to accompany preeclampsia. Many women with normal pregnancies may also report subjective visual disturbances associated with an increase or decrease in refractive error, as well as extraocular changes including ptosis.13 Interestingly, in many cases of preeclampsia, patient complaints of visual changes are not evidenced on ophthalmic or systemic examination.
In a previous case series of patients with preeclampsia, fundus examinations have revealed multiple yellow-white patches during the acute phase of the disease. Angiography of these patients reveals choroidal nonfilling, leakage of dye from the optic disc and deep retinal lesions, and retinal pigment epithelium (RPE) window defects. The areas of leakage correlate with findings on color fundus photography, suggesting that choroidal vascular insufficiency may be present in preeclampsia, and responsible for serous retinal detachments.14
The choroidal vasculature supplies blood to the retinal photoreceptors and is known to be responsive to vascular endothelial growth factor (VEGF),15 which is also known to be upregulated in preeclampsia.16,17 We hypothesize that acute vasospasm in sPE will result in a thickened, edematous choroid as compared to normotensive postpartum controls. To test this hypothesis, we designed this study to characterize visual changes associated with severe preeclampsia. Enhanced depth imaging spectral-domain optical coherence tomography (EDI SD-OCT) is a well-described method of imaging that allows for higher-resolution visualization of the choroid.18
Methods
Study Population
This study received approval through the ethics review committee of Columbia University Medical Center's (CUMC's) Institutional Review Board. Written informed consent was obtained from all subjects and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. This was an incident case-control study of two groups of postpartum women who were inpatients at CUMC and were matched by ethnicity and parity: those diagnosed with sPE or eclampsia (preeclampsia cases) and a control group of normotensive patients. A reference group of normotensive, nongravid women was additionally recruited to permit an evaluation of visual changes in the absence of pregnancy. All three groups were recruited between December 2012 and May 2013.
Inclusion criteria for cases included a diagnosis of sPE or eclampsia, which was defined by previously described criteria.19 Controls included women who were normotensive before, during, and after delivery. Women with a clinical diagnosis of chronic or gestational hypertension, or women diagnosed with pregestational or gestational diabetes, were excluded from both the case and control groups. Both cases and controls were recruited during the postpartum period.
All subjects underwent an ophthalmologic examination by a retinal specialist, axial length measurement, color fundus photography, and confocal scanning laser ophthalmoscopy (including autofluorescence and infrared imaging) and EDI SD-OCT. Enhanced depth imaging SD-OCT is a method that has been previously described in the literature, and allows for better visualization of the choroid.18 Both eyes of each patient were included in the study.
Image Acquisition and Analysis
High-resolution digital color fundus photographs were taken with an FF 450plus with VISUPAC camera (Carl Zeiss Meditec, Dublin, CA, USA). Autofluorescence (AF), infrared (IR), and EDI SD-OCT imaging were obtained by scanning laser ophthalmology (SLO) imaging (Heidelberg Spectralis HRA+OCT version 1.7.0.0; Heidelberg Engineering, Heidelberg, Germany). For AF images, the instrument used blue laser light at 488 nm for illumination and a barrier filter at 500 nm. The IR images were obtained at 810 nm. The Heidelberg Spectralis was used to perform EDI SD-OCT imaging. Seven macular sections, each comprising 100 averaged scans, were obtained in the 5×15-degree rectangle centered on the macula. Images were taken until a clear posterior margin of the choroid was visualized. Horizontal foveal line scans and volume scans were used for measurements of the subfoveal choroidal thickness and retinal macular volume (RMV), respectively.
Infrared, AF, and SD-OCT images were viewed with Heidelberg software (Spectralis Viewing Module 5.4.6.0; Heidelberg Engineering, Heidelberg, Germany). Autofluorescence images were graded on a four-point ordinal scale (0 = normal, 1 = mild, 2 = moderate, 3 = pronounced). Stippled changes were defined by using criteria similar to those of reticular macular disease.20
The best EDI SD-OCT image of each eye with a clear posterior margin of the choroid was chosen for analysis. Choroidal thickness was defined as the distance between the outer portion of the hyperreflective line corresponding to the RPE to the inner surface of the sclera21 beneath the fovea, and was measured with the Heidelberg Eye Explorer interactive software's manual calipers tool, which provides measurements to the closest micrometer. Images were reviewed by two retinal specialists (SB, SHT), one of whom (SHT) was masked to diagnosis of the patient. An example of this is provided in Figure 1. Retinal macular volume was assessed by using a grid, 6 mm in diameter, centered at the fovea, using Heidelberg Spectralis Eye Explorer's automated segmentation program. Eyes with subretinal fluid (six eyes) were excluded from this arm of the study.
Figure 1.
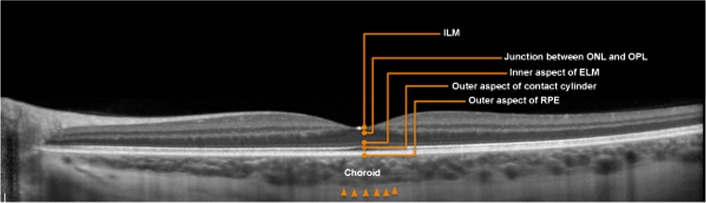
Retinal layers and the choroid. Choroidal thickness was defined as the outer portion of the hyperreflective line corresponding to the RPE to the inner surface of the sclera, which is demarcated by red arrows. CT, choroidal thickness; ELM, external limiting membrane; ILM, internal limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer.
Axial length measurements were performed by using IOLMaster (Carl Zeiss Meditec). Choroidal thickness is known to decrease with myopia; thus, elongated axial length was adjusted according to measurements observed by Flores-Moreno et al.22 In this study, the mean axial length was 23 mm ± SD of 0.7 mm (range, 22.0–28.6 mm). Adjustments in axial length were made for all subjects with an axial length greater than 23.7 mm, with 25.9 μm added to the measured choroidal thickness for every 1-mm increase above 23.7 mm.
Statistical Analysis
Statistical analyses were performed by creating generalized linear regression models based on the method of generalizing estimating equations23 to examine the choroidal thickness and RMV in relation to the case-control and nongravid groups. Models were corrected for intracluster correlation owing to assessments on both eyes within a subject. Failure to correct for clustering between two eyes within a woman would lead to imprecise variance estimates, and consequently incorrect statistical inferences. All models were adjusted for the confounding effects of maternal age, parity, body mass index, and race. Interrater agreement between the two raters on choroidal thickness measurements was calculated by using intraclass correlation coefficient.
Results
Patient Demographics and Clinical Characteristics
The study included 49 subjects: 15 with sPE, 15 controls, and 19 reference subjects. Table 1 lists demographic characteristics. All subjects were phakic. Examination and imaging were both performed on the same day for each patient; dates ranged from postpartum day 1 to postpartum day 7 for the sPE group and from postpartum day 1 to postpartum day 2 for the normotensive postpartum group.
Table 1.
Demographic Comparison Across the Three Groups: Severe Preeclampsia, Normotensive Postpartum, and Normotensive Nongravid Women
|
Severe Preeclampsia,
n
= 15 |
Normotensive Postpartum,
n
= 15 |
Normotensive Nongravid,
n
= 19 |
P
Value |
|
| Age, y | 32.7 ± 7.6 | 31.9 ± 6.6 | 27.9 ± 5.1 | 0.068 |
| African American, % | 20 | 13 | 5 | 0.151 |
| Nulliparous, % | 53 | 53 | 79 | 0.193 |
| Smokers (past or current), % | 7 | 0 | 5 | |
| Gestational age at delivery, wk | 32.5 ± 4.9 | 38.6 ± 2.4 | N/A | |
| Days post partum | 2 (1–7) | 1 (1–2) | N/A | 0.0001 |
| Cesarean delivery, % | 67 | 20 | N/A | |
| Body mass index, kg/m2 | 34.3 ± 9.0 | 29.7 ± 4.6 | N/A | |
| Best corrected visual acuity, logMAR | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.452 |
| Axial length, mm | 22.9 ± 0.8 | 23.9 ± 1.9 | 25.5 ± 1.0 | 0.125 |
| Visual disturbances, % | 47 | 7 | 0 |
For days post partum, range is given in parentheses. N/A, not applicable.
Features Noted on EDI SD-OCT
Mean choroidal thickness and RMV in all three groups are listed in Table 2. Mean choroidal thickness and RMV in sPE cases were significantly different from control eyes (P = 0.021 and P = 0.006, respectively). This difference persisted even after adjustments for potential confounders. A difference in mean choroidal thickness and RMV could not be identified between the control and reference groups (P = 0.764 and P = 0.870, respectively). Representative images of choroidal thickness measurements and RMV measurements are demonstrated in Figures 2 and 3, respectively. Analysis of these measures is seen in Figure 4.
Table 2.
Mean Choroidal Thickness and Retinal Macular Volume Across the Three Groups of Severe Preeclampsia, Normotensive Postpartum, and Normotensive Nongravid Women
|
Mean ± Standard Deviation |
|||
|
Severe Preeclampsia |
Normotensive Postpartum |
Normotensive Nongravid |
|
| Choroidal thickness, μm | 425 ± 90 | 354 ± 140 | 363 ± 82 |
| Retinal macular volume, mm3 | 9.0 ± 0.4 | 8.7 ± 0.5 | 8.8 ± 0.4 |
Figure 2.
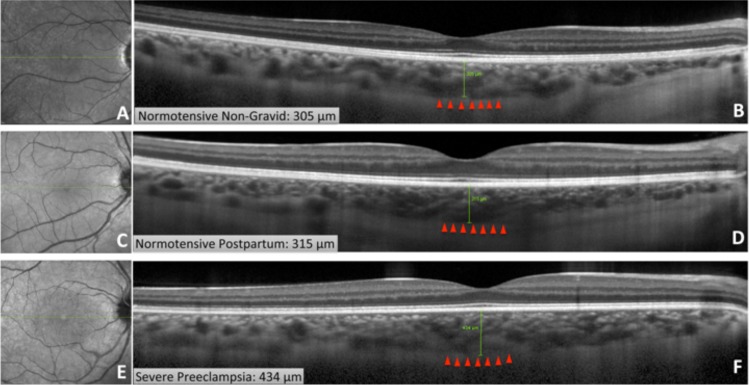
Choroidal thickness measurements. Infrared and EDI SD-OCT imaging from subjects in all three groups: a 25-year-old normotensive woman who has not been pregnant in the last year (A, B), 25-year-old normotensive postpartum woman (C, D), and 25-year-old postpartum woman with severe preeclampsia (E, F). EDI SD-OCT revealed choroidal thicknesses of 305 μm in the normotensive nongravid woman (B), 315 μm in the normotensive postpartum woman (D), and 434 μm in the woman with severe preeclampsia.
Figure 3.
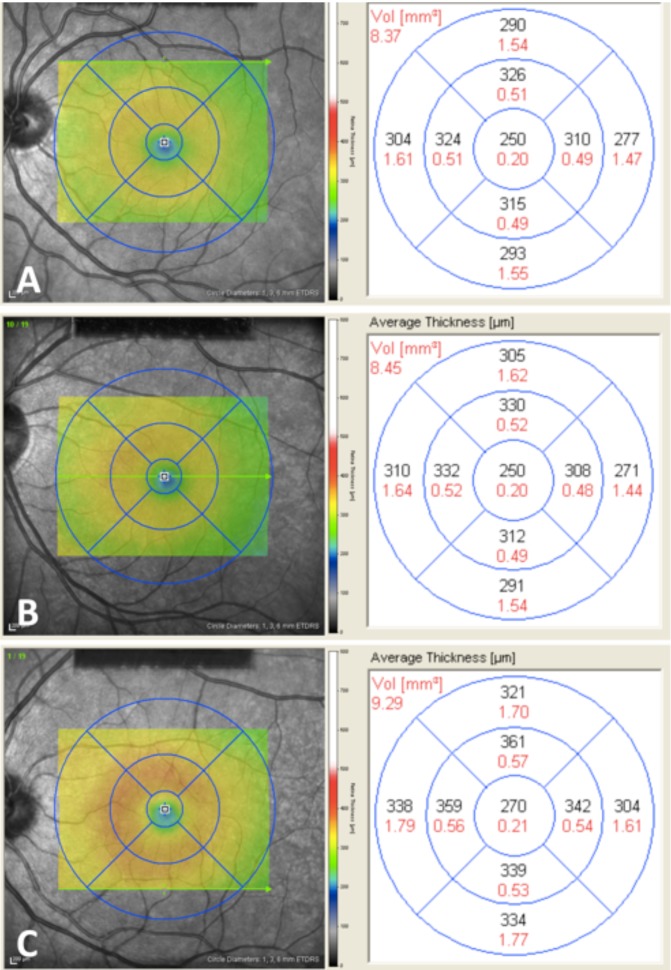
Retinal macular volume measurements. RMV measurements from subjects in all three groups: a 24-year-old normotensive woman who has not been pregnant in the last year with an RMV of 8.4 mm3 (A), 32-year-old normotensive postpartum woman with an RMV of 8.5 mm3 (B), and 26-year-old postpartum woman with severe preeclampsia with an RMV of 9.3 mm3 (C).
Figure 4.
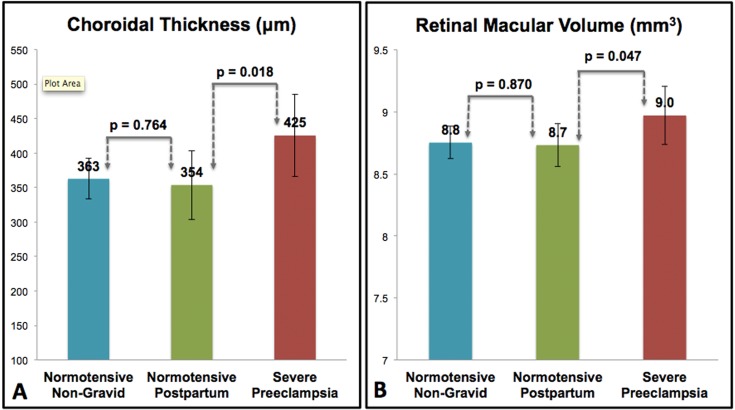
Mean choroidal thickness and RMV are greater in severe preeclampsia. Mean choroidal thickness was significantly thicker in severe preeclampsia than in the normotensive postpartum group (P = 0.018). No difference in choroidal thickness was found between the normotensive postpartum and normotensive nongravid group (P = 0.764) (A). Mean RMV was significantly greater in severe preeclampsia than in the normotensive postpartum group (P = 0.047). No difference in RMV was found between the normotensive postpartum and normotensive nongravid group (P = 0.870) (B).
Interobserver agreement between the two raters (intraclass correlation coefficient) of choroidal thickness values was very high for all three groups: sPE (r = 0.96), control (r = 0.99), and comparison (r = 0.98) measurements (P < 0.001 for all three correlation coefficients).
Features Noted on SLO Imaging
Autofluorescence imaging revealed pronounced stippled changes in 9 of 28 sPE eyes and 2 of 30 control eyes (Supplementary Fig. S1). Two eyes from the sPE group were excluded owing to poor image quality. Additionally, in two patients, retinal lesions noted on AF and IR imaging correlated with disruptions of the inner segment/outer segment boundary on EDI SD-OCT.
Follow-up Visits
Two subjects with sPE returned for follow-up examination within 3 months of delivery, and repeated images were acquired. Both showed a decrease in choroidal thickness (Supplementary Figs. S2, S3).
Preeclampsia Subset Analysis
An analysis within the sPE group was performed to compare the choroidal thickness values of women with and without visual changes. Seven of the 15 women with sPE reported subjective visual changes—which included blurred vision and scintillating scotomas—in the month before hospitalization. Differences in choroidal thickness between those with and without visual changes trended toward significance (P = 0.060).
Discussion
Case reports and series14,24 on ophthalmoscopic findings in women with sPE have previously been published, but to our knowledge, the present analysis is the only quantitative study of choroidal and retinal changes in sPE. We showed that choroidal thickness and retinal volume in sPE are greater than in the normotensive control group. The lack of difference in choroidal thickness between the control and reference groups supports the notion that increase in choroidal thickness is specific to preeclampsia, and is not a result of normal pregnancy-related changes.25,26 Additionally, the correlation of retinal reticular lesions on SLO imaging with disruption of the photoreceptors on EDI SD-OCT represents another unique finding that may represent early, but reversible, retinal ischemia.
Preeclampsia is a multisystemic disorder characterized by vascular changes; however, its effects are inherently distinct from its most essential defining criterion, hypertension. While hypertension is known to cause retinal vascular occlusions and choroidal infarcts (called Elschnig spots), retinal findings in the setting of preeclampsia are more similar to that of disseminated intravascular coagulopathy, which, in a nonpregnant state, has been described to cause serous retinal detachments,27 as well as Purtscher's-like retinopathy.28 Clotting factors are known to be altered in preeclampsia,29,30 which may explain the retinal findings it shares with disseminated intravascular coagulopathy.
The imaging findings in our study are supported by theories on the molecular basis of preeclampsia and its systemic effects. Pregnancies complicated by preeclampsia demonstrate higher serum levels of total VEGF, as compared with normal pregnancies.16 Vascular endothelial growth factor, released by the RPE, controls choroidal fenestrations and “leakiness.” We hypothesize that the choriocapillaris, the layer of the choroid that provides blood supply to the retinal photoreceptors and is VEGF responsive,15 may contribute to visual changes in preeclampsia by resulting in subclinical retinal edema. The reversibility of choroidal thickening seen in the two patients followed up long-term further supports the conclusion that these vascular changes are specific to preeclampsia and are not the consequences of vascular changes during pregnancy.
Conditions other than preeclampsia can cause serous retinal detachments and choroidal thickening. One such condition is central serous retinopathy, which is hypothesized to be due to an overactivation of mineralocorticoid receptor pathway in the choroidal vasculature.31 Although pregnancy is a known risk factor for central serous retinopathy, the authors do not believe that this pathway plays a significant role in the ophthalmic manifestations of preeclampsia for several reasons. First, while increase of cortisol during pregnancy is well established,32,33 a recent study has found no difference in the cortisol levels of normotensive pregnant women and pregnant women with preeclampsia.34 Additionally, the present investigation did not detect a difference in choroidal thickness between pregnancy and nonpregnancy, making the choroidal thickening demonstrated in the severe preeclampsia group unlikely to be due to steroid-related changes. Thus, it appears that while central serous retinopathy and severe preeclampsia share some similar fundus findings, the pathophysiologic mechanisms may be different.
The lack of identifiable difference in choroidal thickness or retinal macular volume between the normotensive postpartum and normotensive nongravid groups was interesting, as pregnancy and the immediate postpartum state are known to be associated with progesterone increase and subsequent drop in the levels of this hormone.35 We speculate that the known physiological volume expansion as a consequence of the initial progesterone surge diminished by the time the present study's subjects were imaged; this may have led to similar choroidal thickness and RMV between the two normotensive groups.
Limitations of the Study
Obtaining high-quality images in subjects with very thick choroids is not always feasible and may have led to underestimated measurements, biasing our results toward the null (i.e., demonstrating less of a difference between the case and control groups). Therefore, the reported group differences are conservative. Furthermore, the small sample size may not have sufficient power to detect differences, that is, differences in choroidal thickness between those with and without subjective visual changes among sPE subjects. Additionally, while all patients were imaged in the immediate postpartum inpatient period, not all were imaged on the same day post partum. Those in the normotensive postpartum group were imaged within 2 days of delivery, while those in the sPE group were imaged on average on postpartum day 2 (range, 1–7 days). While it is possible that choroidal and retinal manifestations of preeclampsia may diminish over time, it is unlikely to happen over this short course of time. Also, the difference in postpartum time may bias toward showing less of a difference, as the increase in choroidal thickening in sPE likely lessens with time.
Lastly, a previous study has found a small diurnal variation of approximately 13 μm in choroidal thickness measurements taken at 8 AM and those taken at 5 PM.36 Although our patients were imaged in this time frame, the authors believe that the variation detected by the mentioned study is minimal in the setting of the present analysis' findings of a mean difference of 119 μm between the sPE and normotensive postpartum groups. Additionally, the statistically insignificant mean difference between the normotensive groups (postpartum and nongravid) was very small at 10 μm, which was also unlikely to be biased by diurnal variation. Additionally, retinal thickness is not known to be associated with this phenomenon.37
We anticipate our imaging markers to be a starting point for further study of the role of total VEGF in sPE. Several studies have reported that serum soluble VEGF receptor 1 (sFlt-1) levels begin to rise approximately 5 weeks before the onset of clinical symptoms of preeclampsia.17,38,39 While it is possible that freely available VEGF-A is reduced in the plasma of pre-eclamptic women owing to increased sFlt-1 levels, VEGF is a key mediator of ischemia-driven angiogenesis and may very well be increased in preeclampsia. This is corroborated by a study by Celik et al.,16 which found that pregnancies complicated by preeclampsia demonstrate higher serum levels of VEGF as compared with normal pregnancies. If these serum changes are in fact responsible for the choroidal changes as our study suggests, perhaps choroidal thickness could be a predictive marker for preeclampsia, and may even enable stratification of different subtypes of sPE.
Additionally, while VEGF levels were of particular interest to our group owing to the known effects of this molecule on the choroid in neovascular age-related macular degeneration and other ophthalmic conditions, several other angiogenic markers have been used for detection and risk assessment in preeclampsia. Most notably, circulating soluble endoglin levels are known to increase before the onset of clinical signs and symptoms of preeclampsia, as the placentae of preeclamptic women release greater levels of this molecule than those of normotensive pregnant women.40,41 Additionally, soluble endoglin has antiangiogenic properties similar to sFlt-1.42,43 Exploration of the role of this molecule as well as placental-derived growth factor in relation to preeclampsia and visual disturbances remains unaddressed.
Conclusions
This study underscores the importance of choroidal and retinal thickening as potential sources of visual disturbances that accompany sPE. This conclusion is supported by novel findings obtained by multimodal imaging in this pregnancy-specific vascular disorder. This is supported by well-described roles of angiogenic and antiangiogenic markers in choroidal physiology as well as in the preeclampsia state.
Acknowledgments
Supported by a grant from the Doris Duke Charitable Foundation to Columbia University, New York, New York (AG), Grants from the National Eye Institute/NIH EY013435 (RA, WL), EY018213 (SHT), and EY019007 (Core Support for Vision Research), unrestricted funds from Research to Prevent Blindness, New York, New York to the Department of Ophthalmology, Columbia University, Foundation for Fighting Blindness, New York Regional Research Center Grant C-NY05-0705-0312 (SHT), Robert L. Burch III Fund, Columbia University (SB), New York Community Trust, Columbia University (SB), and the Silvian Foundation, Columbia University (SB). The authors alone are responsible for the content and writing of the paper.
Disclosure: A. Garg, None; R.J. Wapner, None; C.V. Ananth, None; E. Dale, None; S.H. Tsang, None; W. Lee, None; R. Allikmets, None; S. Bearelly, None
References
- 1. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010; 376: 631–644 [DOI] [PubMed] [Google Scholar]
- 2. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009; 33: 130–137 [DOI] [PubMed] [Google Scholar]
- 3. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013; 28: 1–19 [DOI] [PubMed] [Google Scholar]
- 4. Souwer ET, Blaauw J, Coffeng SM, et al. Decreased arterial elasticity in formerly early-onset preeclamptic women. Acta Obstet Gynecol Scand. 2011; 90: 797–801 [DOI] [PubMed] [Google Scholar]
- 5. Bushnell C, Chireau M. Preeclampsia and stroke: risks during and after pregnancy. Stroke Res Treat. 2011; 2011: 858134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008; 21: 521–526 [DOI] [PubMed] [Google Scholar]
- 7. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009; 113: 1299–1306 [DOI] [PubMed] [Google Scholar]
- 8. Publications Committee The Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011; 205: 191–198 [DOI] [PubMed] [Google Scholar]
- 9. Myatt L, Clifton R, Roberts J, et al. Can changes in angiogenic biomarkers between the first and second trimesters of pregnancy predict development of pre-eclampsia in a low-risk nulliparous patient population? BJOG. 2013; 120: 1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013; 122: 1122–1131 [DOI] [PubMed] [Google Scholar]
- 11. Royburt M, Seidman DS, Serr DM, Mashiach S. Neurologic involvement in hypertensive disease of pregnancy. Obstet Gynecol Surv. 1991; 46: 656–664 [DOI] [PubMed] [Google Scholar]
- 12. Roos NM, Wiegman MJ, Jansonius NM, Zeeman GG. Visual disturbances in (pre)eclampsia. Obstet Gynecol Surv. 2012; 67: 242–250 [DOI] [PubMed] [Google Scholar]
- 13. Garg P, Aggarwal P. Ocular changes in pregnancy. Nepal J Ophthalmol. 2012; 4: 150–161 [DOI] [PubMed] [Google Scholar]
- 14. Fastenberg DM, Fetkenhour CL, Choromokos E, Shoch DE. Choroidal vascular changes in toxemia of pregnancy. Am J Ophthalmol. 1980; 89: 362–368 [DOI] [PubMed] [Google Scholar]
- 15. Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris: evidence for a trophic paracrine relation. Am J Pathol. 1999; 155: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Celik H, Avci B, Isik Y. Vascular endothelial growth factor and endothelin-1 levels in normal pregnant women and pregnant women with pre-eclampsia. J Obstet Gynaecol. 2013; 33: 355–358 [DOI] [PubMed] [Google Scholar]
- 17. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. New Engl J Med. 2004; 350: 672–683 [DOI] [PubMed] [Google Scholar]
- 18. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500 [DOI] [PubMed] [Google Scholar]
- 19. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2008; 183: S1–S22 [PubMed] [Google Scholar]
- 20. Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009; 148: 733–743.e732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009; 147: 801–810 [DOI] [PubMed] [Google Scholar]
- 22. Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013; 155: 314–319.e311 [DOI] [PubMed] [Google Scholar]
- 23. Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993; 14: 43–68 [DOI] [PubMed] [Google Scholar]
- 24. Gass DM, Pautler SE. Toxemia of pregnancy pigment epitheliopathy masquerading as a heredomacular dystrophy. Trans Am Ophthalmol Soc. 1985; 83: 114–130 [PMC free article] [PubMed] [Google Scholar]
- 25. Weinreb RN, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol. 1988; 105: 258–260 [DOI] [PubMed] [Google Scholar]
- 26. Ziai N, Ory SJ, Khan AR, Brubaker RF. Beta-human chorionic gonadotropin, progesterone, and aqueous dynamics during pregnancy. Arch Ophthalmol. 1994; 112: 801–806 [DOI] [PubMed] [Google Scholar]
- 27. Cogan DG. Ocular involvement in disseminated intravascular coagulopathy. Arch Ophthalmol. 1975; 93: 1–8 [DOI] [PubMed] [Google Scholar]
- 28. Viola F, Vezzola D, Villani E, Mapelli C, Barteselli G, Ratiglia R. Purtscher-like retinopathy in septicemic disseminated intravascular coagulation associated with nephrotic syndrome. Eur J Ophthalmol. 2013; 23: 601–603 [DOI] [PubMed] [Google Scholar]
- 29. Lox CD, Dorsett MM, Hampton RM. Observations on clotting activity during pre-eclampsia. Clin Exp Hypertens B. 1983; 2: 179–190 [DOI] [PubMed] [Google Scholar]
- 30. Heilmann L, Rath W, Pollow K. Hemostatic abnormalities in patients with severe preeclampsia. Clin Appl Thromb Hemos. 2007; 13: 285–291 [DOI] [PubMed] [Google Scholar]
- 31. Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012; 122: 2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011; 96: 1533–1540 [DOI] [PubMed] [Google Scholar]
- 33. Scott EM, McGarrigle HH, Lachelin GC. The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. J Clin Endocrinol Metab. 1990; 71: 639–644 [DOI] [PubMed] [Google Scholar]
- 34. Sinha S, Singh GP, Gupta K, Kumar S, Gupta A. Effect of preeclampsia on insulin sensitivity. Int J Appl Basic Med Res. 2014; 4: 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kristiansson P, Wang JX. Reproductive hormones and blood pressure during pregnancy. Hum Reprod. 2001; 16: 13–17 [DOI] [PubMed] [Google Scholar]
- 36. Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina. 2014; 34: 385–393 [DOI] [PubMed] [Google Scholar]
- 37. Jo YJ, Heo DW, Shin YI, Kim JY. Diurnal variation of retina thickness measured with time domain and spectral domain optical coherence tomography in healthy subjects. Invest Ophthalmol Vis Sci. 2011; 52: 6497–6500 [DOI] [PubMed] [Google Scholar]
- 38. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111: 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003; 88: 2348–2351 [DOI] [PubMed] [Google Scholar]
- 40. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. New Engl J Med. 2006; 355: 992–1005 [DOI] [PubMed] [Google Scholar]
- 41. Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006; 12: 642–649 [DOI] [PubMed] [Google Scholar]
- 42. Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006; 21: 3052–3054 [DOI] [PubMed] [Google Scholar]
- 43. Jerkic M, Rodriguez-Barbero A, Prieto M, et al. Reduced angiogenic responses in adult Endoglin heterozygous mice. Cardiovasc Res. 2006; 69: 845–854 [DOI] [PubMed] [Google Scholar]