Abstract
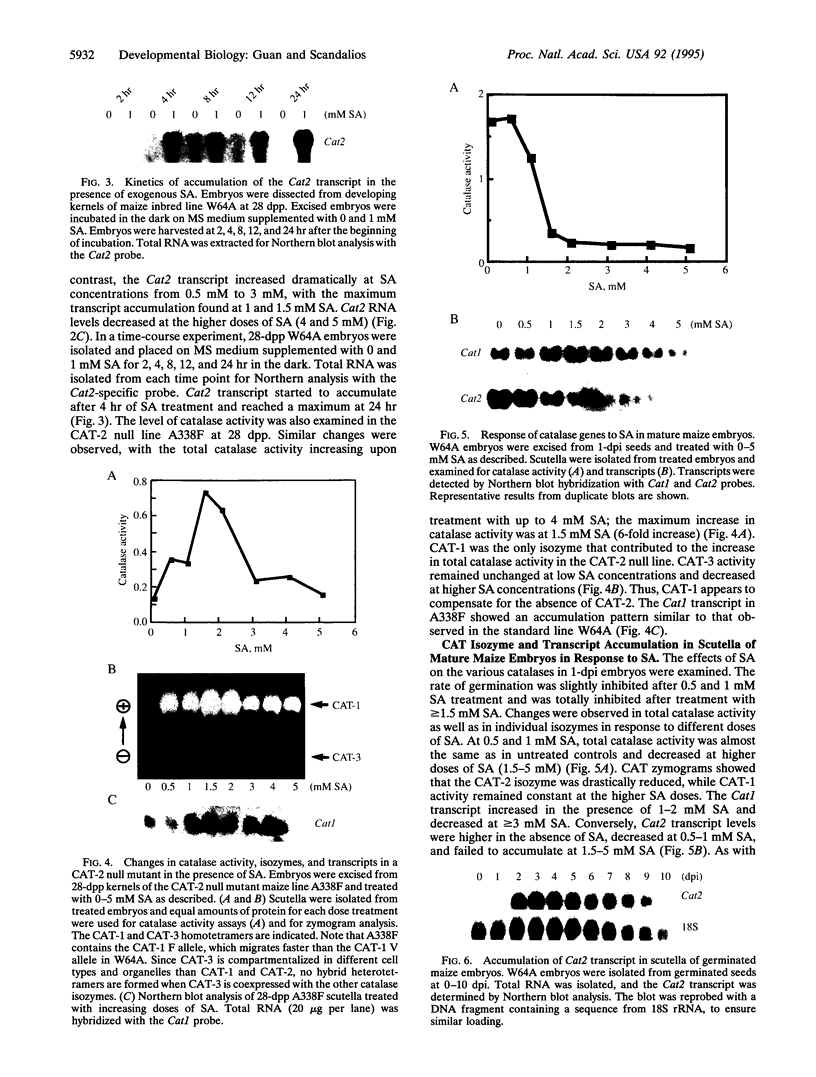
The response of the maize catalase genes (Cat1, Cat2, and Cat3) to salicylic acid (SA) was examined at two distinct developmental stages: embryogenesis and germination. A unique, germination-related differential response of each maize catalase gene to various doses of SA was observed. During late embryogenesis, total catalase activity in scutella increased dramatically with 1 mM SA treatment. The accumulation of Cat2 transcript and CAT-2 isozyme protein provided the major contribution to the observed increase in total catalase activity. This increase was paralleled by the enhanced growth of germinated embryos at that stage. In a CAT-2 null mutant line, a full compensation of total catalase activity by the CAT-1 isozyme was observed in the presence of SA. This suggests that catalase is important for maintenance of normal cellular processes under stress conditions. SA at 1 mM, which enhances growth of precociously germinated embryos, appeared to inhibit seed germination at 1 day after inhibition. Furthermore, Cat2 transcript accumulation was inhibited at this stage. SA is probably not a direct signal for the induction of the catalase genes. Other signals, possibly germination-related regulator(s), might be responsible for the induction of the catalase genes. The effect of SA on the activity of purified catalase protein was also examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethards L. A., Skadsen R. W., Scandalios J. G. Isolation and characterization of a cDNA clone for the Cat2 gene in maize and its homology with other catalases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6830–6834. doi: 10.1073/pnas.84.19.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Klessig D. F. Identification of a soluble salicylic acid-binding protein that may function in signal transduction in the plant disease-resistance response. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8179–8183. doi: 10.1073/pnas.88.18.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Silva H., Klessig D. F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993 Dec 17;262(5141):1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Cohn M. A., Jones K. L., Chiles L. A., Church D. F. Seed Dormancy in Red Rice : VII. Structure-Activity Studies of Germination Stimulants. Plant Physiol. 1989 Mar;89(3):879–882. doi: 10.1104/pp.89.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T., Friedrich L., Vernooij B., Negrotto D., Nye G., Uknes S., Ward E., Kessmann H., Ryals J. Requirement of salicylic Acid for the induction of systemic acquired resistance. Science. 1993 Aug 6;261(5122):754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matters G. L., Scandalios J. G. Effect of elevated temperature on catalase and superoxide dismutase during maize development. Differentiation. 1986;30(3):190–196. doi: 10.1111/j.1432-0436.1986.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Sabre M., Scandalios J. G. Expression of the maize Cat3 catalase gene is under the influence of a circadian rhythm. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6853–6857. doi: 10.1073/pnas.87.17.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Wadsworth G. J., Scandalios J. G. Characterization of catalase transcripts and their differential expression in maize. Biochim Biophys Acta. 1988 Nov 10;951(1):104–116. doi: 10.1016/0167-4781(88)90030-9. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G., Chang D. Y., McMillin D. E., Tsaftaris A., Moll R. H. Genetic regulation of the catalase developmental program in maize scutellum: Identification of a temporal regulatory gene. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5360–5364. doi: 10.1073/pnas.77.9.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios J. G. Genetic control of multiple molecular forms of catalase in maize. Ann N Y Acad Sci. 1968 Jun 14;151(1):274–293. doi: 10.1111/j.1749-6632.1968.tb11896.x. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. Subunit dissociation and recombination of catalase isozymes. Proc Natl Acad Sci U S A. 1965 May;53(5):1035–1040. doi: 10.1073/pnas.53.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Evidence for processing of maize catalase 2 and purification of its messenger RNA aided by translation of antibody-bound polysomes. Biochemistry. 1986 Apr 22;25(8):2027–2032. doi: 10.1021/bi00356a029. [DOI] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaftaris A. S., Scandalios J. G. Spatial pattern of catalase (Cat2) gene activation in scutella during postgerminative development in maize. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5549–5553. doi: 10.1073/pnas.83.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth G. J., Scandalios J. G. Differential expression of the maize catalase genes during kernel development: the role of steady-state mRNA levels. Dev Genet. 1989;10(4):304–310. doi: 10.1002/dvg.1020100405. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Scandalios J. G. Differential response of maize catalases and superoxide dismutases to the photoactivated fungal toxin cercosporin. Plant J. 1992 May;2(3):351–358. doi: 10.1111/j.1365-313x.1992.00351.x. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Scandalios J. G. Differential response of maize catalases to abscisic acid: Vp1 transcriptional activator is not required for abscisic acid-regulated Cat1 expression. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8842–8846. doi: 10.1073/pnas.89.18.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]









