Abstract
Background
An advance directive is a document specifying a person's preferences for treatment, should he or she lose capacity to make such decisions in the future. They have been used in end‐of‐life settings to direct care but should be well suited to the mental health setting.
Objectives
To examine the effects of advance treatment directives for people with severe mental illness.
Search methods
We searched the Cochrane Schizophrenia Group's Register (February 2008), the Cochrane Library (Issue 1 2008), BIOSIS (1985 to February 2008), CINAHL (1982 to February 2008), EMBASE (1980 to February 2008), MEDLINE (1966 to February 2008), PsycINFO (1872 to February 2008), as well as SCISEARCH and Google ‐ Internet search engine (February 2008). We inspected relevant references and contacted first authors of included studies.
We updated this search on 17 May 2012 and added the results to the awaiting classification section of the review.
Selection criteria
We included all randomised controlled trials (RCTs), involving adults with severe mental illness, comparing any form of advance directive with standard care for health service and clinical outcomes.
Data collection and analysis
We extracted data independently. For homogenous dichotomous data we calculated fixed‐effect relative risk (RR) and 95% confidence intervals (CI) on an intention‐to‐treat basis. For continuous data, we calculated weighted mean differences (WMD) and their 95% confidence interval again using a fixed‐effect model.
Main results
We were able to include two trials involving 321 people with severe mental illnesses. There was no significant difference in hospital admission (n=160, 1 RCT, RR 0.69 0.5 to 1.0), or number of psychiatric outpatient attendances between participants given advanced treatment directives or usual care. Similarly, no significant differences were found for compliance with treatment, self harm or number of arrests. Participants given advanced treatment directives needed less use of social workers time (n=160, 1 RCT, WMD ‐106.00 CI ‐156.2 to ‐55.8) than the usual care group, and violent acts were also lower in the advanced directives group (n=160, 1 RCT, RR 0.27 CI 0.1 to 0.9, NNT 8 CI 6 to 92). The number of people leaving the study early were not different between groups (n=321, 2 RCTs, RR 0.92 CI 0.6 to 1.6).
The addition of 11 studies to awaiting classification section of the review may alter the conclusions of the review once assessed.
Authors' conclusions
There are too few data available to make definitive recommendations. More intensive forms of advance directive appear to show promise, but currently practice must be guided by evidence other than that derived from randomised trials. More trials are indicated to determine whether higher intensity interventions, such as joint crisis planning, have an effect on outcomes of clinical relevance.
Keywords: Adult, Humans, Advance Directives, Commitment of Mentally Ill, Mental Disorders, Mental Disorders/therapy, Patient Admission, Randomized Controlled Trials as Topic
Plain language summary
Advance treatment directives for people with severe mental illness
An advance treatment directive is a document that specifies a person’s future preferences for treatment, should he or she lose the mental ability to make treatment decisions (lose capacity). They have traditionally been used to stipulate treatment in end‐of‐life situations. However, people with mental health problems can also have periods where they are unable to make treatment decisions, and an advance statement could help with choosing suitable medication, saying who should look after children and specifying choices in other areas of their life and treatment.
This review looks at whether having an advance statement leads to less hospitalisation (either voluntary or involuntary), less contact with mental health services and whether there is an improvement in general functioning. Two studies were found, involving 321 people. Both took place in England. One trial involved the person concerned making a joint crisis plan in collaboration with the psychiatrist, care coordinator and project worker (high intensity), while the other required filling in a booklet called ‘preferences for care’ (low intensity). Both studies were compared to the usual care in the area concerned.
Since the interventions were quite different, and not all outcomes were measured by both studies, it is quite difficult to compare the trials. Those who filled in the booklet showed no decrease in admission to hospital (voluntary or involuntary) or contact with out‐patient services, when compared to usual care. The high intensity group showed no differences in voluntary admissions compared to those in usual care, but were less likely to be hospitalised involuntarily, or assessed under the Mental Health Act. They were also less likely to be violent. There was no difference in use of psychiatric out‐patient services by those in the intervention groups. These are small studies and more research is needed, but it is suggested that using an advance treatment directive could be an alternative to community treatment orders. (Plain language summary prepared for this review by Janey Antoniou of RETHINK, UK www.rethink.org)
Background
Description of the intervention
An advance directive is a document that specifies a person's preferences for treatment, should he or she lose capacity to make treatment decisions in the future. They are sometimes termed 'Ulysses directives' in reference to the Greek hero's encounter with the Sirens. The Sirens lived on a rocky island in the sea, and were notorious for their enchanted singing, luring many unwary sailors to shipwreck. Ulysses instructed his crew to stuff their ears with wax and tie him to the mast of his ship so he could hear their song but not be lured onto the rocks while under their spell. Advance directives allow the physician to respect the patient's prior competent instructions when these conflict with those expressed while incompetent (Ritchie 1998). Mental health advance directives are intended to convey a person's preferences for psychiatric treatment should the person become incompetent in the future and unable to do so for themselves.
Advance directives have been most commonly used in end‐of‐life care settings to direct medical care treatment decisions. They are, however, well‐suited to mental health settings. Severe mental illnesses, such as psychosis, mania, or severe depression, are characterized by alternating periods of competence and incompetence. Advance directives provide people with these illnesses the opportunity to convey their treatment preferences when they are competent.
There are two general forms of mental health advance directives, the instructional directive and the proxy directive (US GAO 1995). A third form, the hybrid directive, combines the advantages of the instructional and proxy directives (La Fond 2002).
Instructional mental health advance directives communicate instructions for treatment providers in the event of a mental health crisis, should the patient become incompetent and unable to do so themselves. They may contain decisions regarding hospitalisation, methods for handling emergencies (such as use of restraint, seclusion, or sedation), medication (including types of medications to be used, dosages, methods and timing of administration), treatment approaches (such as electroconvulsive therapy or psychotherapy), persons to be notified in the event of hospitalisation, persons responsible for childcare, personal, and financial matters, and medical care issues (Srebnik 1999 a).
Proxy directives are health care power of attorney documents, which allow the patient to designate someone else to make decisions on his or her behalf should the patient become incompetent. As it is difficult to anticipate future events with enough specificity to provide adequate instructions, proxy directives are used more frequently than instructional directives. Proxy directives allow the designated proxy to take into account the actual circumstances of the patient's situation. Depending upon the terms of the directive, the proxy will make decisions using a 'substituted judgment' standard, or using a 'best‐interest' standard (Srebnik 1999).
Hybrid directives name an individual who is authorised to make treatment decisions on behalf of the patient while also providing instructions to that person, combining the specificity of the instructional directive with the flexibility of the proxy decision‐maker (La Fond 2002, Srebnik 1999).
How the intervention might work
The effects of advance directives in the mental health setting are unclear, including the degree of subsequent compliance (Srebnik 1999). Authors have suggested benefits in the following areas: a. Enhanced autonomy and choice for patients, with subsequent benefits to physical and psychological well‐being; b. Improved family relationships through the reduction of conflict around treatment; c. Increased acceptance by service providers of patient autonomy; and/or d. Reduced service use including hospital admissions, bed‐days, use of mental health legislation and contact with the criminal justice system.
Why it is important to do this review
There has, however, been little in the way of research into the effects of advance directives in any of these areas. We do not know the circumstances that promote compliance with directives and this is complicated by the fact that compliance is generally not dichotomous. Some aspects of directives may be complied with in some circumstances. There are more data on the effects on health service use, but results have been equivocal (Papageorgiou 2002, Henderson 2004). Some of the disparities in results may be due to differences in the sample including selection bias or in the type of intervention (Kisely 2005). More intense interventions, including a lengthy interview with patients and their carers (Henderson 2004) appear to be more effective than providing patients with a booklet for completion (Papageorgiou 2002).
Other concerns about advance directives include situations where patients may agree to some treatments in their advance directives, but not others, even when they may be more appropriate for the patient (Ritchie 1998). One solution is to involve third parties in the agreement (Henderson 2004). Although this may be associated with better outcomes in terms of health service use, this may add to their complexity and undermine the very autonomy that advance directives are designed to protect (Ritchie 1998). Another problem concerns the issue of who should decide whether a patient is actually competent when they formulate their advance directive ‐ the treating physician or the courts (Ritchie 1998). A related issue is the patient's cognitive and mental state when making an advance directive. Competence relies on an individual's ability to process, evaluate and apply information but, for example, feelings of hopelessness secondary to depression may lead individuals to underestimate the effectiveness of available treatments. Solutions include careful documentation of competence at the time of the directive, or the addition of a rider stating that in the event of incompetence, the patient's proxy would be mandated to override their treatment decisions not judged to be in the patient's best interests.
Objectives
We examined the effects of advance treatment directives compared with standard care for people with severe mental illness.
Methods
Criteria for considering studies for this review
Types of studies
This review was limited to randomised controlled trials (RCTs) as these remain the least biased method of evaluating effects of all types of interventions. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
We included adults with severe mental illness (schizophrenia and other psychotic disorders, bipolar disorder, or depression with psychotic features) however diagnosed, who were managed in a community setting. We did not consider substance abuse a severe mental disorder in its own right, however, studies were eligible if they included people with dual diagnoses, that is those with severe mental illness and substance abuse.
Types of interventions
1. For an intervention to be accepted as an advance directive, it had to be described using the following terms:
a. Advance directive b. Joint crisis planning c. Advance crisis planning d. Anticipatory psychiatric planning e. Ulysses directive
2. Standard care We defined this as any usual care not involving an advance directive.
Types of outcome measures
We grouped outcomes into the short term (up to 12 weeks), medium term (13 to 26 weeks) and long term (over 26 weeks).
Primary outcomes
1. Health service contact and utilisation 1.1 Overall psychiatric admission to hospital 1.2 Involuntary psychiatric admission to hospital 1.3 Voluntary psychiatric admission to hospital 1.4 Mean days spent in a psychiatric unit per month
2. Response 2.1 No clinically important response as defined by the individual studies (e.g. global impression less than much improved or less than 50% reduction on a rating scale)
3. Global state 3.1 Specific ‐ imprisonment, police contact and arrests
Secondary outcomes
1. Health service contact and utilisation 1.2 Psychiatric outpatient service use ‐ outpatient visits, psychiatric contacts, day hospital/centre contacts 1.3 Remaining in contact with psychiatric services ‐ leaving the study early 1.4 Other health service use ‐ non‐psychiatric admissions, outpatient visits, primary care visits
2. Leaving the studies early (any reason, adverse events, inefficacy of treatment)
3. Global state 3.1 No clinically important change in global state (as defined by individual studies) 3.2 Relapse (as defined by the individual studies)
4. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 4.1 No clinically important change in general mental state score 4.2 Average endpoint general mental state score 4.3 Average change in general mental state score 4.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia) 4.5 Average endpoint specific symptom score 4.6 Average change in specific symptom score
5. General functioning 5.1 No clinically important change in general functioning 5.2 Average endpoint general functioning score 5.3 Average change in general functioning score 5.4 Specific ‐ employment 5.5 Specific ‐ accommodation status
6. Quality of life/satisfaction with treatment 6.1 No clinically important change in general quality of life 6.2 Average endpoint general quality of life score 6.3 Average change in general quality of life score
7. Cognitive functioning 7.1 No clinically important change in overall cognitive functioning 7.2 Average endpoint of overall cognitive functioning score 7.3 Average change of overall cognitive functioning score
8. Adverse effects 8.1 Number of participants with at least one adverse effect 8.2 Clinically important specific adverse effects (cardiac effects, death, movement disorders, prolactin increase and associated effects, weight gain, effects on white blood cell count) 8.3 Average endpoint in specific adverse effects 8.4 Average change in specific adverse effects
Search methods for identification of studies
We used the following strategies without language restriction.
Electronic searches
1. Cochrane Schizophrenia Group Trials Register (February 2008) using the phrase: [((*advance* AND *directive* ) OR (*joint* AND *crisis* AND *planning*)) OR (*advance * AND *crisis* AND *planning*) OR (*anticipatory* AND *psychiatric* AND planning*) OR (*Ulysses* AND *directives *) in the title, abstract, index terms of REFERENCE, or intervention of STUDY]
The Schizophrenia Groups trials register is based on regular searches of BIOSIS Inside; CENTRAL; CINAHL; EMBASE; MEDLINE and PsycINFO; the hand searching of relevant journals and conference proceedings, and searches of several key grey literature sources. A full description is given in the Group's module.
2. Cochrane Library (Issue 1, 2008) using the phrase: [(advance NEAR directive) OR (joint NEAR crisis NEAR planning) OR (advance NEAR crisis NEAR planning) OR (anticipatory NEAR psychiatric NEAR planning) OR (Ulysses NEAR directives)]
3. Biological Abstracts (1980 to February 2008) using the phrase: [(advance AND directive) OR (joint AND crisis AND planning) OR (advance AND crisis AND planning) OR (anticipatory AND psychiatric AND planning) OR (Ulysses AND directives)]
4. CINAHL (1982 to February 2008) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials combined with: [(crisis N3 planning) OR (anticipatory N3 psychiatric N3 planning) OR (Ulysses N3 directive) OR (MH "noncompliance (NANDA)") OR (MH "Advance directives +")]
5. EMBASE (1980 to February 2008) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials combined with: [(advance AND directive) OR (('joint'/exp OR 'joint') AND crisis AND ('planning'/exp OR 'planning')) OR (advance AND crisis AND ('planning'/exp OR 'planning')) OR (anticipatory AND psychiatric AND ('planning'/exp OR 'planning')) OR (ulysses AND directive)]
6. MEDLINE (1966 to February 2008) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials combined with: [(advance directive) OR (joint crisis planning) OR (advance crisis planning) OR (anticipatory psychiatric planning) OR (ulysses directive)]
7. PsycINFO (1872 to February 2008) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials combined with: [(advance N3 directive) OR (advance N3 crisis N3 planning) OR (joint N3 crisis N3 planning) OR (anticipatory N3 psychiatric N3 planning) OR (Ulysses N3 directive)]
8. Google ‐ Internet search engine (February 2008) We searched the Internet to identify any relevant publications using the following terms: advance directive, joint crisis planning, advance crisis planning, anticipatory psychiatric planning, or Ulysses directives.
9. Cochrane Schizophrenia Group Trials Register (May 2012)
We updated this search. The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (17 May 2012). The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches and conference proceedings (see group module).
Trials identified through the searching activities are each assigned to awaiting classification of relevant review titles.
Searching other resources
1. Reference searching We also inspected the references of all identified studies (including those rejected from the review) for more studies.
2. Personal contact We contacted the first author of each included study and known experts who had published reviews in the field for information regarding unpublished trials and extra data on the published trials.
Data collection and analysis
Selection of studies
We (LAC, SK) independently inspected all reports. In order to prevent any bias, we printed out a list of all titles and abstracts excluding the author's names, institutions, and journal titles. We resolved any disagreement by discussion, and where doubt remained, we acquired the full article for further inspection. Once the full articles were obtained, we independently decided whether the studies met the review criteria. A record of all rejected papers and the reasons for rejection was kept.
Data extraction and management
1. Data extraction LAC and SK independently undertook data extraction. Any disagreements were discussed, the decisions documented and where necessary, the authors of the studies contacted to help resolve the issue.
2. Management We extracted data onto standard, simple forms.
3. Scales A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid, and are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore continuous data from rating scales were included only if the measuring instrument had been described in a peer‐reviewed journal.
Assessment of risk of bias in included studies
We assessed the methodological quality of included studies using the criteria described in the Cochrane Handbook (Higgins 2006), which is based on the degree of allocation concealment. Poor concealment has been associated with overestimation of treatment effect (Schulz 1995) Category A includes studies in which allocation has been randomised and concealment is explicit. Category B studies are those which have randomised allocation but in which concealment is not explicit. Category C studies are those in which allocation has neither been randomised nor concealed. Only trials that are stated to be randomised (categories A or B of the handbook) will be included in this review. The categories are defined below:
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment).
When disputes arose as to which category a trial should be allocated, again resolution was attempted by discussion. When this was not possible we did not enter the data and the trial was added to the list of those awaiting assessment until further information could be obtained.
Measures of treatment effect
1. Binary data Where possible, efforts were made to convert outcome measures to binary data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It was generally assumed that if there had been a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS,Kay 1986), this could be considered as a clinically significant response (Leucht 2005a, Leucht 2005b). Similarly, we considered a rating of 'at least much improved' according to the Clinical Global Impression Scale (Guy 1976) as a clinically significant response. It was recognised that for many people, especially those with chronic or severe illness, a less rigorous definition of important improvement (e.g. 25% on the BPRS) would be equally valid. If individual patient data were available, the 50% cut‐off was used for the definition in the case of non‐chronically ill people and 25% for those with chronic illness. If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2. Continuous data 2.1 Normal distribution Continuous data on outcomes in trials relevant to mental health issues are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data we applied the following standards to continuous final value endpoint data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from zero, the standard deviation, when multiplied by two, should be less than the mean (otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution ‐ Altman 1996); In cases with data that are greater than the mean they were entered into 'Other data' table as skewed data. If a scale starts from a positive value (such as PANSS, which can have values from 30 to 210) the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. We reported non‐normally distributed data (skewed) in the 'other data types' tables.
For change data (mean change from baseline on a rating scale) it is impossible to tell whether data are non‐normally distributed (skewed) or not, unless individual patient data are available. After consulting the ALLSTAT electronic statistics mailing list, we entered change data in RevMan analyses and reported the finding in the text to summarise available information. In doing this, we assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew.
2.2 Final endpoint value versus change data Where both final endpoint data and change data were available for the same outcome category, only final endpoint data were presented. We acknowledge that by doing this much of the published change data may be excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. Where studies reported only change data we contacted authors for endpoint figures.
3. General Where possible, we entered data in such a way that the area to the left of the line of no effect in the forest plots indicated a favourable outcome for advance treatment directives.
Unit of analysis issues
1. Cluster trials Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a unit of analysis error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes Type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect=1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
Dealing with missing data
In the event of more than 35% of those originally randomised being lost to follow‐up, the data from that particular outcome were not included in this review. We conducted an intention‐to‐treat analysis, including all participants who were randomised, and in the case of (Papageorgiou 2002), only those who had been discharged from hospital. We assumed that those who dropped out had the negative outcome.
Assessment of heterogeneity
Firstly, we considered all the included studies within any comparison to judge for clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. We supplemented this by using primarily the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 50%, we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2003). When heterogeneous results were found, we investigated the reasons for this; where heterogeneity substantially altered the results these data were not summated, but presented separately and reasons for heterogeneity investigated.
Assessment of reporting biases
Again, according to the protocol (Campbell 2006), we planned to plot data from all included studies in a funnel graph (plotting trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias (Egger 1997). However, with only 2 studies included in the review, we were unable to use this technique.
Data synthesis
Where possible we employed a fixed‐effect model for analyses. We understand that there is no closed argument for preference for use of fixed or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random‐effects does put added weight onto the smaller of the studies ‐ those trials that are most vulnerable to bias. For this reason we favour using fixed‐effect models
For binary outcomes we calculated the relative risk (RR) and its 95% confidence interval (CI) based on the fixed‐effect model. Relative Risk is more intuitive (Boissel 1999) than odds ratios and odds ratios tend to be interpreted as RR by clinicians (Deeks 2000) This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number needed to harm (NNH).
For continuous outcomes we estimated a weighted mean difference (WMD) between groups based on a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
If data are clearly heterogeneous we checked that data are correctly extracted and entered and that we had made no unit of analysis errors. If the high levels of heterogeneity remained we did not undertake a meta‐analysis at this point for if there is considerable variation in results, and particularly if there is inconsistency in the direction of effect, it may be misleading to quote an average value for the intervention effect.
Sensitivity analysis
We investigated factors that may lead to differences between the results of individual studies using sensitivity analyses. We specified potential sources of heterogeneity in advance. These included: variations of types of intervention (e.g. advance directives ‐ instructional, proxy, or hybrid, joint crisis planning, advance crisis planning, or anticipatory psychiatric planning), intensity of intervention (low vs. high intensity e.g. information booklet and checklist vs. family meeting with patient present), and variations in methodological quality such as adequate descriptions of reasons for dropout (e.g. high vs. low methodological quality).
Results
Description of studies
Results of the search
Our search in the Cochrane Schizophrenia Group's Register (February 2008) and the other databases identified 485 potentially relevant papers, of which we identified 54 papers for further inspection. Only two studies met the inclusion criteria for this review.
Included studies
We were able to include two studies involving 321 participants (Henderson 2004 a, n=160, Papageorgiou 2002, n=161).
1. Design Both included studies were described as randomised controlled trials. Neither study used double blind methodology due to the nature of the intervention. Henderson 2004 a, however, described blinding of the investigator to allocation.
2. Study length Both studies were long term (Henderson 2004 a ‐ 15 months duration, Papageorgiou 2002 ‐ 12 months).
3. Setting Henderson 2004 a took place within community mental health teams in South London and Kent. Papageorgiou 2002 was also based in London but within inner city acute psychiatric services.
4. Participants Both trials included participants with severe mental illness. Overall, there were more men (58%) than women randomised. The mean age of participants were mid to late thirties. Henderson 2004 a used the (OPCRIT) criteria to classify the participants' mental illness. No operationally defined criteria were reported by the Papageorgiou 2002 study.
5. Interventions Henderson 2004 a employed a joint crisis plan formulated by the patient, care coordinator, psychiatrist and project worker. The plan contained contact information, details of mental and physical illnesses, treatments, indicators for relapse, and advance statements of preferences for care in the event of future relapse. The directives were formulated by patients along with carers, friends, or advocates in conjunction with their mental health care providers. Participants in the (Papageorgiou 2002) study were provided with a booklet entitled "Preferences for care", in which participants were encouraged to complete seven statements outlining future preferences for treatment. Participants could complete and sign the statements themselves, or dictate their wishes to a researcher.
6. Outcomes The main outcomes in the Henderson 2004 a study were admission to hospital, bed days, and use of the Mental Health Act over 15‐month follow‐up. Secondary outcomes included adverse events such as self harm and violence.
Papageorgiou 2002 captured rate of compulsory readmissions over 12‐month follow‐up as the main outcome measure. Secondary outcomes included time spent in hospital compulsorily or voluntarily, reported symptoms of mental illness according to the Basis‐32, prescribing, patients' satisfaction with service delivery, and patients' ability to make decisions for themselves.
6.1 Basis‐32 (Eisen 1994) The BASIS‐32 measures the change in self‐reported symptoms over the course of treatment. The instrument assesses treatment outcomes from the patient perspective, for a wide range of symptoms and problems that occur across the diagnostic spectrum. Scoring the 32 items provides summary and sub‐scale (Relation to Self and Others, Depression and Anxiety, Daily Living and Role Functioning, Impulsive and Addictive Behavior, Psychosis) scores of how patients feel before and after receiving care. The survey measures the degree of difficulty experienced by the patient over a one‐week period on a five‐point scale ranging from no difficulty to extreme difficulty. Papageorgiou 2002 reported data from this scale.
6.2 Hospital Service Satisfaction Scale The Hospital Service Satisfaction Scale is an adapted brief version of the Verona Satisfaction Scale (Ruggeri 1993), which is an instrument aimed at measuring expectations and satisfaction of patients, relatives, and professionals with community‐based psychiatric services in a multidimensional, sensitive, valid and reliable way. It is not clear whether the Hospital Service Satisfaction Scale itself was validated. Papageorgiou 2002 reported data from this scale.
7. Missing outcomes We found no data for the following outcomes: social functioning, imprisonment, employment time to relapse quality of life, self esteem, or carer/family satisfaction
Excluded studies
1. Excluded studies We excluded 52 studies from the review. Many were not randomised trials (n=37), and several were people not with mental health issues but those at the end of their lives (n=9). For two the studies were of interventions other than those of interest, in three outcomes were not of interest to this review, and for one study we were unable to obtain data for meta‐analysis.
2.Studies awaiting assessment There are 11 studies awaiting assessment.
3.Ongoing Studies We know of no ongoing studies.
Risk of bias in included studies
1. Loss to follow‐up All participants in the Henderson 2004 a study were included in the data analyses using an intention‐to‐treat analysis. No systematic differences were present in the participants who left the study early between the two study groups. Papageorgiou 2002 reported the reasons for participant withdrawal, with all participants accounted for, and no systematic differences emerged between intervention groups.
Allocation
Henderson 2004 a determined allocation sequence using minimisation, stratified by team and severity of patients' condition to ensure even distribution of those features. When predictability of allocation became possible due to batches of similar patients being allocated, the authors reassigned the allocation of one patient chosen at random before reverting to minimisation. Papageorgiou 2002 allocated patients randomly using a block design, stratified according to whether this was the patient's first ever or subsequent sectioning. Henderson 2004 a reported that only 36% of eligible patients agreed to participate.
Blinding
Henderson 2004 a reported that one investigator collected follow‐up data and was blinded to treatment group. Neither participants nor staff could be blinded to allocation due to the nature of the intervention. Papageorgiou 2002 stated that they were unable to blind the research assistants to the patients' allocation as they assisted patients in the intervention group with their directives. The trial authors noted that it was unlikely that systematic bias was introduced, as the primary outcome was compulsory hospital admission and was not based on assessment by the researchers.
Incomplete outcome data
The studies reported losses to follow‐up of approximately 25% in both intervention and control groups. Henderson 2004 a conducted intention‐to‐treat analyses of all participants randomised. Papageorgiou 2002 conducted analyses for all participants randomised except for those never eventually discharged (one in the intervention group, four in the control group). In this review, in the case of Papageorgiou 2002, we used a sensitivity analysis to determine if including those who were never discharged made any difference to the results. As including the five people never discharged made no difference to the results, they have been omitted.
Selective reporting
The first authors of both studies were helpful in providing additional data for the review. Due to skew, however, Papageorgiou 2002 reported grouped medians rather than means.
Other potential sources of bias
We did not identify any other potential sources of bias.
Effects of interventions
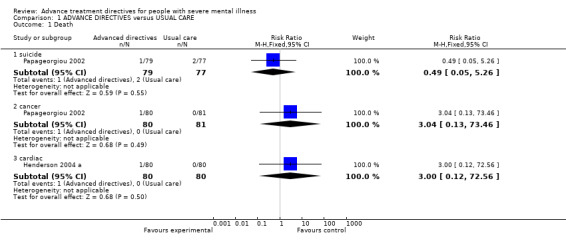
1. Comparison: ADVANCE DIRECTIVES versus USUAL CARE 1.1 Death We found no significant difference in death by suicide (Papageorgiou 2002, n=156, RR 0.49 CI 0.1 to 5.3). One participant in Henderson 2004 a died of a longstanding cardiac condition (n=160, RR 3.00 CI 0.12 to 72.6), and one participant in Papageorgiou 2002 died from cancer (n=161, RR 3.04 CI 0.13 to 73.5).
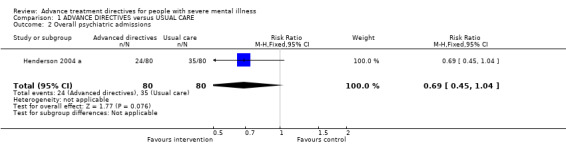
1.2 Overall psychiatric admissions Only Henderson 2004 a reported overall psychiatric admissions. We found a reduction in the intervention group (n=160, RR 0.69 0.5 to 1.0) compared with the control but this failed to reach statistical significance (p=0.08).
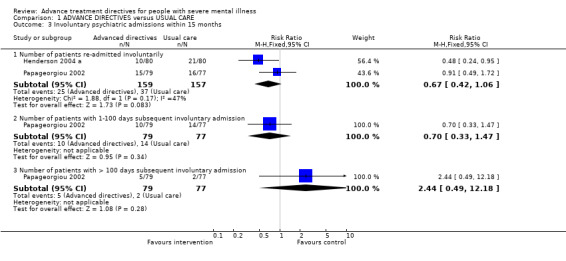
1.3 Involuntary psychiatric admissions within 15 months 1.3.1 Number of participants re‐admitted involuntarily Overall, we found no significant difference in compulsory readmissions between the intervention and treatment groups (n=316, 2 RCTs, RR 0.67 CI 0.4 to 1.1).
1.3.2 Number of participants with 1‐100 days subsequent involuntary admission We found that participants in the intervention group were no less likely to have short (1‐100 days) subsequent involuntary admissions than those in the control group (Papageorgiou 2002 , n=156, RR 0.70 CI 0.3 to 1.5).
1.3.3 Number of participants with >100 days subsequent involuntary admission Similarly we found participants with advance directives were no less likely to have long (more than 100 days) subsequent involuntary admission than those in the control group (Papageorgiou 2002, n=156, RR 2.44 CI 0.5 to 12.2).
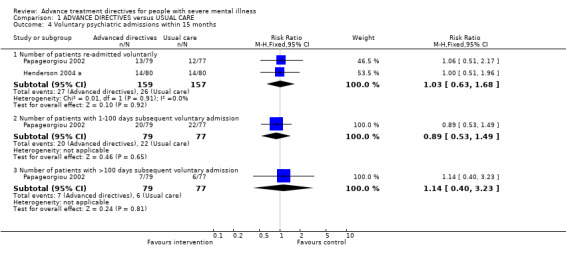
1.4 Voluntary admissions within 15 months 1.4.1 Number of participants re‐admitted voluntarily We found no significant difference between the intervention and control groups in terms of the number of participants with voluntary re‐admissions (n=316, 2 RCTs, RR 1.03 CI 0.6 to 1.7).
1.4.2 Number of participants with 1‐100 days subsequent voluntary admission Papageorgiou 2002 investigated whether there were any effects of advance directives on voluntary short admissions (<100 days). We found data to be equivocal between intervention and control groups for voluntary admissions of fewer than 100 days (n=156, RR 0.89 CI 0.5 to 1.5).
1.4.3 Number of participants with >100 days subsequent voluntary admission Papageorgiou 2002 also reported voluntary long admissions (>100 days). Again, we found no significant differences between intervention and control groups for voluntary admissions of greater than 100 days (n=156, RR 1.14 CI 0.4 to 3.2).
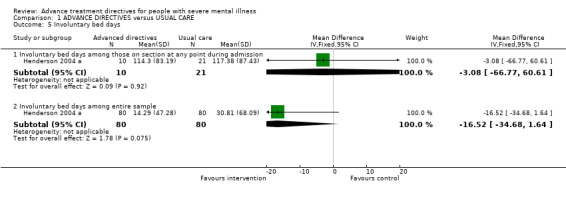
1.5 Involuntary bed days We found no significant differences between participants given advanced treatment directives (Henderson 2004 a, n=31, WMD ‐3.08 CI ‐66.8 to 60.6) and the usual care group whilst on section at any point during their admission. Averaging involuntary bed days among the entire study population (n=160) did not achieve a statistically significant difference between intervention and control groups (n=160, WMD ‐16.52 CI ‐34.7 to 1.6).
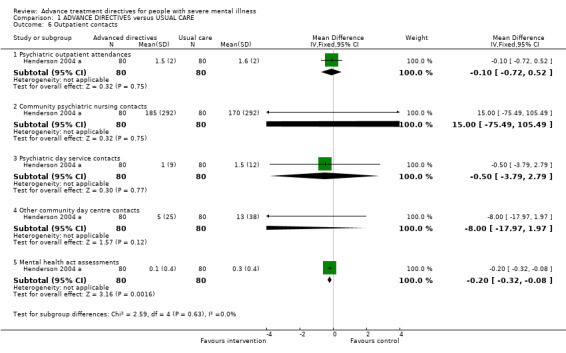
1.6 Outpatient contacts Advanced treatment directives did not significantly reduce the number of psychiatric outpatient attendances compared with those given usual care (Henderson 2004 a cited in Flood 2006, n=160, WMD ‐0.10 CI ‐0.72 to 0.52), or community psychiatric nurse contacts (n=160, 1 RCT, WMD 15.00 CI ‐75.5 to 105.5), nor the number of psychiatric day service contacts (n=160, 1 RCT, WMD ‐0.50 CI ‐3.8, 2.8), and other community day centre contacts (n=160, 1 RCT, WMD ‐8.00 CI ‐18.0, 2.0). We found participants given advanced treatment directives were significantly less likely to have been assessed under the Mental Health Act (n=160, WMD ‐0.20 CI ‐0.3 to ‐0.1).
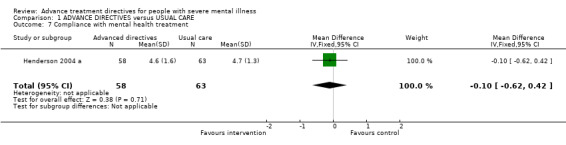
1.7 Compliance with mental health treatment Data concerning compliance with mental health treatment (scored on a scale from 0 to 6) were available from Henderson 2004 a. We found no significant difference between advanced treatment directives (n=121, WMD ‐0.10 CI ‐0.6 to 0.4) and usual care.
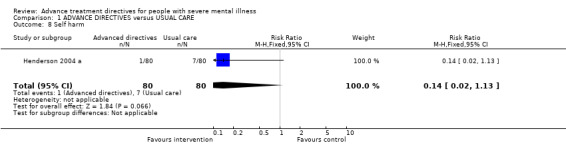
1.8 Self harm Only Henderson 2004 a reported data for self harm. We found no significant difference in self harm between intervention and control groups (n=160, RR 0.14 CI 0.02 to 1.1).
1.9 Violence Again, only Henderson 2004 a reported data for violence as an outcome measure. We found a statistically significant difference in violence between the intervention and control arms (n=160, RR 0.27 CI 0.1 to 0.9), with those in the intervention arm less likely to be violent than those in the control arm (NNT 8 CI 6 to 92).
1.10 Criminal justice contacts These data were only available from the Flood 2006 citation of Henderson 2004 a (n=160). We found no significant difference between the advanced treatment directives and usual care in the number of arrests (WMD ‐0.10 CI ‐0.3 to 0.12), lawyer contacts (WMD 0.00 CI ‐0.5 to 0.5), contacts with the police (WMD ‐0.20 CI ‐0.6 to 0.2), and nights in a police cell (WMD 0.00 CI ‐0.1 to 0.1) or prison (WMD ‐6.60 CI ‐16.7 to 3.5).
1.11 Social services We found data from the Flood 2006 paper favoured advanced treatment directives for the outcome of 'fewer social work hours' (n=160, WMD ‐106.00 CI ‐156.2 to ‐55.8). The number of weeks spent in specialised accommodation (n=160, WMD 0.10 CI ‐0.02 to 0.2) were not significantly different between intervention groups.
1.12 Other health services Henderson 2004 a found that the intervention group spent significantly more days in non‐psychiatric facilities compared to the control group (n=160, WMD 15.50 CI 9.8 to 21.2). Otherwise there were no significant differences between the intervention and control groups in terms of the number of non‐psychiatric outpatient visits (WMD ‐15.00, 95% CI ‐37.1 to 7.1), emergency department visits for either non‐psychiatric problems (WMD ‐0.10 CI ‐0.3 to 0.1) or psychiatric problems (WMD ‐0.10 CI ‐0.2 to 0.04), general practitioner visits (WMD 0.60 CI ‐0.4 to 1.6), or number of different medications prescribed (WMD 0.20 CI ‐0.3 to 0.7).
1.13 Leaving the study early We found no significant differences in attrition rates between advanced treatment directives (n=316, 2 RCTs, RR 1.08 CI 0.8 to 1.4) and those given usual care.
2. Sensitivity analyses and publication bias We were only able to obtain data from a maximum of one study for most outcomes. Therefore we could not undertake sensitivity analyses as described in the protocol for variations in types of intervention (e.g. advance directives (instructional, proxy, or hybrid), joint crisis planning, advance crisis planning, or anticipatory psychiatric planning), intensity of intervention (low versus high intensity of intervention, e.g. information booklet and checklist versus family meeting with patient and clinician present), and variations in methodological quality such as adequate descriptions of reasons for dropout (high versus low methodological quality). Similarly, it was not possible to examine publication bias given the small number of studies identified.
For the involuntary admissions outcome, however, participants in the comparatively higher‐intensity joint crisis plan intervention group in the Henderson 2004 a study were less likely to be re‐admitted involuntarily after 15 months of follow‐up than their peers in the control group (n=160, RR 0.48 CI 0.2 to 1.0). In contrast, we found no difference in involuntary re‐admissions between the lower‐intensity advance directive intervention and control groups in the Papageorgiou 2002 study (n=156, RR 0.91 CI 0.5 to 1.7).
Data were also available from both included studies for the voluntary admissions outcome. There were no differences between intervention and control groups in either of the studies.
3. Missing outcomes We found no data on social functioning, imprisonment, employment, time to relapse, quality of life, self esteem, accommodation status, or carer/family satisfaction. We were also unable to group outcomes into the short term (up to 12 weeks) and medium term (13 to 26 weeks). All data reported by the two included studies were for long‐term outcomes.
Discussion
Summary of main results
1. General Advance directives are used most often in end‐of‐life care, where they have been shown to increase expression of patient treatment preferences (Hanson 1997). Advance directives are well suited to the mental health setting for the purpose of conveying patients' treatment preferences should they become unable to articulate them in the future. However, few randomised controlled trials to examine the effects of mental health advance directives have been conducted. In this systematic search for trials, only two studies met inclusion criteria. The intervention utilised in the (Henderson 2004 a) study were more intensive, with participants in the intervention group in receipt of joint crisis planning involving patient, family, friend, carer or other and the project worker. By contrast, patients in the (Papageorgiou 2002) study received a booklet containing statements of preferences for future treatment.
2. Specific ‐ Comparison: ADVANCE DIRECTIVES versus USUAL CARE 2.1 Death During the long term follow up there were few deaths. Three that did occur were attributed to suicide but were not suggestive of any effect of advance directives.
2.2 Psychiatric admissions ‐ primary outcome Results for overall admissions were only available from Henderson 2004 a and these failed to achieve statistical significance (p=0.08). In this small trial (n=160) there were, nevertheless, less admissions in the advance directive group (24 vs 35). This finding alone is encouraging of the need for further trials.
Both studies provided data for the number of participants re‐admitted involuntarily over the follow‐up period. While the combined estimate did not demonstrate a significant difference in involuntary re‐admissions between intervention and control groups, there was also some suggestion that advance directives did decrease these events. The Henderson 2004 a study did indicate that participants in the intervention group were less likely to be hospitalised involuntarily during the follow‐up period. This might have been because the low participation rate in the Henderson 2004 a study (36%) could have lead to selection of participants with better prognoses. Another explication may be the intensity of the intervention. In the Henderson 2004 a study, plans were developed at a 30‐60 minute meeting with the researcher, treating team, patient, and invited relative. In Papageorgiou 2002 participants were only given booklets with seven statements regarding future preferences for treatment for completion. Nevertheless, this finding also suggests a moderate effect of the advance directives.
Data concerning length of subsequent involuntary admissions were only available from Papageorgiou 2002. This study found no significant difference between intervention and control groups. Papageorgiou 2002 noted that there were fewer participants involuntarily re‐admitted than predicted in both arms. While it would appear that intensity of treatment may be associated with an effect on subsequent involuntary admissions, it is problematic to draw firm conclusions from such limited data ‐ with the exception that more studies are indicated.
We found no difference between intervention and control groups for either the number of participants re‐admitted voluntarily, or for the length of voluntary admissions. In the case of Papageorgiou 2002, the lack of evidence of a difference may be due to the lower intensity of the intervention. For the Henderson 2004 a study, its power is reduced by a lower than anticipated admission rate in the control group.
2.3 Involuntary bed days Data were only available in this format from Henderson 2004 a. We found no evidence to suggest a difference in involuntary bed days between intervention and control groups, either among those on a section at any point during their admission, or among the entire sample. However, the rate of hospital admission among the control group is lower than expected based upon their earlier pilot study results, reducing the power of the study to detect a difference between the groups.
2.4 Outpatient contacts Overall, advance treatment directives did not reduce the number of outpatient contacts when assessed in various settings, but participants were less likely to be assessed under the Mental Health Act. This single finding is important but should be replicated.
2.5 Compliance with mental health treatment There is no evidence of effect of advance directives (in the form of joint crisis planning) on compliance with mental health treatment, as measured on a scale from 1 to 6. Again, data for this outcome were only available from one study (Henderson 2004 a), and more data is needed before conclusions can be drawn.
2.6 Self harm There is no evidence of an effect of advance directives (as joint crisis planning) on episodes of self harm. Data for this outcome were only available from one study (Henderson 2004 a).
2.7 Other outcomes Participants in the intervention group were less likely to commit acts of violence than those in the control group. By contrast, they had more inpatient days in non‐psychiatric facilities. The explanations for these findings are unclear. On one hand, they may represent a good outcome in that participants required less social work assistance as they were better. Alternatively, it could mean that they were not receiving sufficient service. The same uncertainty applies to the finding that participants with advance directives spent more inpatient days in non‐psychiatric facilities. Either way, data for these outcomes were only available from Henderson 2004 a and so require replication.
2.8 Leaving the study early
Long‐term studies in people with mental illnesses typically suffer from high study attrition ‐ the studies reported losses to follow up of approximately 25% in both the intervention and control groups. No differences were found between the two intervention groups. Participants were followed up in both studies with most being accounted for, although part of the data is based on an intention‐to‐treat analysis, and therefore assumptions were made for participants at their last assessment. This leads to potentially biased results.
Overall completeness and applicability of evidence
1. Applicability The two studies included participants with severe mental illness (diagnosis of psychosis or bipolar affective disorder without psychotic symptoms, psychiatric inpatients), where recurrence of illness is common and for whom advance directives would be most appropriate.
2. Homogeneity In the studies there is methodological heterogeneity due to differences in intensity of the interventions offered in the two studies. The Chi‐square test for statistical heterogeneity for the main outcome, number of participants re‐admitted involuntarily, did not reach significance (Chi‐square=1.88, df=1, p=0.17), probably due to the very small number of studies. The I‐square statistic for the main outcome, which approximates the proportion of total variation in study estimates due to heterogeneity rather than chance (sampling error), was 47%, indicating a moderate level of statistical heterogeneity. Chi‐square test for statistical heterogeneity for the other main outcome, number of participants re‐admitted voluntarily, also did not reach significance (Chi‐square=0.01, df=1, p=0.91). The I‐square statistic for number of participants with voluntary re‐admissions was 0%, indicating no statistical heterogeneity.
Quality of the evidence
We had hoped to report on the effects of advance directives on social functioning, imprisonment, employment, time to relapse, quality of life, self esteem, accommodation status, and carer/family satisfaction, however data were not reported for these outcomes. The included studies did not report data for many of the same outcomes, and reported the main outcomes differently, thus precluding several meta‐analytic analyses. Also, the included trials are both small (largest n=160) and are likely underpowered due to fewer than anticipated admissions post‐intervention.
Potential biases in the review process
Advance directive interventions are varied, encompassing interventions of varying intensity. We had planned to assess the contribution of the intensity of the intervention on the identified outcomes by means of sensitivity analyses. However, only two trials were identified, of which one was high‐ and the other low‐intensity.
Agreements and disagreements with other studies or reviews
We know of no other systematic reviews in this area.
Authors' conclusions
Implications for practice.
1. For people with severe mental illness Evidence for the effects of mental health advance directives is limited by the paucity of data available from randomised controlled trials. In a non‐randomised study examining the effects of advance directives on working alliance and treatment satisfaction, participants in the intervention group showed significantly greater improvement in their working relationship with their clinicians, and were more likely to report satisfaction with their mental health treatment at one‐month follow‐up (Swanson 2006c). Currently it is not possible to recommend advance treatment directives for people with severe mental illness due to the lack of supporting data.
2. For clinicians Results of this review suggest that more intensive interventions, such as joint crisis planning, may be of benefit in terms of reduction of involuntary readmissions or assessments under the Mental Health Act. This is consistent with the literature on advance directives in end‐of‐life care Successful end‐of‐life treatment planning has been associated with a process‐ rather than product‐oriented approach and has been directed by the family or community rather than the physician (Prendergast 2001). However, caution in interpreting this finding is necessary, based on the lack of available randomised controlled trial data.
A qualitative analysis of a randomised controlled trial of psychiatric advance directives (PADs) reported that while PADs were tools for empowerment and self‐determination, limited knowledge of PADs among service providers and difficulty communicating PADs to inpatient staff limited their usefulness (Kim 2007).
3. For policy makers There are limited data available from which to inform policy. However, high intensity advance directives, such as joint crisis planning, may offer promise. Although not an outcome of this review, the Henderson 2004 a study also reported that use of a joint crisis plan was associated with lower costs on average than usual care, although the differences did not reach statistical significance (Flood 2006). Intensive advance directives, such as joint crisis planning, may provide a more promising alternative to controversial community treatment orders.
Implications for research.
1. General This review highlighted the need for good quality randomised controlled trials to assess the effectiveness of advance directives in the mental health setting. Better reporting of both the included and excluded studies would have meant that more data were available for this review. Future studies should fully comply with CONSORT guidance (Moher 2001).
2. Specific More randomised controlled trials are required, investigating the differing levels of intensity associated with various types of advance directives. Future trials must be adequately powered for a variety of outcomes, including compulsory and voluntary admissions (number of admissions and lengths of stay), social functioning, mental state, quality of life, and satisfaction with mental health services, both in the short and long term. One suggested design for a study is outlined in Table 1. Additionally, further study is required to measure whether advance directives accurately represent patient wishes.
1. Suggested design for future trial.
| Methods | Participants | Interventions | Outcomes | Notes |
| Allocation: randomised, clearly described. Blinding: single (due to nature of intervention), tested. Duration: 12‐18 months |
Diagnosis: people with serious mental illnesses. N=300.* |
1. High intensity advance directive (in person meeting for co‐negotiation of advance directive with patient, care co‐ordinator, and carer, friend, or advocate). 2. Low intensity advance directive (pamphlet and checklist). |
Health service outcomes, admission, type of admission, bed days, outpatient contacts. General functioning: self harm, violence, criminal justice contact, quality of life, satisfaction. Leaving early. |
* powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. |
What's new
| Date | Event | Description |
|---|---|---|
| 17 May 2012 | Amended | Update search of Cochrane Schizophrenia Group's Trial Register (see Search methods for identification of studies), 11 studies added to awaiting classifciation. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 8 February 2010 | Amended | Plain Language Summary added |
| 5 August 2009 | Amended | Contact details updated. |
| 21 July 2008 | Amended | Converted to new review format. |
Acknowledgements
LAC is employed by the Capital District Health Authority, Halifax, Nova Scotia, Canada. SK is employed by Griffith University, Queensland, Australia. Both have appointments at Dalhousie University, Halifax, Nova Scotia, Canada.
Data and analyses
Comparison 1. ADVANCE DIRECTIVES versus USUAL CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 suicide | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.26] |
| 1.2 cancer | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.46] |
| 1.3 cardiac | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.56] |
| 2 Overall psychiatric admissions | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.04] |
| 3 Involuntary psychiatric admissions within 15 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Number of patients re‐admitted involuntarily | 2 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.42, 1.06] |
| 3.2 Number of patients with 1‐100 days subsequent involuntary admission | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.33, 1.47] |
| 3.3 Number of patients with > 100 days subsequent involuntary admission | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.49, 12.18] |
| 4 Voluntary psychiatric admissions within 15 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Number of patients re‐admitted voluntarily | 2 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.63, 1.68] |
| 4.2 Number of patients with 1‐100 days subsequent voluntary admission | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 4.3 Number of patients with >100 days subsequent voluntary admission | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.23] |
| 5 Involuntary bed days | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Involuntary bed days among those on section at any point during admission | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐3.08 [‐66.77, 60.61] |
| 5.2 Involuntary bed days among entire sample | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐16.52 [‐34.68, 1.64] |
| 6 Outpatient contacts | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Psychiatric outpatient attendances | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.72, 0.52] |
| 6.2 Community psychiatric nursing contacts | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [‐75.49, 105.49] |
| 6.3 Psychiatric day service contacts | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.79, 2.79] |
| 6.4 Other community day centre contacts | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐17.97, 1.97] |
| 6.5 Mental health act assessments | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.32, ‐0.08] |
| 7 Compliance with mental health treatment | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.62, 0.42] |
| 8 Self harm | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.13] |
| 9 Violence | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.94] |
| 10 Criminal justice contacts | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Arrests | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.32, 0.12] |
| 10.2 Contacts with lawyer | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
| 10.3 Contacts with police officer | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.62, 0.22] |
| 10.4 Nights in police cell | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.11, 0.11] |
| 10.5 Nights in prison | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐6.6 [‐16.68, 3.48] |
| 11 Social services | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Social work hours | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐106.0 [‐156.24, ‐55.76] |
| 11.2 Specialised accommodation weeks | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.02, 0.22] |
| 12 Other health services | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Non‐psychiatric inpatient days | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 15.5 [9.79, 21.21] |
| 12.2 Non‐psychiatric outpatient attendances | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐37.06, 7.06] |
| 12.3 Accident and emergency contacts (non‐psychiatric) | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.32, 0.12] |
| 12.4 Accident and emergency contacts (psychiatric) | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.24, 0.04] |
| 12.5 General practitioner contacts | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.38, 1.58] |
| 12.6 Number of different medications prescribed | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.30, 0.70] |
| 13 Leaving the study early | 2 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.38] |
1.1. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 1 Death.
1.2. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 2 Overall psychiatric admissions.
1.3. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 3 Involuntary psychiatric admissions within 15 months.
1.4. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 4 Voluntary psychiatric admissions within 15 months.
1.5. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 5 Involuntary bed days.
1.6. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 6 Outpatient contacts.
1.7. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 7 Compliance with mental health treatment.
1.8. Analysis.
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 8 Self harm.
1.9. Analysis.
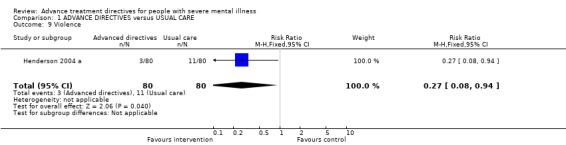
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 9 Violence.
1.10. Analysis.
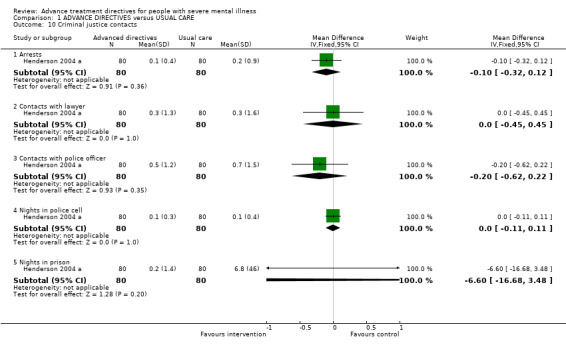
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 10 Criminal justice contacts.
1.11. Analysis.
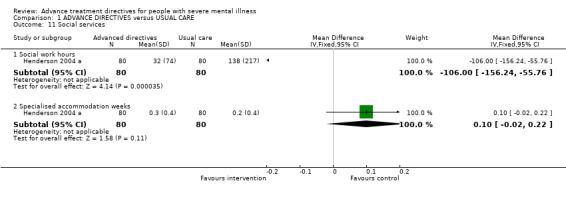
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 11 Social services.
1.12. Analysis.
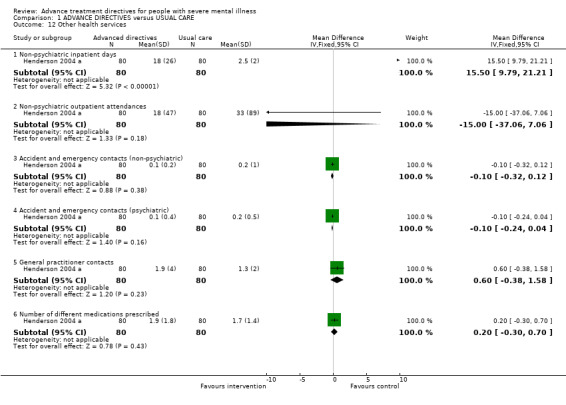
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 12 Other health services.
1.13. Analysis.
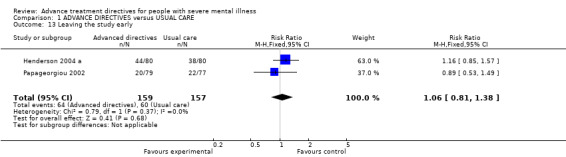
Comparison 1 ADVANCE DIRECTIVES versus USUAL CARE, Outcome 13 Leaving the study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Henderson 2004 a.
| Methods | Allocation: randomised. Blindness: single. Duration: 15 months. | |
| Participants | Diagnosis: psychotic illness or non‐psychotic bipolar disorder (OPCRIT criteria). N=160. Age: mean age 39 years. Sex: 94 M, 66 F. Inclusion criteria: patients in contact with local community health team; admission to inpatient psychiatric service at least once in previous 2 years. Exclusion criteria: unable to give informed consent due to mental incapacity; insufficient command of English; current inpatients excluded to avoid potential coercion. Setting: Community, Southern England. | |
| Interventions | Intervention: joint crisis planning including patient, carer, friend or advocate, care co‐ordinator (if possible) and project worker. N=80. Control: participants received information leaflets about local services, mental illness and treatments, the Mental Health Act, local provider organisations, and relevant policies. N=80. | |
| Outcomes | Primary outcomes: admission to hospital and length of time spent in hospital. Secondary outcome: objective coercion (i.e., compulsory treatment under the Mental Health Act). Other: sociodemographic variables, clinical details, history of adverse events (e.g., self harm, harm to others), and compliance with mental health treatment (rated by care co‐ordinator on 7‐point rating scale). | |
| Notes | Data collected from case notes, the computerised patient administration system, Mental Health Act Office data, and interviews with patients and care co‐ordinators. Follow up conducted 15 months after randomisation. Due to the nature of the interventions, neither staff nor participants could be blinded to allocation. However, one investigator collected follow‐up data, and was blinded to treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated using minimization, stratified by team and by severity of patients' condition to ensure even distribution of these features. Predictability of allocation avoided by random reassignment of one patient per batch. |
| Allocation concealment (selection bias) | Low risk | Allocation not revealed to investigator. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | One investigator collected follow‐up data and was blinded to treatment group. Double‐blinding not possible due to nature of intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses. |
| Selective reporting (reporting bias) | Low risk | Reported all data. |
| Other bias | High risk | Rate of hospital admission among control group lower than expected from pilot study. Only 36% of eligible patients agreed to participate. However, had high rate of follow‐up for reported outcomes. |
Papageorgiou 2002.
| Methods | Allocation: randomised, stratified according to number of times sectioned, using sealed envelopes. Blindness: not blinded. Duration: 12 months. | |
| Participants | Diagnosis: "inpatients receiving compulsory psychiatric treatment under sections 2, 3,or 4 of the Mental Health Act 1983 for England and Wales" (no diagnostic criteria reported). N=161. Age: mean age 36 years. Sex: 93 M, 63 F. Inclusion criteria: inpatients receiving compulsory psychiatric treatment (under sections 2, 3, or 4 of the Mental Health Act 1983 for England and Wales), age 18 years and over, able to read English. Exclusion criteria: patients under other specialised sections, those about to be transferred to other orders or other hospitals, and those with organic brain disease. Setting: in‐patients, Inner London. | |
| Interventions | Intervention: participants provided with a booklet containing seven statements concerning preferences for future treatment; patients were encouraged to complete and sign the booklets, or dictate their preferences to the researcher; copies were provided to the key worker, general practitioner, and filed with the hospital and general practice records; all patients received standard community psychiatric care (planned and provided by a multidisciplinary community psychiatric team). N=80. Control: usual care, consisting of standard community psychiatric treatment (as above). N=81. | |
| Outcomes | Primary outcome: number of people compulsorily re‐admitted under the Mental Health Act during follow‐up. 12‐month follow‐up: Basis‐32. self‐report questionnaire designed for patients with mental illness. Hospital Service Satisfaction Scale. Questions on the use of the advance directive. Questions for consultant psychiatrists and key workers on their awareness of the directive, its use, and whether it could be improved. Prescribing data (from case notes). | |
| Notes | Data regarding voluntary and involuntary admissions collected from hospital records for the 5 years before baseline and 12 months of follow‐up. Due to the nature of the intervention, it was impossible to mask research assistants to patients' allocation. However, primary outcome of admission was not likely affected by systematic bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation using block design in blocks of 12 (6 experimental, 6 control), stratified by first ever or subsequent sectioning. Block randomisation in known block sizes allows for identification of allocation of at least the 12th participant per block if block size is known to investigators. |
| Allocation concealment (selection bias) | Unclear risk | Allocation provided in sealed envelopes. Independent colleague selected next envelope. As above, is possible to identify allocation of at least the 12th participant per block. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "It was impossible to mask the research assistants to the patients' allocation as they were required to assist patients to make a directive in those allocated to the intervention group. However, systematic bias was unlikely as the primary outcome concerned compulsory hospital admission and was not based on any later assessment by the researcher." Bias possible for secondary outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis, with exception of 5 (1 intervention, 4 control) patients not discharged from hospital. |
| Selective reporting (reporting bias) | Low risk | Reported all data. |
| Other bias | High risk | In both arms, fewer than expected patients were compulsorily re‐admitted, leading to lower statistical power than anticipated. Potential for lack of sustained awareness of directives throughout the 12 months of follow‐up. Key workers in one of the two psychiatric services were often not allocated before patients were discharged, potentially reducing the effect of the directives. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amering 2005 | Allocation: not randomised. |
| Anderson 2003 | Allocation: not randomised. |
| Appelbaum 2004 | Allocation: not randomised. |
| Atkinson 2004 | Allocation: not randomised. |
| Backlar 1997 | Allocation: not randomised. |
| Bracken 2001 | Allocation: not randomised. |
| Brown 1999 | Allocation: randomised. Participants: end‐of‐life patients. |
| Campbell 2000 | Allocation: not randomised. |
| Dawson 2001 | Allocation: not randomised. |
| Ditto 2001 | Allocation: randomised. Participants: end‐of‐life patients. |
| Elbogen 2006 | Allocation: not randomised. |
| Froman 2005 | Allocation: randomised. Participants: end‐of‐life patients. |
| Geller 2000 | Allocation: not randomised. |
| Griffith 2006 | Allocation: not randomised. |
| Gutheil 2005 | Allocation: randomised. Participants: end‐of‐life patients. |
| Heiman 2004 | Allocation: cluster randomised. Participants: end‐of‐life patients. |
| Henderson 1999 | Allocation: Cochrane review of randomised trials. Participants: people with a diagnosis of psychotic illness. Interventions: patient‐held clinical information. |
| Joshi 2003 | Allocation: not randomised. |
| Kim 2007 a | Allocation: not randomised (qualitative sub‐study of a larger randomised trial). |
| LaFond 2002 | Allocation: not randomised. |
| Lang 1999 | Allocation: not randomised. |
| Loewy 2004 | Allocation: not randomised. |
| McKenna 2000 | Allocation: not randomised. |
| Molloy 2000 | Allocation: randomised. Participants: end‐of‐life patients. |
| Murphy 2000 | Allocation: randomised. Participants: end‐of‐life patients. |
| O'Connell 2005 | Allocation: not randomised. |
| Patel 2004 | Allocation: systematic review. Participants: end‐of‐life patients. |
| Pearlman 2005 | Allocation: randomised. Participants: end‐of‐life patients. |
| Peto 2004 | Allocation: not randomised. |
| Prendergast 2001 a | Allocation: not randomised. |
| Priebe 1997 | Allocation: not randomised. |
| Priebe 1999 | Allocation: randomised. Participants: people with schizophrenia in acute day hospital. Intervention: patient treatment wishes. Outcomes: unable to obtain data for inclusion in meta‐analysis. |
| Rich 2004 | Allocation: not randomised. |
| Rissmiller 2001 | Allocation: not randomised. |
| Ritchie 1998b | Allocation: not randomised. |
| Schouten 2006 | Allocation: not randomised. |
| Springer Loewy 2004 | Allocation: not randomised. |
| Srebnik 1999 a | Allocation: not randomised. |
| Srebnik 2003 | Allocation: not randomised. |
| Srebnik 2005 | Allocation: not randomised. |
| Sutherby 1998 | Allocation: not randomised. |
| Swanson 2000 | Allocation: not randomised. |
| Swanson 2006a | Allocation: not randomised. |
| Swanson 2006b | Allocation: not randomised. |
| Swanson 2006c | Allocation: randomised. Participants: people with severe mental illness. Intervention: psychiatric advance directives (PADs) facilitation. Outcomes: completion of AD, 1‐month working relationship with clinicians, satisfaction with services. |
| Swartz 2006 | Allocation: randomised. Participants: people with severe mental illness. Intervention: psychiatric advance directives (PADs) facilitation. Outcomes: patient preferences and interest in PADs. |
| Tapp 2006 | Allocation: not randomised. |
| Van Dorn 2006 | Allocation: not randomised. |
| Van Os 2004 | Allocation: randomised. Participants: people with schizophrenia. Intervention: use of two‐way communication checklist. |
| VanWilligenburg 2005 | Allocation: not randomised. |
| Wareham 2005 | Allocation: not randomised. |
| Warner 2000 | Allocation: cluster randomised. Participants: people with long‐term mental illness. Intervention: patient held shared care records. |
Characteristics of studies awaiting assessment [ordered by study ID]
Elbogen 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Henderson 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Henderson 2004 b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
NCT00105794 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Papageorgiou 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Ruchlewska 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Sensky 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Swanson 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Thornicroft 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Van Dorn 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Wilder 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Differences between protocol and review
Some reformatting of the protocol has taken place. There has been no substantive changes to methods.
Contributions of authors
Leslie Anne Campbell ‐ jointly formulated the review question, initially developed the search strategy, and wrote the first draft of the review.
Steve Kisely ‐ jointly formulated the review question, and reviewed and provided comments on the search strategy and review.
Sources of support
Internal sources
Dalhousie University, Halifax, Nova Scotia, Canada.
Health Outcomes Unit, Capital District Health Authority, Halifax, Nova Scotia, Canada.
Griffith University, Queensland, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Henderson 2004 a {published data only}
- Flood C, Byford S, Henderson C, Leese M, Thornicroft G, Sutherby K, Szmukler G. Joint crisis plans for people with psychosis: economic evaluation of a randomised controlled trial. BMJ 2006;333:729‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C, Flood C, Leese M, Thornicroft G, Sutherby K, Szmukler G. Effect of joint crisis plans on use of compulsory treatment in psychiatry: single blind randomised controlled trial. BMJ 2004;329:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papageorgiou 2002 {published data only}
- Papageorgiou A, King M, Janmohamed A, Davidson O, Dawson J. Advance directives for patients compulsorily admitted to hospital with serious mental illness: randomised controlled trial. British Journal of Psychiatry 2002;181:513‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amering 2005 {published data only}
- Amering M, Stastny P, Hopper K. Psychiatric advance directives: qualitative study of informed deliberations by mental health service users. Br J Psychiatry 2005;186:247‐52. [DOI] [PubMed] [Google Scholar]
Anderson 2003 {published data only}
- Anderson N. Dr. Jekyll's waiver of Mr. Hyde's right to refuse medical treatment: Washington's new law authorizing mental health care advance directives needs additional protection. Washington Law Review 2003;78(3):795‐829. [PubMed] [Google Scholar]
Appelbaum 2004 {published data only}
- Appelbaum PS. Psychiatric advance directives and the treatment of committed patients. Psychiatric Services 2004;55:751‐52, 763. [DOI] [PubMed] [Google Scholar]
Atkinson 2004 {published data only}
- Atkinson JM, Garner HC, Gilmour WH. Models of advance directives in mental health care: stakeholder views. Social Psychiatry and Psychiatric Epidemiology 2004;39(8):673‐80. [DOI] [PubMed] [Google Scholar]
Backlar 1997 {published data only}
- Backlar P. Ethics in community mental health care: anticipatory planning for psychiatric treatment is not quite the same as planning for end‐of‐life care. Community Mental Health Journal 1997;33:261‐68. [DOI] [PubMed] [Google Scholar]
Bracken 2001 {published data only}
- Bracken P, Thomas P. Postpsychiatry: a new direction for mental health. BMJ 2001;322:724‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown JB, Beck A, Boles M, Barrett P. Practical methods to increase use of advance medical directives. Journal of General Internal Medicine 1999;14(1):21‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2000 {published data only}
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, Tyrer P. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 2001 {published data only}
- Dawson J, King M, Papageorgiou A, Davidson O. Legal pitfalls of psychiatric research. British Journal of Psychiatry 2001;178:67‐70. [DOI] [PubMed] [Google Scholar]
Ditto 2001 {published data only}
- Ditto PH, Danks JH, Smucker WD, Bookwala J, Coppola KM, Dresser R, Fagerlin A, Gready RM, Houts RM, Lockhart LK, Zyzanski S. Advance directives as acts of communication: a randomized controlled trial. Archives of Internal Medicine 2001;161(3):421‐30. [DOI] [PubMed] [Google Scholar]
Elbogen 2006 {published data only}
- Elbogen EB, Swartz MS, Dorn R, Swanson JW, Kim M, Scheyett A. Clinical decision making and views about psychiatric advance directives. Psychiatric Services 2006;57:350‐5. [DOI] [PubMed] [Google Scholar]
Froman 2005 {published data only}
- Froman RD, Owen SV. Randomized study of stability and change in patients' advance directives. Research in Nursing and Health 2005;28(5):398‐407. [DOI] [PubMed] [Google Scholar]
Geller 2000 {published data only}
- Geller JL. The use of advance directives by persons with serious mental illness for psychiatric treatment. Psychiatric Quarterly 2000;71:1‐13. [DOI] [PubMed] [Google Scholar]
Griffith 2006 {published data only}
- Griffith R. Making decisions for incapable adults 2: advanced decisions refusing care. British Journal of Community Nursing 2006;11(4):162‐6. [DOI] [PubMed] [Google Scholar]
Gutheil 2005 {published data only}
- Gutheil IA, Heyman JC. Communication between older people and their health care agents: results of an intervention. Health and Social Work 2005;30(2):107‐16. [DOI] [PubMed] [Google Scholar]
Heiman 2004 {published data only}
- Heiman H, Bates DW, Fairchild D, Shaykevich S, Lehmann LS. Improving completion of advance directives in the primary care setting: a randomized controlled trial. American Journal of Medicine 2004;117(5):318‐24. [DOI] [PubMed] [Google Scholar]
Henderson 1999 {published data only}
- Henderson C, Laugharne R. Patient held clinical information for people with psychotic illnesses. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001711] [DOI] [PubMed] [Google Scholar]
Joshi 2003 {published data only}
- Joshi KG. Psychiatric advance directives. Journal of Psychiatric Practice 2003;9(4):303‐6. [DOI] [PubMed] [Google Scholar]
Kim 2007 a {published data only}
- Kim MM, Dorn RA, Scheyett AM, Elbogen EE, Swanson JW, Swartz MS, McDaniel LA. Understanding the personal and clinical utility of psychiatric advance directives: a qualitative perspective. Psychiatry: Interpersonal & Biological Processes 2007;70(1):19‐29. [DOI] [PubMed] [Google Scholar]
LaFond 2002 {published data only}
- Fond JQ, Srebnik D. The impact of mental health advance directives on patient perceptions of coercion in civil commitment and treatment decisions. International Journal of Law and Psychiatry 2002;25:537‐55. [DOI] [PubMed] [Google Scholar]
Lang 1999 {published data only}
- Lang MA, Davidson L, Bailey P, Levine MS. Clinicians' and clients' perspectives on the impact of assertive community treatment. Psychiatric Services 1999;50:1331‐40. [DOI] [PubMed] [Google Scholar]
Loewy 2004 {published data only}
- Lowey EH. Advance directives: good, bad, or indifferent. Wien Klin Wochenschr 2004;116(13):411‐16. [DOI] [PubMed] [Google Scholar]
McKenna 2000 {published data only}
- McKenna BG, Simpson AIF, Coverdale JH. What is the role of procedural justice in civil commitment?. Australian and New Zealand Journal of Psychiatry 2000;34:671‐6. [DOI] [PubMed] [Google Scholar]
Molloy 2000 {published data only}
- Molloy DW, Guyatt GH, Russo R, Goeree R, O'Brien BJ, Bédard M, Willan A, Watson J, Patterson C, Harrison C, Standish T, Strang D, Darzins PJ, Smith S, Dubois S. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA 2000;283(11):1437‐44. [DOI] [PubMed] [Google Scholar]
Murphy 2000 {published data only}
- Murphy P, Kreling B, Kathryn E, Stevens M, Lynn J, Dulac J. Description of the SUPPORT intervention. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Journal of the American Geriatrics Society 2000;48(5 Suppl):154‐61. [PubMed] [Google Scholar]
O'Connell 2005 {published data only}
- O'Connell MJ, Stein CH. Psychiatric advance directives: perspectives of community stakeholders. Administration Policy in Mental Health 2005;32(3):241‐65. [DOI] [PubMed] [Google Scholar]
Patel 2004 {published data only}
- Patel RV, Sinuff T, Cook DJ. Influencing advance directive completion rates in non‐terminally ill patients: a systematic review. Journal of Critical Care 2004;19(1):1‐9. [DOI] [PubMed] [Google Scholar]
Pearlman 2005 {published data only}
- Pearlman RA, Starks H, Cain KC, Cole WG. Improvements in advance care planning in the Veterans Affairs System: results of a multifaceted intervention. Archives of Internal Medicine 2005;165:667‐74. [DOI] [PubMed] [Google Scholar]
Peto 2004 {published data only}
- Peto T, Srebnik D, Zick E, Russo J. Support needed to create psychiatric advance directives. Administration and Policy in Mental Health 2004;31(5):409‐19. [DOI] [PubMed] [Google Scholar]
Prendergast 2001 a {published data only}
- Prendergast TJ. Advance care planning: pitfalls, promise, progress. Critical Care Medicine 2001;29(2 Suppl):34‐9. [DOI] [PubMed] [Google Scholar]
Priebe 1997 {published data only}
- Priebe S, Broker M. Prediction of hospitalizations by schizophrenia patients' assessment of treatment: an expanded study. Journal of Psychiatric Research 1999;33:113‐19. [DOI] [PubMed] [Google Scholar]
Priebe 1999 {published data only}
- Priebe S, Gruyters T. A pilot trial of treatment changes according to schizophrenic patients' wishes. Journal of Nervous and Ment Disease 1999;187(7):441‐3. [DOI] [PubMed] [Google Scholar]
Rich 2004 {published data only}
- Rich BA. Current legal status of advance directives in the United States. Wien Klin Wochenschr 2004;116(13):420‐26. [DOI] [PubMed] [Google Scholar]
Rissmiller 2001 {published data only}
- Rissmiller DJ, Musser E, Rhoades W, Rissmiller FR, Steer RA. A survey of use of a durable power of attorney to admit geropsychiatric patients. Psychiatric Services 2001;52:98‐100. [DOI] [PubMed] [Google Scholar]
Ritchie 1998b {published data only}
- Ritchie J, Sklar R, Steiner W. Advance directives in psychiatry: resolving issues of autonomy and competence. International Journal of Law and Psychiatry 1998;21:245‐60. [DOI] [PubMed] [Google Scholar]
Schouten 2006 {published data only}
- Schouten R. Commentary: Psychiatric advance directives as tools for enhancing treatment of the mentally ill the mentally ill Commentary: Psychiatric advance directives as tools for enhancing treatment of the mentally ill Commentary: Psychiatric advance directives as tools for enhancing treatment of the mentally ill. Journal of the American Academy of Psychiatry and the Law 2006;34(1):58‐60. [PubMed] [Google Scholar]
Springer Loewy 2004 {published data only}
- Springer Loewy R. Written advance directives: Theuth's blessing... or... curse?. Wien Klin Wochenschr 2004;166(13):439‐41. [DOI] [PubMed] [Google Scholar]
Srebnik 1999 a {published data only}
- Srebnik DS, Fond JQ. Advance directives for mental health treatment. Psychiatric Services 1999;50:919‐25. [DOI] [PubMed] [Google Scholar]
Srebnik 2003 {published data only}
- Srebnik DS, Russo J, Sage J, Peto T, Zick E. Interest in psychiatric advance directives among high users of crisis services and hospitalization. Psychiatric Services 2003;54:981‐86. [DOI] [PubMed] [Google Scholar]
Srebnik 2005 {published data only}
- Srebnik DS, Rutherford LT, Peto T, Russo J, Zick E, Jaffe C, Holtzheimer P. The content and clinical utility of psychiatric advance directives. Psychiatric Services 2550;56(5):592‐98. [DOI] [PubMed] [Google Scholar]
Sutherby 1998 {published data only}
- Sutherby K, Szmukler G. Crisis cards and self‐help crisis initiatives. Psychiatric Bulletin 1998;22:4‐7. [Google Scholar]
Swanson 2000 {published data only}
- Swanson JW, Tepper MC, Backlar P, Swartz MS. Psychiatric advance directives: an alternative to coercive treatment?. Psychiatry 2000;63:160‐72. [DOI] [PubMed] [Google Scholar]
Swanson 2006a {published data only}
- Swanson JW, McCrary S, Swartz MS, Dorn RA, Elbogen EB. Overriding Psychiatric Advance Directives: factors Associated with psychiatrists' decisions to preempt patients' advance refusal of hospitalization and medication. Law and Human Behaviour 2006;31(1):77‐90. [DOI] [PubMed] [Google Scholar]
Swanson 2006b {published data only}
- Swanson J, Swartz M, Ferron J, Elbogen E, Dorn R. Psychiatric advance directives among public mental health consumers in five U.S. cities: prevalence, demand, and correlates. Journal of the American Academy of Psychiatry and the Law 2006;34(1):43‐57. [PubMed] [Google Scholar]
Swanson 2006c {published data only}
- Swanson JW, Swartz MS, Elbogen EB, Dorn RA, Ferron J, Wagner HR, McCauley BJ, Kim M. Facilitated psychiatric advance directives: a randomized trial of an intervention to foster advance treatment planning among persons with severe mental illness. American Journal of Psychiatry 2006;163:1943‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swartz 2006 {published data only}
- Swartz MS, Swanson JW, VanDorn RA, Elbogen EB, Shumway M. Patient preferences for psychiatric advance directives. International Journal of Forensic Mental Health 2006;5:67‐81. [Google Scholar]
Tapp 2006 {published data only}
- Tapp A. Advance directives. Can Nurse 2006;102(2):26. [PubMed] [Google Scholar]
Van Dorn 2006 {published data only}
- Dorn RA, Swartz MS, Elbogen EB, Swanson JW, Kim M, Ferron J, McDaniel LA, Scheyett AM. Clinicians' attitudes regarding barriers to the implementation of psychiatric advance directives. Administration Policy in Mental Health 2006;33(4):449‐60. [DOI] [PubMed] [Google Scholar]
Van Os 2004 {published data only}
- Os J, Altamura AC, Bobes J, Gerlach J, Hellewell JS, Kasper S, Naber D, Robert P. Evaluation of the Two‐Way Communication Checklist as a clinical intervention. Results of a multinational, randomised controlled trial. British Journal of Psychiatry 2004;184:79‐83. [DOI] [PubMed] [Google Scholar]
VanWilligenburg 2005 {published data only}
- Willigenburg T, Delaere P. Protecting autonomy as authenticity using ulysses contracts. Journal of Medicine and Philosophy 2005;30(6):691. [DOI] [PubMed] [Google Scholar]
Wareham 2005 {published data only}
- Wareham P, McCallin A, Diesfeld K. Advance directives: the New Zealand context. Nursing Ethics 2005;12(4):349‐59. [DOI] [PubMed] [Google Scholar]
Warner 2000 {published data only}
- Warner JP, King M, Blizard R, McClenahan Z, Tang S. Patient‐held shared care records for individuals with mental illness: randomised controlled evaluation. British Journal of Psychiatry 2000;177:319‐24. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Elbogen 2007 {published data only}
- Elbogen EB, Swanson JW, Appelbaum PS, Swartz MS, Ferron J, Dorn RA, et al. Competence to complete psychiatric advance directives: effects of facilitated decision making. Law and Human Behavior 2007;31(3):275‐89. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2001 {published data only}
- Henderson C. An exploratory trial of joint crisis plans for people with psychotic illnesses. National Research Register 2001; Vol. 1.
Henderson 2004 b {published data only}
- Henderson C, Flood C, Leese M, Thornicroft G, Sutherby K, Szmukler G. The joint crisis plan on the use of compulsion in psychiatric treatment: a RANDOMized controlled trial. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
NCT00105794 2005 {published data only}
- NCT00105794. Psychiatric advance directives for improved healthcare. http://www.clinicaltrials.gov 2005.
Papageorgiou 2004 {published data only}
- Papageorgiou A, Janmohamed A, King M, Davidson O, Dawson J. Advance directives for patients compulsorily admitted to hospital with serious mental disorders: directive content and feedback from patients and professionals. Journal of Mental Health 2004;13(4):379‐88. [Google Scholar]
Ruchlewska 2009 {published data only}
- Ruchlewska A, Mulder CL, Smulders R, Roosenschoon BJ, Koopmans G, Wierdsma A. The effects of crisis plans for patients with psychotic and bipolar disorders: a RANDOMised controlled trial. BMC Psychiatry 2009;9(41):8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sensky 2000 {published data only}
- Sensky T. A pilot study of an intervention to improve communications between carers of people with schizophrenia and the multi‐professional mental health care team. National Research Register 2000.
Swanson 2008 {published data only}
- Swanson JW, Swartz MS, Elbogen EB, Dorn RA, Wagner HR, Moser LA, et al. Psychiatric advance directives and reduction of coercive crisis interventions. Journal of Mental Health 2008;17(3):255‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornicroft 2010 {published data only}
- Thornicroft G, Farrelly S, Birchwood M, Marshall M, Szmukler G, Waheed W, et al. Crimson crisis plan impact: Subjective and objective coercion and engagement protocol: A RANDOMised controlled trial of joint crisis plans to reduce compulsory treatment of people with psychosis. Trials 2010;11:102. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dorn 2008 {published data only}
- Dorn RA, Swanson JW, Swartz MS, Elbogen E, Ferron J. Reducing barriers to completing psychiatric advance directives. Administration and Policy in Mental Health and Mental Health Services Research 2008;35(6):440‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilder 2010 {published data only}
- Wilder CM, Elbogen EB, Moser LL, Swanson JW, Swartz MS. Medication preferences and adherence among individuals with severe mental illness and psychiatric advance directives. Psychiatric Services 2010;4:380‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Campbell 2006
- Campbell LA, Kisely SR. Advance treatment directives for people with severe mental illness. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder CE. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisen 1994
- Eisen SV, Dill DL, Grob MC. Reliability and validity of a brief patient‐report instrument from psychiatric outcome evaluation. Hospital and Community Psychiatry 1994;45(3):242‐7. [DOI] [PubMed] [Google Scholar]
Flood 2006
- Flood C, Byford S, Henderson C, Leese M, Thornicroft G, Sutherby K, Szmukler G. Joint crisis plans for people with psychosis: economic evaluation of a randomised controlled trial. BMJ 2006;333:729‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology, revised. Rockville, MD: National Institute of Mental Health, 1976:218–22. [DHEW Publ No ADM 76‐338] [Google Scholar]
Hanson 1997
- Hanson LC, Tulsky JA, Danis M. Can clinical interventions change care at the end of life?. Annals of Internal Medicine 1997;126(5):381‐8. [DOI] [PubMed] [Google Scholar]
Henderson 2004
- Henderson C, Flood C, Leese M, Thornicroft G, Sutherby K, Szmukler G. Effect of joint crisis plans on use of compulsory treatment in psychiatry: single blind randomised controlled trial. BMJ 2004;329:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Collaboration, 2006..
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Kim 2007
- Kim MM, Dorn RA, Scheyett AM, Elbogen EE, Swanson JW, Swartz MS, McDaniel LA. Understanding the personal and clinical utility of psychiatric advance directives: a qualitative perspective. Psychiatry: Interpersonal & Biological Processes 2007;70(1):19‐29. [DOI] [PubMed] [Google Scholar]
Kisely 2005
- Kisely S. A joint crisis plan negotiated with mental health staff significantly reduces compulsory admission and treatment in people with severe mental illness. Evidence Based Mental Health 2005;8:17. [DOI] [PubMed] [Google Scholar]
La Fond 2002
- Fond JQ, Srebnik D. The impact of mental health advance directives on patient perceptions of coercion in civil commitment and treatment decisions. International Journal of Law and Psychiatry 2002;25:537‐55. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. Clinical implications of Brief Psychiatric Rating Scale Scores 2005;187:366‐71. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Prendergast 2001
- Prendergast TJ. Advance care planning: pitfalls, progress, promise. Critical Care Medicine 2001;29(2 Suppl):34‐9. [DOI] [PubMed] [Google Scholar]
Ritchie 1998
- Ritchie J, Sklar R, Steiner W. Advance directives in psychiatry: resolving issues of autonomy and competence. International Journal of Law and Psychiatry 1998;21:245‐60. [DOI] [PubMed] [Google Scholar]
Ruggeri 1993
- Ruggeri M, Dall'Agnola R. The development and use of the Verona Expectations for Care Scale (VECS) and the Verona Service Satisfaction Scale (VSSS) for measuring expectations and satisfaction with community‐based psychiatric services in patients, relatives and professionals. Psychological Medicine 1993;23(2):411‐23. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Srebnik 1999
- Srebnik DS, Fond JQ. Advance directives for mental health treatment. Psychiatric Services 1999;50:919‐25. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
US GAO 1995
- US General Accounting Office. Patient self‐determination act: Providers offer information on advance directives, but effectiveness uncertain. Report to the ranking minority member, subcommittee on health, committee on ways and means, House of Representatives; 1995.


