Pericentrin (PLP) is a centrosomal protein required for organizing pericentriolar material. It requires interaction with calmodulin (CaM) for proper centrosome targeting both in vitro and in vivo. In addition, the PLP-CaM interaction is critical for mechanosensory neuron function but not for functional sperm.
Abstract
Pericentrin is a critical centrosomal protein required for organizing pericentriolar material (PCM) in mitosis. Mutations in pericentrin cause the human genetic disorder Majewski/microcephalic osteodysplastic primordial dwarfism type II, making a detailed understanding of its regulation extremely important. Germaine to pericentrin's function in organizing PCM is its ability to localize to the centrosome through the conserved C-terminal PACT domain. Here we use Drosophila pericentrin-like-protein (PLP) to understand how the PACT domain is regulated. We show that the interaction of PLP with calmodulin (CaM) at two highly conserved CaM-binding sites in the PACT domain controls the proper targeting of PLP to the centrosome. Disrupting the PLP-CaM interaction with single point mutations renders PLP inefficient in localizing to centrioles in cultured S2 cells and Drosophila neuroblasts. Although levels of PCM are unaffected, it is highly disorganized. We also demonstrate that basal body formation in the male testes and the production of functional sperm does not rely on the PLP-CaM interaction, whereas production of functional mechanosensory neurons does.
INTRODUCTION
Centrosomes are cellular organelles required for microtubule (MT) organization during both interphase and mitosis (Kellogg et al., 1994). They are highly complex structures made up of hundreds of proteins assembled into a core pair of centrioles and surrounding pericentriolar material (PCM). Our understanding of the role and regulation of almost all of these proteins is extremely limited. One class of centrosomal proteins that has drawn significant attention comprises those that may act to scaffold proteins within the PCM. These scaffolds, which include the proteins centrosomin (Cnn; Megraw et al., 1999; Fong et al., 2008; Choi et al., 2010) and possibly Cep152/Asterless (Varmark et al., 2007; Fu and Glover, 2012; Lawo et al., 2012; Mennella et al., 2012), have been suggested to recruit a series of other proteins to the centrosome. Pericentrin is believed to be the main PCM scaffolding protein that directly recruits the MT nucleation machinery—the γ-tubulin ring complex (Dictenberg et al., 1998; Takahashi et al., 2002; Zimmerman et al., 2004). In addition, Pericentrin has been linked to ciliary function and human genetic disorders such as Majewski/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) and Seckel syndrome (Jurczyk et al., 2004; Anitha et al., 2008; Jackson et al., 2008; Rauch et al., 2008; Delaval and Doxsey, 2010; Willems et al., 2010; Muhlhans et al., 2011). Therefore understanding all aspects of pericentrin regulation and function is critical to understanding these diseases.
Pericentrin's function in organizing PCM has been recognized for some time (Doxsey et al., 1994; Takahashi et al., 2002; Haren et al., 2009; Matsuo et al., 2010). Germane to this function is its ability to properly target and localize to centrosomes, potentially attaching directly to the centriole wall. This localization is mediated by a highly conserved domain called pericentrin-AKAP450-centrosome targeting (PACT) located at the C-terminus of pericentrin and its homologue, CG-NAP/AKAP450 (Gillingham and Munro, 2000). The PACT domain in isolation is sufficient for centriole targeting (Gillingham and Munro, 2000; Martinez-Campos et al., 2004). In fact, the PACT domain has been used as a tool to ectopically drive the localization of other proteins to the centriole (Kishi et al., 2009; Januschke et al., 2013). Although sufficient for centriole localization, it is not known whether the PACT domain is necessary for pericentrin localization and function. However, two pieces of evidence are highly suggestive of its necessity: 1) Overexpression of the PACT domain in cultured cells reduces the efficiency of PCM recruitment to centrosomes, suggesting that it acts as a dominant negative to endogenous pericentrin (Gillingham and Munro, 2000). 2) All but one of the cases of primordial dwarfism mapped to pericentrin are nonsense mutations that terminate translation upstream of the PACT domain (Rauch et al., 2008; Willems et al., 2010). Even the one isolated case results from a single amino acid deletion, lysine 3154, in the PACT domain (Kantaputra et al., 2011). Together these data point to a critical role for the PACT domain in the function of pericentrin, but a direct test of this hypothesis has not been performed.
Drosophila PLP, the orthologue to human pericentrin (Pcnt), was identified through its homology to the PACT domain (Kawaguchi and Zheng, 2004; Martinez-Campos et al., 2004). PLP is also similar to Pcnt in function, as loss of PLP affects mitotic PCM architecture and cilia formation (Martinez-Campos et al., 2004). Furthermore, the spatial arrangements of Pcnt and PLP in the centrosome are identical, with the PACT domain positioned near the centriole wall and the N-terminus extending away in a radial manner (Fu and Glover, 2012; Lawo et al., 2012; Mennella et al., 2012; Sonnen et al., 2012). Given all these similarities, PLP serves as a valuable model to study pericentrins.
Pioneering work in fungi demonstrated that calmodulin (CaM) functions at the spindle pole body as a critical partner of Spc110 (Saccharomyces cerevisiae; Geiser et al., 1993; Stirling et al., 1994, 1996; Sundberg et al., 1996) and PCP1 (Schizosaccharomyces pombe; Flory et al., 2002), the functional orthologues of pericentrin. The binding sites for CaM at the C-terminus of PCP1 are highly conserved and are located within the PACT domain (Flory et al., 2002). In fact, an interaction between PACT and CaM has also been shown in both humans and Drosophila (Flory et al., 2000; Gillingham and Munro, 2000; Kawaguchi and Zheng, 2004), and biochemical analysis indicates that mutants within the CaM-binding sites can completely abrogate CaM's interaction with the C-terminus of Pcnt (Flory et al., 2000; Gillingham and Munro, 2000). Furthermore, loss of the PACT-CaM interaction reduces the efficiency of PACT centrosome targeting (Gillingham and Munro, 2000). These results led to the long-standing hypothesis that this interaction is required for the proper localization and function of Pcnt and PLP. In this study, we directly test this hypothesis by investigating the importance of CaM in the function of PLP at centrosomes and basal bodies in Drosophila.
RESULTS
PLP requires CaM for efficient centrosome localization in vitro
Given the known interaction between the PACT domain and CAM (Gillingham and Munro, 2000), we hypothesized that CaM is essential for PLP's function at the centrosome. We began by investigating the relative localization of endogenous PLP and CaM in interphase and mitosis in Drosophila S2 cells. In mitosis, CaM accumulates on the entire centrosome, whereas PLP localization is more compact (Supplemental Figure S1A), consistent with CaM's known spindle pole localization (Zavortink et al., 1983) and PLP's known proximity to the centriole wall (Fu and Glover, 2012; Mennella et al., 2012). In interphase, PLP staining remained compact at centrioles, whereas CaM was undetectable (Supplemental Figure S1A), suggesting that PLP might not require CaM for localization in interphase. Of interest, green fluorescent protein (GFP)–CaM does localize to interphase centrioles (Supplemental Figure S1B), raising the possibility that the antibody used might not detect the centriole CaM pool due to its low levels or poor antibody access to the CaM epitope. Alternatively, GFP-CaM overexpression might force CaM onto the centriole in interphase in a nonphysiological manner.
To determine whether loss of CaM affected centrosomal PLP localization to interphase and mitotic centrioles, we reduced CaM protein levels using double-stranded RNA (dsRNA)–mediated interference in Drosophila S2 cells (Figure 1A and Supplemental Figure S1, C and D). Depletion of CaM revealed two very distinct phenotypes: a reduction in the average number of centrioles per cell (Figure 1B) and a reduction in PLP levels at both interphase and mitotic centrioles (Figure 1C and Supplemental Figure S1D). Identical results were obtained using Kc cells, another Drosophila cell line (Supplemental Figure S2, A–C). The reduction in centriole number indicates that CaM is required for centriole duplication, as previously reported (Matsumoto and Maller, 2002; Dobbelaere et al., 2008). These results also indicate that CaM is necessary for the efficient localization of PLP to interphase and mitotic centrioles, as 50% of the remaining centrioles in CaM-knockdown cells show a reduction or a complete absence of PLP, a phenotype not seen in control Sas6-knockdown cells (Figure 1C and Supplemental Figure S2C). Of interest, CaM appears to be required for PLP localization to interphase centrioles, even though CaM is not detectable by antibody staining. This suggests the presence of very low levels of centriolar CaM during interphase. However, we cannot rule out the possibility that the reduced level of PLP at interphase centrosomes is the result of a defect in PLP recruitment during the previous mitosis or the consequence of an indirect effect of CaM loss, given its importance for many cellular processes.
FIGURE 1:
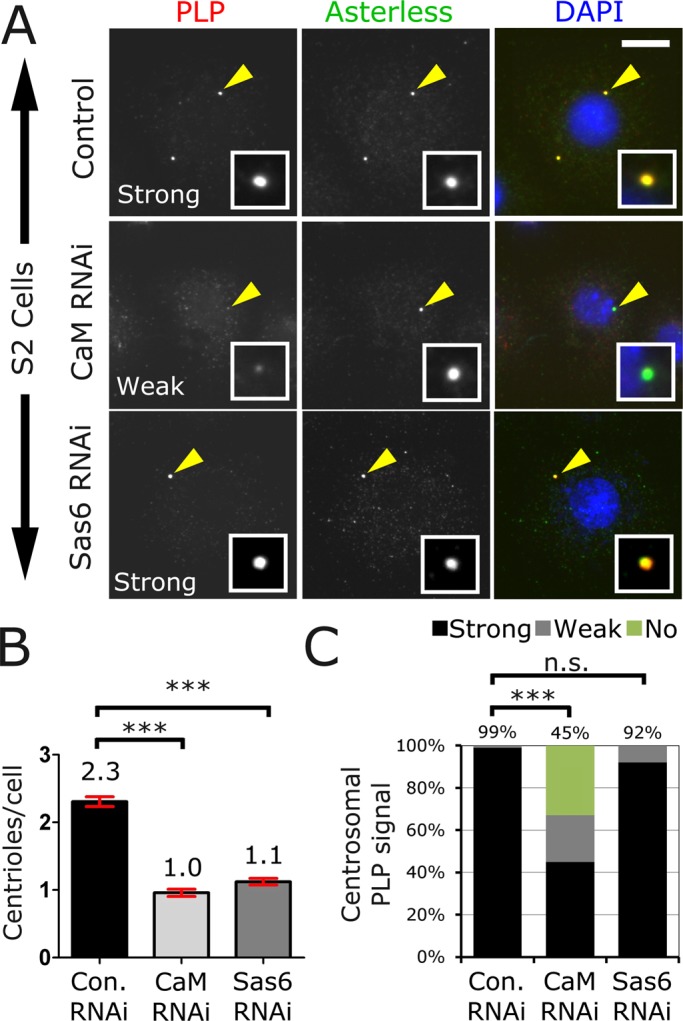
Calmodulin is required for centriole duplication and PLP targeting. (A) Drosophila S2 cells treated with dsRNA against control, CaM, or Sas6 were fixed and stained for PLP (red), Asterless (green), and DNA (blue). Bar, 5 μm. (B) The average number of centrioles per cell was determined for each condition. Mean is indicated on top of each bar, and SE is indicated with red brackets. Both Sas6 and CaM knockdown caused a significant reduction in the number of centrioles/cell compared with controls (analysis of variance [ANOVA] test, ***p < 0.001, three independent experiments, 200 cells counted per condition per experiment). (C) PLP localization strength was determined in a blind experiment and was classified as strong, weak, or no localization (three independent experiments were performed, and at least 200 cells were scored for each). The percentage of “strong” localization is shown above each column. CaM knockdown significantly reduces PLP localization to centrosomes (ANOVA followed by a paired Turkey test, ***p < 0.001, n.s., not significant).
PLP's interaction with CaM relies on the CBD2 within the PACT domain
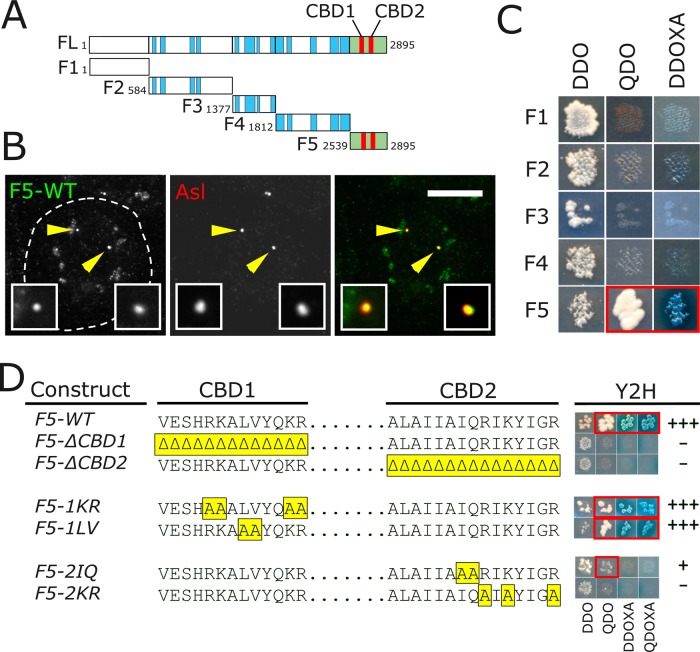
Given the lack of information regarding the 12 putative PLP isoforms, we selected PLPPF for our studies (Supplemental Figure S3A). Previously known as the “long” isoform, PLPPF contains a total of 13 exons (only missing the very small exons 2, 10, and 12). We show that PLPPF is a reasonable selection because it localizes to centrosomes (Supplemental Figure S3B) and fully rescues plp− null animals (see later discussion). We will refer to PLPPF as PLP for the remainder of the article. To identify a direct interaction between PLP and CaM, we truncated PLP into five fragments (PLPF1–PLPF5), taking care not to disrupt predicted coiled-coil domains (Figure 2A). These fragments were N-terminally GFP tagged and transfected into S2 cells (Supplemental Figure S4A). As expected, PLPF5 (containing the PACT domain) localized to centrioles (Figure 2B). Of interest, PLPF4 localized to centrosomes at low frequency in S2 cells (Supplemental Figure S4B) but did not show centrosomal localization in transgenic animals expressing GFP::PLPF4 (Supplemental Figure S4C). Taken together, these results confirm that the PACT domain is the major centrosome-targeting domain in PLP. Coimmunoprecipitation from transfected S2 cells shows that CaM interacts with PLPF5 but fails to interact with PLPF1–PLPF4 (Supplemental Figure S4D). Yeast two-hybrid (Y2H) analysis of CaM and each of the five PLP fragments indicates that PLPF5 and CaM directly interact (Figure 2C).
FIGURE 2:
PLP binds CaM through CBD2 within the PACT domain. (A) PLPPF was divided into five fragments at the indicated amino acid positions. F5 (green) includes the PACT domain, which contains CBD1 and CBD2 (red). Blue blocks indicate regions of predicted coiled-coil. (B) S2 cell transfected with F5-GFP (green) and costained for Asl (red). Insets, enlargements of the indicated centrioles (arrows). Bar, 5 μm. (C) Y2H showing direct interaction of CaM and F5 as indicated by growth on SD –Ade –His –Leu –Trp (QDO) and growth and blue color on SD –Leu –Trp +Aureobasidin A + X-α-Gal (DDOXA). (D) Amino acid sequences of CBD1 and CBD2. The yellow regions indicate the mutated residues. The Y2H column shows a picture and empirically judged strength of the interaction for each. Growth on QDO and growth and color on DDOXA and SD –Ade –His –Leu –Trp + Aureobasidin A + X-α-Gal (QDOXA) plates indicates interaction.
The PACT domain contains two highly conserved CaM-binding sites, deletion of which reduces PACT targeting efficiency (Gillingham and Munro, 2000). Using a Web-based prediction program (http://calcium.uhnres.utoronto.ca/), we refined the location of the CaM-binding sites within PLP. We defined the conserved CaM Binding Domain 1 (CBD1; VESHRKALVYQKR) and CBD2 (ALAIIAIQRIKYIGR) as the regions with residues with the highest “score” of 7–9 (Yap et al., 2000). These CBDs are conserved among species, with several residues absolutely conserved from flies to humans and yeast (Supplemental Figure S5). We first generated deletion mutations of CBD1 or CBD2; these deletions clearly disrupt the PLPF5-CaM interaction as shown by Y2H (Figure 2D). However, it is very likely that these deletions dramatically disrupt the entire structure of the PACT domain. We therefore generated point mutations at conserved lysine (K) and arginine (R) residues in CBD1 and CBD2, which had been shown to disrupt Spc110 (yeast pericentrin) and human Pcnt interaction with CaM (Flory et al., 2000; Gillingham and Munro, 2000). Mutating the Ks and Rs to alanines in CBD2 (PLPF5-2KR) but not in CBD1 (PLPF5-1KR) disrupted the PLPF5-CaM interaction (Figure 2D). As controls, we mutated adjacent residues in each of the CBDs. The PLPF5-1LV mutant did not disrupt the interaction with CaM, whereas the PLPF5-2IQ reduced the interaction but did not eliminate it (Figure 2D). To determine whether these mutants can interact with CaM in cells, we first attempted coimmunoprecipitation (Co-IP) experiments by co-overexpressing CaM and each of the mutants in S2 cells. We noticed great variability in the stability of the mutant constructs, precluding a direct comparison between the mutants and control immunoprecipitations (Supplemental Figure S6A). Therefore we developed a comitochondrial-targeting (Co-MT) assay in which we express both GFP::CaM and mito-RFP-X (where X is any of the PLPF5 mutants; see Materials and Methods) from a single plasmid. In this assay, the PLPF5 fragments are much more stable, presumably because they are anchored to the surface of the mitochondria (Supplemental Figure S6B). We then determined whether GFP-CaM could be drawn onto the mitochondria by interacting with each of the mutant alleles. Using the Co-MT assay, we show that PLPF5-2KR was unable to recruit GFP-CaM to the mitochondria, whereas PLPF5-2IQ was almost indistinguishable from PLPF5-WT (Supplemental Figure S6C), confirming that PLPF5-2KR is unable to interact with CaM. In summary, we successfully generated a mutant that disrupts the PLP-CaM interaction (PLPF5-2KR), allowing us to test the importance of this interaction.
The PLP-CaM interaction is required for targeting PLP to the centrosome
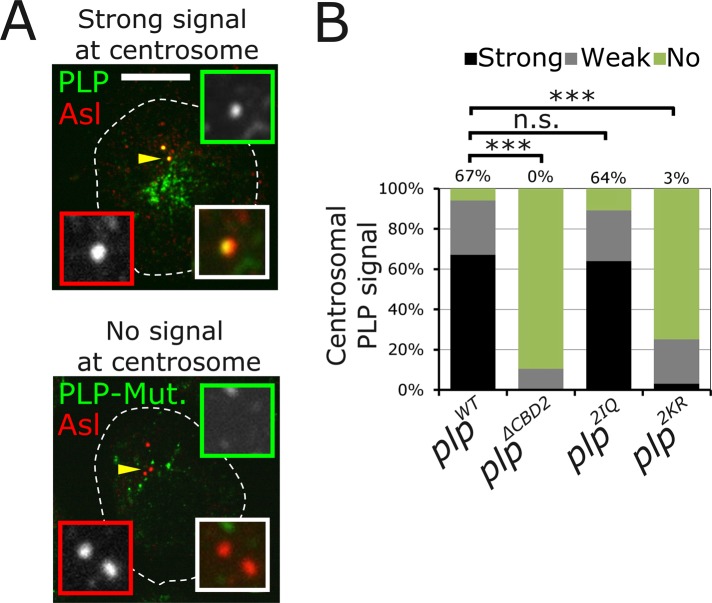
Our depletion of CaM by RNA interference (RNAi) suggests a direct role for CaM in localizing PLP to the centrosome, but possible pleiotropic effects that indirectly influence PLP localization could not be dismissed. To test whether the PLP-CaM interaction is critical, we engineered the PLPF5-2KR and PLPF5-2IQ mutations into full-length PLP to produce PLP2KR and PLP2IQ. To test the effect of these mutations on PLP, we transfected GFP fusions of each into S2 cells. We used an empirical measurement in a blind experiment to classify the strength of PLP localization to the centrosome as strong, weak, or no localization (Figure 3). There was a clear reduction in the centrosome-targeting efficiency of PLP2KR, whereas the control PLP2IQ was indistinguishable from PLPWT (Figure 3, A and B). These data support a model in which CaM interaction at CBD2 is required for PLP targeting to the centrosome.
FIGURE 3:
PLP centrosome targeting is disrupted by mutating CBD2. (A) Representative images of S2 cells transfected with GFP-PLPPF or each of the CBD2 mutants (green). Cells were stained for Asl (red) to identify centrioles. The localization strength of GFP on the centriole was determined empirically to be strong (top), no signal (bottom), or weak (anything between these two levels). Bar, 5 μm. (B) PLP localization strength at the centrosomes was determined for indicated genotypes. At least three independent experiments were performed, and >150 cells were scored. The percentage of “strong” localization is indicated above each column. PLP localization was significantly reduced in PLP∆CBD2 and PLP2KR (ANOVA followed by a paired Turkey test, ***p < 0.001) but was normal in PLP2IQ (n.s., not significant).
The PLP-CaM interaction is necessary for PLP localization and function at the centrosome in the fly
Although the PLP localization data in S2 cells suggest an important role for the PLP-CaM interaction, its physiological importance is not clear. We therefore turned to the animal model, Drosophila. Previous work using complete loss-of-function mutations documented three important roles for PLP in Drosophila: 1) a role in centrosome maturation (PCM recruitment), 2) a role in maintaining centriole integrity in meiotic cells and the proper formation of motile cilia during spermatogenesis, and 3) a role in building sensory organ cilia (Martinez-Campos et al., 2004). We hypothesized that PLP requires an interaction with CaM for one or more of these functions. Our approach was to perform a detailed comparison between animals with a complete loss-of-function mutation for PLP with animals expressing mutant PLP that lacks the ability to bind CaM (PLP2KR).
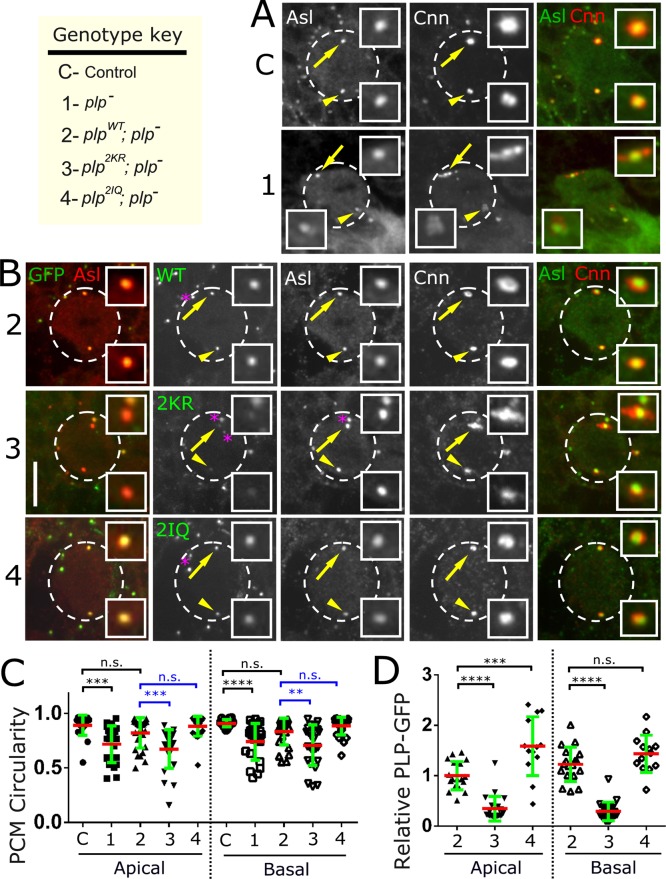
We began by characterizing animals expressing the plp2172 allele, a P-element insertion between exons 6 and 7 that does not produce any detectable protein, with an antibody raised against the N-terminus of PLP (Supplemental Figure S7A). We will refer to plp2172/Df(3L)BrdR15 as plp− throughout. To determine the effects of PLP loss of function on centrosome maturation, we analyzed the central brain neuroblasts (NBs) from control and plp− mutant flies for the ability of centrosomes to recruit the PCM component Cnn during mitosis (Figure 4A). Given the known differences between apical and basal PCM amounts and behavior in these cells (Rebollo et al., 2007; Rusan and Peifer, 2007), we measured the levels of Cnn at each centrosome independently. plp− NBs showed a significant reduction in Cnn at both the apical and basal centrosomes in metaphase (Figure 4A and Supplemental Figure S8, genotype C vs. genotype 1). We also noticed a general disorganization of the PCM throughout mitosis (Figure 4A), similar to what was described previously (Martinez-Campos et al., 2004). This disorganization seems to be more prominent in NBs, as we were unable to detect disorganization of PCM in mitotic (metaphase) spermatogonia (Supplemental Figure S7B). To quantify the disorganization in NB, we measured centrosome circularity (see Materials and Methods). In control NBs, centrosomes are fairly circular (0.9, where 1 is perfectly circular), whereas in plp− NBs, centrosomes are much more disorganized, showing significantly reduced circularity (0.7; Figure 4C, C vs. Figure 1). In the mutant there is frequently additional PCM outside of the measured region. As such, this circularity measurement is likely an underestimate of the disorganization because it measures only PCM that is contiguous with the centrosome. This disorganization was seen in both apical and basal centrosomes equally and is also seen in plp5 (Supplemental Figure S7C), a hypomorphic allele (Martinez-Campos et al., 2004) resulting from a nonsense mutation we mapped to residue Q1900 (Supplemental Figure S3A). Of interest, despite the disorganization of the centrosomes, previous reports indicate that the centrosomes in plp− neuroblasts can assemble functional spindles (Martinez-Campos et al., 2004; Lerit and Rusan, 2013). Together these data confirm that PLP is required for proper PCM levels and organization during mitosis.
FIGURE 4:
The PLP-CaM interaction is required for proper PCM organization in neuroblasts. (A) Metaphase NBs from control (genotype C) and plp− (genotype 1) flies were stained for Asl (green) to detect centrioles and Cnn (red) to detect PCM. Enlargements of the apical (arrow) and basal (arrowhead) centrosomes. (B) NBs from animals expressing the indicated transgenes (GFP, green) in the mutant plp− background. NBs were strained for Asl (red, left) to detect centriole and Cnn (red, right) to detect PCM. All numbers refer to the genotype key (top left corner). Images are maximum intensity projections of the entire NB volume. Bar, 10 μm. (C, D) Circularity and GFP measurements were performed on at least 15 apical and 15 basal centrosomes for the indicated genotypes. Two independent ANOVA tests (blue and green bars above the graph) followed by a paired Turkey test were performed for each of the apical and basal centrosomes. (C) Circularity measurements show a significant centrosome shape change in plp− (genotype 1, ***p < 0.001, ****p < 0.0001) when compared with control (genotype C), but this was rescued by plpWT (genotype 2, n.s., not significant). Circularity was disrupted in the plp2KR mutant (genotype 3, ***p < 0.001, **p < 0.01) but not the plp2IQ mutant (genotype 4, n.s., not significant). (D) Centrosomal GFP signal was normalized to apical plpWT levels (genotype 2). plp2KR levels are significantly reduced on the apical and basal centrosomes (genotype 3, ****p < 0.0001). plp2IQ levels were higher than those of plpWT on the apical centrosome (genotype 4, ***p < 0.001), which could be attributed to the slightly higher expression of the plp2IQ transgene. No significant difference was measured on the basal plp2IQ centrosome.
To test our hypothesis that PLP requires its interaction with CaM for localization and function in vivo, we generated transgenic animals expressing each of three GFP-tagged transgenes driven by the ubiquitin promoter: 1) wild-type PLP::GFP (referred to as plpWT), 2) PLP-2IQ::GFP (referred to as plp2IQ), and 3) PLP-2KR::GFP (referred to as plp2KR). We selected transgenic animals that produce similar amounts of protein, as analyzed by Western blotting (Supplemental Figure S9A) and by measuring the cytoplasmic levels of GFP fluorescence (Supplemental Figure S9B). We next introduced each of these transgenes into the plp− background to produce the following genotypes: 1) plpWT; plp− (rescue fly), 2) plp2IQ; plp− (control mutant), and 3) plp2KR; plp− (CaM interaction mutant). Inexplicably, on a Western blot, all three transgenes in the mutant background show a detectable band at the endogenous PLP size (Supplemental Figure S9A, blue asterisks). However, detailed PCR genotyping confirms that the endogenous PLP locus is disrupted in all three genotypes (Supplemental Figure S9C). It may be that this lower band represents a truncation of the GFP-tagged protein or that plp2172 produces a protein product, normally undetectable, that is somehow stabilized in the presence of a transgene. Most important for this study, whatever its origin, this product is nonfunctional, as it is unable to rescue the plp− mutant phenotype (see later discussion).
To determine the effect of disrupting the PLP-CaM interaction on PLP localization and centrosome function in NBs, we analyzed brains from plpWT; plp−, plp2IQ; plp−, and plp2KR; plp− flies (Figure 4B). Analysis of apical and basal mitotic centrosomes revealed a significant (∼70%) decrease in plp2KR levels as compared with plpWT (Figure 4D, genotype 2 vs. genotype 3). Despite this decrease in plp2KR at the centrosome, analysis of PCM shows no reduction in Cnn levels on either the apical or basal centrosomes (Supplemental Figure S8B, genotype 5 vs. genotype 6). However, centrosome circularity (shape) was significantly altered in plp2KR; plp− NBs as compared with plpWT; plp− (Figure 4C, genotype 2 vs. genotype 3) and plp2IQ; plp− (Figure 4C, genotype 2 vs. genotype 4). Of note, the expression of the plp2KR transgene in a wild-type background shows a slight dominant effect that increases PCM levels (Supplemental Figure S8, genotype 2 vs. genotype 3) but has no dominant effect on centrosome circularity (Supplemental Figure S9D, genotype 2 vs. genotype 3). Taken together, our data in the animal strongly indicate that the PLP-CaM interaction is required to target/anchor PLP to the centrosome and organize PCM but is not required to recruit the proper amount of PCM.
The PLP-CaM interaction is critical for mechanosensation but dispensable for motile sperm formation
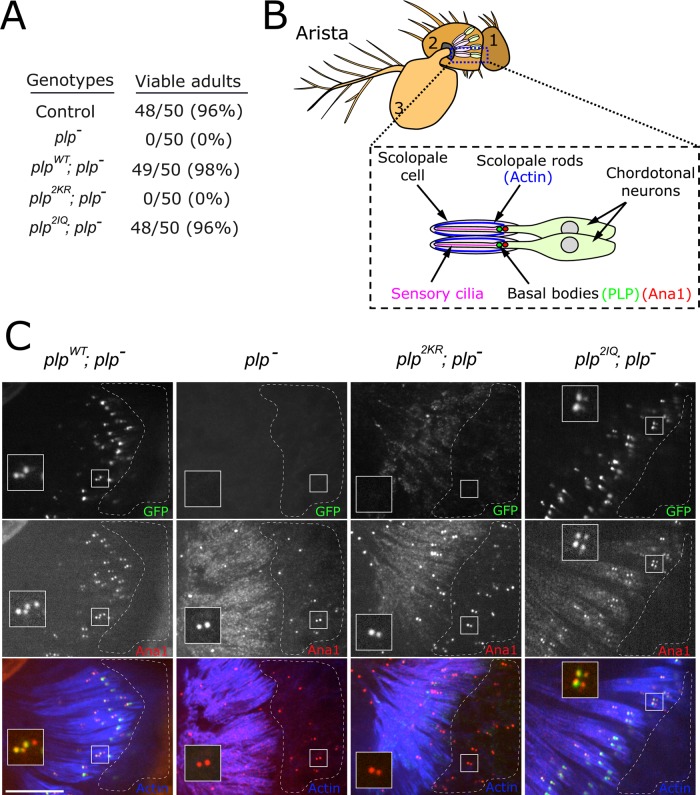
A second phenotype previously described in plp− animals is the “uncoordinated” phenotype, resulting from the failed formation of mechanosensory cilia (Martinez-Campos et al., 2004). These plp− flies often fail to eclose properly, but if they manage to do so, they lie incapacitated. To determine whether the PLP-CaM interaction is required for mechanosensation, we assayed adult flies from the following genotypes for coordination: plpWT; plp−, plp2KR; plp−, and plp2IQ; plp−. Of importance, both plpWT and plp2IQ completely rescue fly viability, allowing us to generate stable stocks of plpWT; plp− and plp2IQ; plp− (Figure 5A). In contrast, plp2KR; plp− flies display severe uncoordination, indistinguishable from the plp− mutant flies. To investigate the cause of this phenotype further, we imaged the basal bodies (marked by the centriole protein Ana1::tdTomato; Blachon et al., 2009) of the chordotonal neurons in the Drosophila Johnston's organ (Figure 5, B and C). Each chordotonal neuron contained two centrioles as marked by Ana1::tdtomato, but PLPWT protein was enriched on the centriole adjacent to the scolopale rod. This localization suggests that PLP is localized to the basal body and not the daughter centriole, consistent with a role for PLP in basal body function. As expected, PLP does not appear to be required for centriole formation/duplication (Lerit and Rusan, 2013), as centrioles were found in the plp2KR chordotonal neurons. However, unlike the controls (plpWT and plp2IQ), plp2KR centrioles were located within the cell bodies and not near the scolopale rods (dashed region, Figure 5C), similar to the complete loss-of-function plp− mutant. Of importance, centrioles in plp2KR neurons did not recruit PLP2KR protein. These data indicate that the PLP-CaM interaction is critical for PLP function in neuronal basal bodies. Further work will be required to determine whether PLP serves a role in the centriole-to-basal body transition or its role is to assist in proper basal body membrane docking and positioning. Either way, these data offer an explanation for the absence of cilia in plp− mutants (Martinez-Campos et al., 2004).
FIGURE 5:
PLP requires interaction with CaM to localize to neuronal basal bodies. (A) Flies of the indicated genotype were determined to be viable if they fully eclosed and were motile in the vial. One hundred percent of the plp− and plp2KR individuals were incapacitated and scored “not viable.” (B) Diagram indicating the location of the chordotonal neurons in the second antennal segment. (C) Actin (blue), PLP::GFP expressed from the indicated transgenes (green), and Ana1::tdTomato in chordotonal neurons. Inset, basal bodies of chordotonal neurons. Unlike plpWT and plp2IQ, the plp2KR allele was incapable of localizing to basal bodies, although centrioles still formed and were randomly positioned in the area of the neural cell bodies (dashed region). Bar, 10 μm.
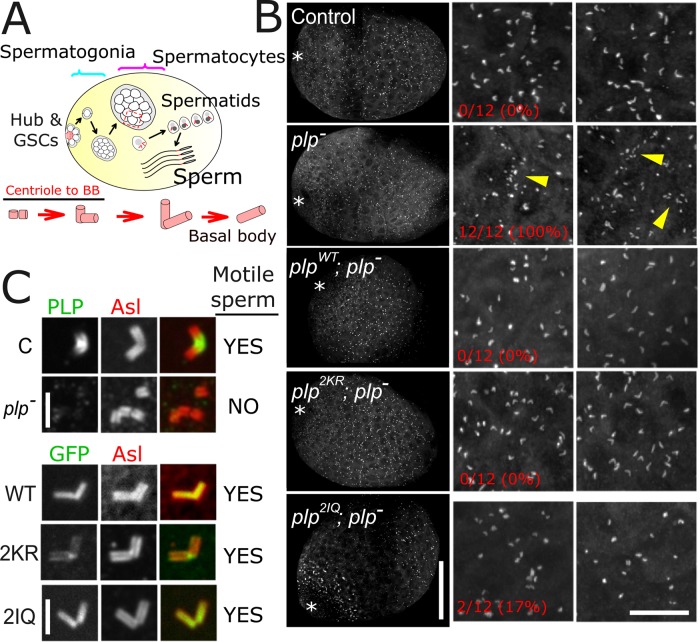
The third and final characterized role for PLP is in the proper formation of motile sperm (Martinez-Campos et al., 2004). Commonly, mutations in centrosome proteins that have an effect on mechanosensation also display defects in male fertility due to defective basal body formation (Martinez-Campos et al., 2004; Basto et al., 2006; Blachon et al., 2008). Given that plp2KR was unable to localize to neuronal basal bodies or rescue uncoordination, we expected plp2KR; plp− animals to produce immotile sperm, similar to the plp− mutants. We dissected male testes and analyzed the centriole-to-basal body transition (Figure 6A and Supplemental Figure S10). We found that the plp− mutants display the previously reported giant centriole fragmentation phenotype (n = 12/12, 100%), although normal basal bodies can also be found in abundance (Figure 6, B and C). Unexpectedly, plp2KR completely rescues the centriole fragmentation phenotype, with centrioles reaching normal lengths in mitosis and meiosis (Figure 6, B and C, n = 0/12, 0% fragmented centrioles). Furthermore, sperm dissected from plp2KR; plp− seminal vesicles were motile, similar to plpWT; plp− controls. Unfortunately, fertility could not be tested because these uncoordinated flies are unable to mate. Unlike sensory neuron basal bodies, we found that the plp2KR was able to localize to sperm basal bodies, albeit at low levels (Figure 6C). Together these data suggest that giant centriole formation and the production of functional sperm can proceed with very little PLP localized to the centriole and do not rely on the PLP-CaM interaction.
FIGURE 6:
PLP does not require interaction with CaM for its role in spermatogenesis. (A) Cartoon representation of the centriole-to–basal body (BB) transition during spermatogenesis. (B) Testes from third-instar larvae of the indicated genotypes (12 testes from six larvae each). Testes were visually scored to contain abnormal centriole fragments, as clearly seen in the plp− testes. The number of testes in which abnormal centriole fragments were observed over the total number scored is indicated on the left for each genotype. All the transgenes, including plp2KR, fully rescued the plp− centriole fragmentation. Asterisks indicate the location of the stem cell niche, termed the hub. At this stage, the gross morphology of testes of all genotypes appeared normal. The two right columns are random enlargements from the low-magnification image, intended to highlight the fragmentation phenotype. Bars, 50 μm (left), 15 μm (right enlargement). (C) Enlargements of a single centriole pair from spermatocytes of each of the indicated genotypes. Control and plp− testes were stained for endogenous PLP (green), and the transgenes were imaged using the GFP signal. Sperm motility was determined by eye through a stereomicroscope after disruption of seminal vesicles. Bar, 2 μm.
DISCUSSION
Pericentrin has been recognized for many years for its role in scaffolding centrosomal proteins (Doxsey, 1994). A key aspect of this role is likely its physical orientation within the centrosome, where the PACT domain is anchored near the centriole wall and the N-terminus extends outward (Fu and Glover, 2012; Lawo et al., 2012; Mennella et al., 2012). Understanding how the PACT domain is tethered and regulated is important for understanding the function of pericentrin. One possible method of regulating the PACT domain is through its highly conserved CaM-binding domains (CBD1 and CBD2). Our work investigates the role of CaM binding in the context of full-length pericentrin. More important, we demonstrate the importance of this interaction in an animal model system by genetically removing endogenous PLP and providing PLP carrying specific mutants from transgenes expressing normal amounts of protein.
We began by investigating the effect of the loss of CaM on PLP centrosome localization. Although complicated by possible indirect effects, CaM knockdown reduces the level of PLP at centrioles in cultured cells. We attempted similar experiments in the animal but were unsuccessful in analyzing tissue from zygotic homozygous-null CaM mutation (CaMn339) or RNAi knockdown (see Materials and Methods) because they die as early larvae. We also attempted to generate clones of CaM-null cells using the hs-FLP system (see Materials and Methods) but were unable to recover any cam− clones, suggesting that cells lacking CaM do not survive. We concluded from these harsh experiments that our hypothesis must be addressed using a more precise method of perturbing the PLP-CaM interaction.
The CBDs in the PACT domain show high evolutionary conservation, suggesting not only similar CaM-binding properties among species, but also similar regulation and function. Deletion of either CBD1 or CBD2 disrupts the PACT-CaM interaction, suggesting a cooperative binding model. This is consistent with the analysis of the Pcnt PACT domain (Gillingham and Munro, 2000). However, the point mutations generated here revealed a stronger dependence on CBD2, suggesting that CBD1 is insufficient for CaM binding but might serve to modulate the CaM-CBD2 interaction. For the purposes of this study, we sought to generate a PLP allele that completely lacks the ability to bind CaM, and we were successful with the plp2KR allele. We note that although this mutant disrupts the interaction of PLP with CaM, it remains a formal possibility, as is the case with any mutation in any protein, that the amino acid changes we generated in the PACT domain might disrupt the interaction of PLP with a hitherto-unidentified binding partner. Our overexpression analysis in cultured cells and our replacement of endogenous PLP with mutant transgenes in animals reveal that the conserved CBDs in the PACT domain serve a critical role in PLP targeting and centrosome function in vivo. Although this mutant reduced the amount of PLP localized to the centrosome by 70%, some PLP was still able to localize to the centrosome. This indicates that there must be an additional, although less robust, mechanism for recruiting PLP to the centrosome that does not require the PLP-CaM interaction.
Our results uncovered roles for PLP that depend on its CaM interaction and others that do not (Table 1). Therefore the plp2KR mutant is a true separation-of-function allele that begins to tease apart the complex function of PLP. As it relates to centrosome function, we show that the PLP-CaM interaction is important for organizing the PCM but is not essential for recruiting the proper amount of PCM. There are two possible explanations for this. First, it is possible that only a small amount of PLP (as seen with plp2KR; plp−) at the centriole is required to recruit, or load, PCM into the maturing centrosome, but a larger amount of PLP at the centriole (as seen with plpWT; plp− and plp2IQ; plp−) is required to stabilize the overall centrosome structure. In this case, the main role of the PLP-CaM interaction would be to efficiently recruit PLP to the centrosome, and the PLP-CaM interaction would not directly be involved in organizing the PCM. The second possible explanation is that CaM's interaction within the PACT domain is directly required for organizing Cnn. In this case, the main role of the PLP-CaM interaction would not be in recruiting PLP or Cnn, but instead it would be important to directly influence the organization of Cnn. Although our data do not directly support this second mechanism, it is worth noting that CDK5RAP2 (the Cnn human orthologue) interacts with CaM (Wang et al., 2010) within a C-terminal region called Cnn motif 2 (CM2), which is conserved in Drosophila (Zhang and Megraw, 2007). One speculative model is that CaM is required to directly organize Cnn within the PCM by forming a tricomplex of Cnn-CaM-PLP, but this tricomplex would not be required for the initial recruitment of Cnn within the centriole vicinity. This model is worth exploring further through a dual structure–function study of PLP and Cnn. In addition, although this study focused on the role of the PLP-CaM interaction during mitosis, PLP has a recently reported negative regulatory role in PCM recruiting during interphase in Drosophila neuroblasts (Lerit and Rusan, 2013). If and how the PLP-CaM interaction plays a role during interphase and in the changes in the centrosome as it enters mitosis from interphase will be an interesting subject for future exploration.
TABLE 1:
Summary of the findings of this study.
| Genotype | CaM Interaction? | Viable adults? | Centrosome targetting? | Circular centrosomes? | Normal PCM levels? | Motile sperm? | Coordinated files? |
| Control | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| plp– | – | No | – | No | No | No | No |
| plpWT; plp– | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| plp2KR; plp– | No | No | ∼30% | No | Yes | Yes | No |
These results show that PLP's function in organizing PCM and producing functional mechanosensory cilia rely on its interaction with CaM, whereas its function to recruit normal levels of PCM and generate motile cilia does not.
A role for pericentrin has also been documented in ciliogenesis in human cultured cells and Drosophila mechanosensory neurons and sperm (Jurczyk et al., 2004; Martinez-Campos et al., 2004). In addition, CaM is a well-known axonemal component (Gitelman and Witman, 1980; Stommel et al., 1982) and is important for ciliary function (AbouAlaiwi et al., 2009; DiPetrillo and Smith, 2010; Plotnikova et al., 2012). For these reasons, we investigated the importance of the PLP-CaM interaction for cilia function. Previous work showed that loss of PLP disrupts mechanosensation due to the loss of sensory organ mechanocilia, resulting in uncoordinated movements (Martinez-Campos et al., 2004). The plp2KR; plp− flies also display an uncoordinated behavior indistinguishable from that of the null mutants, indicating that the PLP-CaM interaction is required for mechanosensation. We show that a likely cause of this defect is the inability of PLP2KR to localize to neuronal centrioles and/or a loss of the localization of these centrioles to the base of the scolopale rods. It will be interesting to further investigate these centrioles to determine whether they have developed into neuronal basal bodies or whether they are fully formed basal bodies that are unable to migrate and anchor to the cortex. The results of such experiments will help assign a more precise role for PLP in nucleating neuronal axonemes.
Finally, our analysis of the centriole-to–basal body transition during male spermatogenesis revealed the most surprising result of this study. We fully expected that the PLP-CaM interaction would be required for PLP function in building these basal bodies but were surprised to find that the plp2KR allele fully rescues basal body formation, basal body integrity, and sperm motility. This suggests that the role of PLP in basal body formation might be cell type specific. This is not surprising, as basal body structure in each system is unique. The long basal bodies of the spermatid might only require low levels of PLP, whereas the smaller basal bodies of the sensory neurons might require high PLP levels. Another difference is that neuronal axoneme formation relies on intraflagellar transport (IFT), whereas formation of the sperm tail axoneme does not (Han et al., 2003; Sarpal et al., 2003). Consistent with a role in building IFT-dependent cilia, silencing pericentrin in RPE1 cells results in a reduction in the targeting of several IFT proteins to the basal body and a loss of primary cilia (Jurczyk et al., 2004). Therefore the PLP-CaM interaction might play a critical role in cilia/flagella built using IFT, and thus only the cilia in sensory neurons are affected.
In summary, we assigned critical roles for the PLP-CaM interaction in vivo, revealing a complicated, cell type–specific mechanism at work. Future work to identify other players, including the proteins that form the “docking site” for PACT, will be critical to begin to understand the interplay between pericentrin, CaM, Cnn, and these docking proteins. Posttranslational modifications, specific cell cycle control, protein isoforms, and cell type specificity are all critical elements to consider as the field moves toward understanding the details of centrosome proteins, including pericentrin.
MATERIALS AND METHODS
Fly stocks
All control (wild-type) flies used in this study are yw. The following strains were used: plp2172 (Spradling et al., 1999), plp5 (Martinez-Campos et al., 2004), Df(3L)BrdR15 (stock 5354; Bloomington Drosophila Stock Center, Bloomington, IN), actin-Gal4 (stock 3954; Bloomington Drosophila Stock Center), CaM-TRiP line (TRiP.HMS01318; stock 34609; Bloomington, IN), FRT42D,camn339 (Heiman et al., 1996), and Ana1-tdTomato (Blachon et al., 2008). To generate the PLP wild-type (plpWT) and mutant transgenes (plp2KR, plp2IQ), we first PCR amplified and directionally cloned full-length PLPPF into pENTR/D vector for use in the Gateway cloning system. Site-directed mutagenesis was then conducted to generate plp2KR and plp2IQ. Each allele was recombined using Gateway reactions into the P-element destination vector pUWG (ubiquitin promoter, C-terminal GFP, DGRC). All transgenic flies were generated by BestGene (Chino Hills, CA) using standard P-element–mediated transformation.
Drosophila cell culture and double-stranded RNAi
Drosophila cell culture, in vitro dsRNA synthesis, and dsRNA treatments were performed as described previously (Rogers and Rogers, 2008). In brief, S2 and Kc cells were cultured in Sf900II medium (Life Technologies, Grand Island, NY) and split every 3–4 d. RNAi was performed in six-well tissue culture plates. Cells (50–90% confluency) were treated with 10 μg of dsRNA in 1 ml of medium and replenished with fresh medium/dsRNA every other day for 4–7 d. The following gene-specific primers were used to amplify DNA templates for RNA synthesis: control (5′-CGCTTTTCTGGATTCATCGAC-3′ and 5′-TGAGTAACCTGAGGCTATGG-3′), PLP (5′-TCAGTTTTTCCAGTGTTTTCTCC-3′ and 5′-TACAAACCAACGAAGAATTGGG-3′), CaM (5′-CGATCAGCTGACAGAGGA-3′ and 5′-AGTATTTCCCCCCACAATCC-3′ ), and Sas6 (5′-TTGAACACCGTACTCTTCTAC-3′ and 5′-CTTGAGGTCCTCGATTTTGT-3′).
Immunofluorescence
S2 and Kc cells were plated on #1.5 concanavalin A–coated coverslips and allowed to spread for 30–60 min. Cells were then briefly washed with phosphate-buffered saline (PBS) and fixed for 15 min in −20ºC methanol. Cells were stained at room temperature in primary antibodies for 1 h and secondary antibodies for 30 min and mounted in Aqua-Poly/Mount (Polysciences, Warrington, PA). Whole brains dissected from third-instar larvae were fixed in 9% formaldehyde in PBSTx (PBS, 0.3% Triton X-100) for 15 min, washed 3× 15 min in PBSTx, and blocked for 1 h in PBT (PBS, 0.1% Tween-20, 1% bovine serum albumin [BSA]). For chordotonal neuron analysis, entire heads of third-day pupae were fixed as for brains for 20 min. Whole antennae were then removed from the heads. For testes imaging, larval or adult testes were dissected and fixed as for brains. Brains or testes were incubated in primary antibody in PBT plus 4% normal goat serum (NGS) overnight at 4ºC. After several washes, samples were further blocked in modified PBT (PBS, 0.1% Tween-20, 2% BSA, and 4% NGS) and incubated for 2 h at room temperature with secondary antibody. Samples were finally washed 3× 15 min in PBST (PBS, 0.1% Tween-20) and mounted in Aqua-Poly/Mount. Primary antibodies used were rabbit anti-PLP (1:5000; Rogers et al., 2008), guinea pig anti-Asterless (1:30,000; gift from G. Rogers, University of Arizona Cancer Center, University of Arizona, Tucson, AZ), mouse anti-CaM (1:500, 05-173; Millipore), and rabbit anti-Cnn (1:2000, gift from T. Megraw (Florida State University, Tallahassee, FL; Heuer et al., 1995). Secondary antibodies were Alexa Fluor 488, 568, or 647 (1:500; Life Technologies). Phalloidin was used at 1:500 and added along with secondary antibodies for 3 h at room temperature with agitation.
Constructs and transfection
The synthesis of cDNA encoding full-length PLPPF was commissioned from GenScript. This plasmid was used to template the PCR amplification of all PLP fragments (1–5) using the following primers: fragment 1 (5′-CACCATGAATCTGTACACTATATACGATTGG-3′ and 5′-AGGCGGATCCTGCTCCTCTTCC-3′), fragment 2 (5′-CACCATGTCCCTCTCCTTGGATGAGTC-3′ and 5′-TGGAGGTAGGGAGGAATGTG-3′), fragment 3 (5′-CACCATGGATCTTCAAGAGCATGCGGG-3′ and 5′-CAGCCGCTCGATTTCGCGC-3′), fragment 4 (5′-CACCATGACGCTGCAGGGTCGTATGGAGG-3′ and 5′-TTCATTGAAGTGTTCCAACTCTG-3′), fragment 5 (5′-CACCATGCGTTTAACCCTGCAAGCCAG-3′ and 5′-ATGATGCCGCGCATGCGCTC-3′). These fragments were directionally cloned into the pENTR/D vector for use in the Gateway cloning system (Life Technologies). Gateway recombination reactions were conducted using the manufacturer's protocols to tag each fragment with GFP or FLAG. For work in S2 cells, the pAWG (actin promoter, C-terminal GFP tag) destination vector was used, whereas the pAFW (actin promoter, N-terminal FLAG tag) destination vector was used to tag these same fragments with a 3xFLAG tag for the Co-IP experiments. Details on the pAWG and pAFW vectors can be found at the Drosophila Genomics Resource Center (DGRC; Bloomington, IN; https://dgrc.cgb.indiana.edu/Home) and the Drosophila Gateway website (http://emb.carnegiescience.edu/labs/murphy/Gateway%20vectors.html). To generate the GFP-40aaLinker-CaM construct, a CaM cDNA with a N-terminal 40 amino acid linker was PCR amplified from an unpublished vector provided by T. Megraw and cloned into the pENTR/D vector. The 40aaLinker-CaM was then tagged using the Gateway system into pAGW (actin promoter, N-terminal GFP tag; DGRC) to generate GFP-40aaLinker-CaM. All deletions and point mutations were generated using the QuikChange II kit (Agilent Technologies) following the manufacturer's protocol. S2 or Kc Cells were transfected in a six-well plate containing a confluent monolayer of cells using Effectene (Qiagen, Venlo, Netherlands) by following the manufacturer's protocol. Cells were processed for immunofluorescence or immunoprecipitation 48 h posttransfection.
For mitochondrial targeting experiments, we modified the Gateway destination vector pAGW (actin promoter, N-terminal GFP tag; DGRC). We digested this vector with StuI and AgeI to remove the sequence for GFP and then ligated into its place a PCR product including the N-terminal 36 amino acids of the Drosophila Tom20 gene (gift of Hong Xu, National Heart, Lung, and Blood Institute, Bethesda, MD), followed immediately by the sequence encoding TagRFP. This new destination vector was named pAT20TRW. We then amplified the promoter, GFP, and calmodulin from the pAGW-40aaLinker-CaM construct described here and inserted it into the MluI site in the backbone of pAT20TRW, making pAT20TRW-AGFP-CaM. Fragments of PLP were then introduced into pAT20TRW-AGFP-CaM by standard Gateway cloning methods. These vectors allowed for simultaneous expression, from the same plasmid, of mitochondrial-targeted TagRFP-fusions of PLP fragments and GFP-calmodulin.
Immunoprecipitation and immunoblotting
S2 cells were cotransfected with GFP-CaM and each of the five FLAG-PLP fragments. Transfected cells were lysed in CLB (50 mM Tris, pH 7.2, 125 mM NaCl, 2 mM dithiothreitol, 0.1% Triton X-100, and 0.1 mM phenylmethanesulfonyl fluoride), precleared by centrifugation, and diluted to 5 mg/ml. Rabbit anti-GFP antibodies (clone ab290; Abcam, Cambridge, UK) were added to the lysate and rocked at 4ºC for 60 min. Then 30 μl of equilibrated protein A Sepharose beads (Sigma-Aldrich, St. Louis, MO) were added to the lysates and rocked for an additional 60 min at 4ºC. Beads were then washed three times in CLB and boiled for 5 min in SDS–PAGE sample buffer. The following primary antibodies were used for the Western blots in the Co-IP experiments: mouse anti-GFP (1:10,000, clone JL-8; Clontech Laboratories, Mountain View, CA) and mouse anti-FLAG (1:1000, clone M2; Sigma-Aldrich).
S2 cell extracts from dsRNA-treated cells (Supplemental Figure S1C) were produced by resuspending cell pellets in PBS plus 0.1%Triton X-100. SDS–PAGE sample buffer was added to the extracts and boiled for 5 min. Western blots were performed to determine knockdown efficiency. Tissue extracts from animals (Figure 4A) were generated by dissecting 20 brains from each genotype, adding them to 50 μl of 1× SDS–PAGE sample buffer, thoroughly grinding them using a microtubule pestle, and boiling the samples for 5 min. The following antibodies were used: mouse anti-calmodulin (1:500, MA3-917; Thermo Scientific), mouse anti–α-tubulin (1:5000, clone DM1a; Sigma-Aldrich), guinea pig anti-Sas6 (1:5000; gift from G. Rogers), and rabbit anti-PLP (1:5000; Rogers et al., 2008).
Microscopy
All images were collected on a Nikon Ti Microscope equipped with a 100× (1.49 numerical aperture, polarization and total internal reflection fluorescence) objective, a CSU-22 spinning disk confocal head (Yokogawa, Tokyo, Japan), and an interline transfer-cooled charge-coupled device camera (CoolSNAP HQ2; Photometrics, Tuscon, AZ). Excitation laser was supplied by a laser merge module equipped with 491-, 561-, and 642-nm solid-state lasers (VisiTech International, Sunderland, UK). Emission filters (Semrock, Rochester, NY) were controlled by a MAC6000 (Ludl Electronic Products, Hawthorne, NY). The system is controlled with MetaMorph software (Molecular Devices, Sunnyvale, CA). For fixed cells, Z-stacks covering the entire volume of each cell were collected by acquiring optical slices spaced by 200 nm; these data are presented as maximum intensity projections.
Fluorescence measurements and statistical analysis
To determine the relative amount of wild-type and mutant PLP localization to centrioles in S2 cells (Figures 1 and 3 and Supplemental Figure S2), we used an empirical judgment of fluorescence intensity in a blind experiment to all genotypes. Cells were visualized on the microscope using epifluorescence to first identify the centriole marker by Asterless staining in the red channel. The filter cube was then switched to the green channel, where a judgment of fluorescence intensity was determined. This intensity was judged as strong, weak, or no signal. Figure 3 shows an example of a strong and no signal. A weak signal was assigned to anything between these two extremes. At least 200 cells per condition per trial were assayed in these experiments. Each independent trial was processed the same day and included both the wild-type (control dsRNA or full-length PLP) and non–wild-type (dsRNA treated or mutant plp alleles) cells. Three independent blind trials on three different days were conducted for each experiment shown in Figures 1 and 3 and Supplemental Figure S2; the average of all three trials is presented.
For fluorescence measurements in neuroblasts, cells of all measured genotypes were dissected and processed the same day. Measurements were always performed relative to the control for that specific day—this constituted a single experiment and was repeated in triplicate. Levels of Cnn and GFP were measured by adding the total intensity through the volume of the centrosome, which was then outlined with a region of interest (ROI) large enough to encompass the entire centrosome, and measurements of the total integrated intensity were collected. Background-integrated intensity was collected from an identical ROI in an adjacent cytoplasmic region and subtracted from the centrosome measurement to obtain a final value. For analysis of centrosome circularity (Figure 4), centrosome fluorescence was outlined by hand and analyzed using the shape descriptors in ImageJ (National Institutes of Health, Bethesda, MD). For measurement of cytoplasmic GFP levels, a region of cytoplasm of neuroblasts away from the centrosomes, nuclei, and all other notable cellular features was selected. The total fluorescence intensity of a 625-pixel2 area of this region in a single confocal plane was measured, providing the total fluorescence of a fixed volume of cytoplasm. These data were normalized to the average total fluorescence intensity of PLPWT (in WT). All images analyzed were digitized at 16 bit, and only a maximum of three-fourths of the full dynamic range of the camera was used to avoid saturation. ImageJ was used for all image analysis. All statistical analysis and plots were performed using Prism software (GraphPad, La Jolla, CA). All figures were assembled using PhotoDraw (Microsoft, Redmond, WA).
Yeast two-hybrid
Fragments of PLP and CAM were introduced into pDEST-pGADT7 and pDEST-pGBKT7 (Rossignol et al., 2007) using the Gateway cloning system (Life Technologies). Before use in cloning, the kanamycin resistance cassette in pDEST-pGBKT7 was replaced with an ampicillin resistance cassette using yeast-mediated recombination. Fragments in pGADT7 or pGBKT7 were transformed into yeast strains Y187 and Y2HGold, respectively (Clontech, Mountain View, CA) using standard techniques. Cultures of yeast carrying these plasmids were grown to OD600 ∼0.5 at 30ºC in SD Leu or SD –Trp media as appropriate to maintain plasmid selection. For mating, 20 μl of a Y187 strain and a Y2HGold strain were added to 100 μl of 2× yeast extract/peptone/dextrose medium in the well of a 96-well plate. Mating cultures were grown for 20–24 h at 30ºC with shaking. Approximately 3 μl of cells were then pinned onto SD –Leu –Trp (DDO) plates using a Multi-Blot Replicator (VP 407AH; V&P Scientific), and plates were grown for 5 d at 30ºC. These plates were replica plated onto four plates: 1) DDO, 2) QDO (SD –ade –leu -trp –ura), 3) DDOXA (SD –leu –trp plates containing Aureobasidin A (Clontech) and X-α-Gal (Gold Biotechnology, St. Louis, MO), 4) QDOXA (SD –ade –leu -trp –ura with Aureobasidin A and X-α-Gal). Plates were grown for 5 d at 30ºC. Interactions were scored based on growth and development of blue color as appropriate. None of the plasmids used in this study was able to drive yeast-two hybrid reporter activity on its own (unpublished data).
CaM loss of function
We attempted to analyze the effect of CaM loss of function in vivo in two ways: first, we used actin-Gal4 to drive expression of uas-CaM RNAi using a TRiP line. No larvae were recovered from this cross at 25 and 29°C, precluding analysis of cam− cells in this manner. The second method was using the FRT flip system to create GFP-negative clones. Flies of the genotype hs-FLP;FRT42D,camn339/FRT42D, GFPnls were heat shocked for 1, 2, or 3 h at 37ºC, either 48 or 72 h after egg laying. Under no condition did we detect any GFP-negative cells. This suggests that cells are simply not viable without CaM, in agreement with data from budding yeast.
Supplementary Material
Acknowledgments
We thank Jeremy Smyth for his comments and suggestions. N.M.R. is supported by the Division of Intramural Research at the National Heart, Lung, and Blood Institute/National Institutes of Health (1ZIAHL006126). G.C.R. is supported by National Cancer Institute/National Institutes of Health (P30CA23074) and GI SPORE (National Cancer Institute/National Institutes of Health, P50CA95060), the National Science Foundation (1158151), and the Arizona Biomedical Research Commission (1210). T.L.M. is supported by the National Institute of General Medical Sciences/National Institutes of Health (R01GM068756).
Abbreviations used:
- CaM
calmodulin
- CBD
calmodulin-binding domain
- GFP
green fluorescent protein
- MT
microtubule
- NB
neuroblast
- PACT
pericentrin-AKAP450-centrosome targeting
- PCM
pericentriolar material
- Pcnt
pericentrin
- PLP
pericentrin-like protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-10-0617) on July 16, 2014.
*These authors contributed equally.
REFERENCES
- AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha A, Nakamura K, Yamada K, Iwayama Y, Toyota T, Takei N, Iwata Y, Suzuki K, Sekine Y, Matsuzaki H, et al. Gene and expression analyses reveal enhanced expression of pericentrin 2 (PCNT2) in bipolar disorder. Biol Psychiatry. 2008;63:678–685. doi: 10.1016/j.biopsych.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Blachon S, Cai X, Roberts KA, Yang K, Polyanovsky A, Church A, Avidor-Reiss T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics. 2009;182:133–144. doi: 10.1534/genetics.109.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, Malicki J, Avidor-Reiss T. Drosophila Asterless and vertebrate Cep152 are orthologs essential for centriole duplication. Genetics. 2008;180:2081–2094. doi: 10.1534/genetics.108.095141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Liu P, Sze SK, Dai C, Qi RZ. CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex. J Cell Biol. 2010;191:1089–1095. doi: 10.1083/jcb.201007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaval B, Doxsey SJ. Pericentrin in cellular function and disease. J Cell Biol. 2010;188:181–190. doi: 10.1083/jcb.200908114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg JB, Zimmerman W, Sparks CA, Young A, Vidair C, Zheng Y, Carrington W, Fay FS, Doxsey SJ. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo CG, Smith EF. Pcdp1 is a central apparatus protein that binds Ca(2+)-calmodulin and regulates ciliary motility. J Cell Biol. 2010;189:601–612. doi: 10.1083/jcb.200912009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Josue F, Suijkerbuijk S, Baum B, Tapon N, Raff J. A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation in Drosophila. PLoS Biol. 2008;6:e224. doi: 10.1371/journal.pbio.0060224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Flory MR, Morphew M, Joseph JD, Means AR, Davis TN. Pcp1p, an Spc110p-related calmodulin target at the centrosome of the fission yeast Schizosaccharomyces pombe. Cell Growth Differ. 2002;13:47–58. [PubMed] [Google Scholar]
- Flory MR, Moser MJ, Monnat RJ, Jr, Davis TN. Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc Natl Acad Sci USA. 2000;97:5919–5923. doi: 10.1073/pnas.97.11.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KW, Choi YK, Rattner JB, Qi RZ. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol Biol Cell. 2008;19:115–125. doi: 10.1091/mbc.E07-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Glover DM. Structured illumination of the interface between centriole and peri-centriolar material. Open Biol. 2012;2:120104. doi: 10.1098/rsob.120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR, Sundberg HA, Chang BH, Muller EG, Davis TN. The essential mitotic target of calmodulin is the 110-kilodalton component of the spindle pole body in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:7913–7924. doi: 10.1128/mcb.13.12.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman SE, Witman GB. Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J Cell Biol. 1980;87:764–770. doi: 10.1083/jcb.87.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Haren L, Stearns T, Luders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One. 2009;4:e5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman RG, Atkinson RC, Andruss BF, Bolduc C, Kovalick GE, Beckingham K. Spontaneous avoidance behavior in Drosophila null for calmodulin expression. Proc Natl Acad Sci USA. 1996;93:2420–2425. doi: 10.1073/pnas.93.6.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer JG, Li K, Kaufman TC. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development. 1995;121:3861–3876. doi: 10.1242/dev.121.11.3861. [DOI] [PubMed] [Google Scholar]
- Jackson AP, Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40:232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januschke J, Reina J, Llamazares S, Bertran T, Rossi F, Roig J, Gonzalez C. Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat Cell Biol. 2013;15:241–248. doi: 10.1038/ncb2671. [DOI] [PubMed] [Google Scholar]
- Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, Peters DJ, Doxsey S. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol. 2004;166:637–643. doi: 10.1083/jcb.200405023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantaputra P, Tanpaiboon P, Porntaveetus T, Ohazama A, Sharpe P, Rauch A, Hussadaloy A, Thiel CT. The smallest teeth in the world are caused by mutations in the PCNT gene. Am J Med Genet A. 2011;155A:1398–1403. doi: 10.1002/ajmg.a.33984. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S, Zheng Y. Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol Biol Cell. 2004;15:37–45. doi: 10.1091/mbc.E03-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Kishi K, van Vugt MA, Okamoto K, Hayashi Y, Yaffe MB. Functional dynamics of Polo-like kinase 1 at the centrosome. Mol Cell Biol. 2009;29:3134–3150. doi: 10.1128/MCB.01663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat Cell Biol. 2012;14:1148–1158. doi: 10.1038/ncb2591. [DOI] [PubMed] [Google Scholar]
- Lerit DA, Rusan NM. PLP inhibits the activity of interphase centrosomes to ensure their proper segregation in stem cells. J Cell Biol. 2013;202:1013–1022. doi: 10.1083/jcb.201303141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J Cell Biol. 2004;165:673–683. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Maller JL. Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts. Science. 2002;295:499–502. doi: 10.1126/science.1065693. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nishimura T, Hayakawa A, Ono Y, Takahashi M. Involvement of a centrosomal protein kendrin in the maintenance of centrosome cohesion by modulating Nek2A kinase activity. Biochem Biophys Res Commun. 2010;398:217–223. doi: 10.1016/j.bbrc.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Megraw TL, Li K, Kao LR, Kaufman TC. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development. 1999;126:2829–2839. doi: 10.1242/dev.126.13.2829. [DOI] [PubMed] [Google Scholar]
- Mennella V, Keszthelyi B, McDonald KL, Chhun B, Kan F, Rogers GC, Huang B, Agard DA. Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nat Cell Biol. 2012;14:1159–1168. doi: 10.1038/ncb2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhans J, Brandstatter JH, Giessl A. The centrosomal protein pericentrin identified at the basal body complex of the connecting cilium in mouse photoreceptors. PLoS One. 2011;6:e26496. doi: 10.1371/journal.pone.0026496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA. Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol Biol Cell. 2012;23:2658–2670. doi: 10.1091/mbc.E11-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008;319:816–819. doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- Rebollo E, Sampaio P, Januschke J, Llamazares S, Varmark H, Gonzalez C. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev Cell. 2007;12:467–474. doi: 10.1016/j.devcel.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Rogers GC. Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat Protoc. 2008;3:606–611. doi: 10.1038/nprot.2008.18. [DOI] [PubMed] [Google Scholar]
- Rossignol P, Collier S, Bush M, Shaw P, Doonan JH. Arabidopsis POT1A interacts with TERT-V(I8), an N-terminal splicing variant of telomerase. J Cell Sci. 2007;120:3678–3687. doi: 10.1242/jcs.004119. [DOI] [PubMed] [Google Scholar]
- Rusan NM, Peifer M. A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol. 2007;177:13–20. doi: 10.1083/jcb.200612140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Sonnen KF, Schermelleh L, Leonhardt H, Nigg EA. 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biol Open. 2012;1:965–976. doi: 10.1242/bio.20122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DA, Rayner TF, Prescott AR, Stark MJ. Mutations which block the binding of calmodulin to Spc110p cause multiple mitotic defects. J Cell Sci. 1996;109:1297–1310. doi: 10.1242/jcs.109.6.1297. [DOI] [PubMed] [Google Scholar]
- Stirling DA, Welch KA, Stark MJ. Interaction with calmodulin is required for the function of Spc110p, an essential component of the yeast spindle pole body. EMBO J. 1994;13:4329–4342. doi: 10.1002/j.1460-2075.1994.tb06753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel EW, Stephens RE, Masure HR, Head JF. Specific localization of scallop gill epithelial calmodulin in cilia. J Cell Biol. 1982;92:622–628. doi: 10.1083/jcb.92.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg HA, Goetsch L, Byers B, Davis TN. Role of calmodulin and Spc110p interaction in the proper assembly of spindle pole body components. J Cell Biol. 1996;133:111–124. doi: 10.1083/jcb.133.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol Biol Cell. 2002;13:3235–3245. doi: 10.1091/mbc.E02-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmark H, Llamazares S, Rebollo E, Lange B, Reina J, Schwarz H, Gonzalez C. Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr Biol. 2007;17:1735–1745. doi: 10.1016/j.cub.2007.09.031. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu T, Shi L, Zhang L, Zheng W, Qu JY, Niu R, Qi RZ. Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J Biol Chem. 2010;285:22658–22665. doi: 10.1074/jbc.M110.105965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M, Genevieve D, Borck G, Baumann C, Baujat G, Bieth E, Edery P, Farra C, Gerard M, Heron D, et al. Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. J Med Genet. 2010;47:797–802. doi: 10.1136/jmg.2009.067298. [DOI] [PubMed] [Google Scholar]
- Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M. Calmodulin target database. J Struct Funct Genomics. 2000;1:8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]
- Zavortink M, Welsh MJ, McIntosh JR. The distribution of calmodulin in living mitotic cells. Exp Cell Res. 1983;149:375–385. doi: 10.1016/0014-4827(83)90350-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Megraw TL. Proper recruitment of gamma-tubulin and D-TACC/Msps to embryonic Drosophila centrosomes requires centrosomin motif 1. Mol Biol Cell. 2007;18:4037–4049. doi: 10.1091/mbc.E07-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell. 2004;15:3642–3657. doi: 10.1091/mbc.E03-11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.