Abstract
Background
Alcohol abuse and dependence can cause a wide variety of cognitive, psychomotor, and visual-spatial deficits. It is questionable whether this condition is associated with impairments in the recognition of affective and/or emotional information. Such impairments may promote deficits in social cognition and, consequently, in the adaptation and interaction of alcohol abusers with their social environment. The aim of this systematic review was to systematize the literature on alcoholics’ recognition of basic facial expressions in terms of the following outcome variables: accuracy, emotional intensity, and latency time.
Methods
A systematic literature search in the PsycINFO, PubMed, and SciELO electronic databases, with no restrictions regarding publication year, was employed as the study methodology.
Results
The findings of some studies indicate that alcoholics have greater impairment in facial expression recognition tasks, while others could not differentiate the clinical group from controls. However, there was a trend toward greater deficits in alcoholics. Alcoholics displayed less accuracy in recognition of sadness and disgust and required greater emotional intensity to judge facial expressions corresponding to fear and anger.
Conclusion
The current study was only able to identify trends in the chosen outcome variables. Future studies that aim to provide more precise evidence for the potential influence of alcohol on social cognition are needed.
Keywords: alcoholism, face, emotional recognition, facial expression, systematic review
Introduction
It is well known that alcoholism can lead, in the long-term, to deficits in neurocognitive functions that involve memory, learning, visual-spatial orientation, psychometricity, and information processing, among other skills.1,2 In addition, previous authors have highlighted pronounced impairments in the recognition of affective and/or emotional information.
Such impairments promote deficits in social cognition and, consequently, in the adaptation and interaction of alcohol abusers with their social environment.3–6 These deficits occur because social cognition is one of the key aspects of emotional adaptive functioning. It is also an important nonverbal social skill that conveys messages about individuals’ emotional states at the moment of interaction, thus providing clues that aid the interpretation of behaviors.7
These deficits, together with the development of interpersonal problems, could become risk factors for the maintenance of an individual’s cycle of alcohol abuse and/or dependence. They may induce relapses to drinking and hinder the processes of withdrawal, treatment, and recovery.8–10
Given this situation, the majority of studies in the area that have aimed to verify and/or measure the presence of such deficits have employed tasks that involve recognition of basic facial expressions, such as anger, disgust, fear, and sadness (negative emotions), and happiness and surprise (positive emotions). These studies have primarily assessed one or more of the following three key variables: accuracy, latency time, and emotional intensity, pointing to some specific changes.
In addition, smaller and more recent studies in this field have monitored brain electrical activity during the performance of cognitive tasks through the use of electroencephalogram instrumentation. These studies have shown that alcoholics exhibit lower brain activation in regions that mediate visual, auditory, and visual-motor processes and deficits in processing anger.11–14
Other studies have investigated the recognition of facial expressions during neuroimaging tests performed via functional magnetic resonance imaging techniques. These studies have indicated, for example, that alcoholics show lower brain activity in the cingulate, orbitofrontal, and insular cortex (regions that mediate emotional processing) during recognition of facial expressions of fear15 and disgust.6 In addition, Marinkovic et al16 showed that alcoholic individuals have lower activation in the right amygdala and hippocampus upon negative and positive stimuli compared with nonalcoholics. These studies suggest that this deficient activation may underlie impaired processing of emotional faces in alcoholic individuals.
Considering the field of knowledge in question and the nonsystematization of findings, the present study consists of a systematic literature review of facial expression recognition by individuals with a history of alcohol abuse and dependence. This aim was achieved by reviewing clinical studies that included accuracy, latency time, and emotional intensity.
Materials and methods
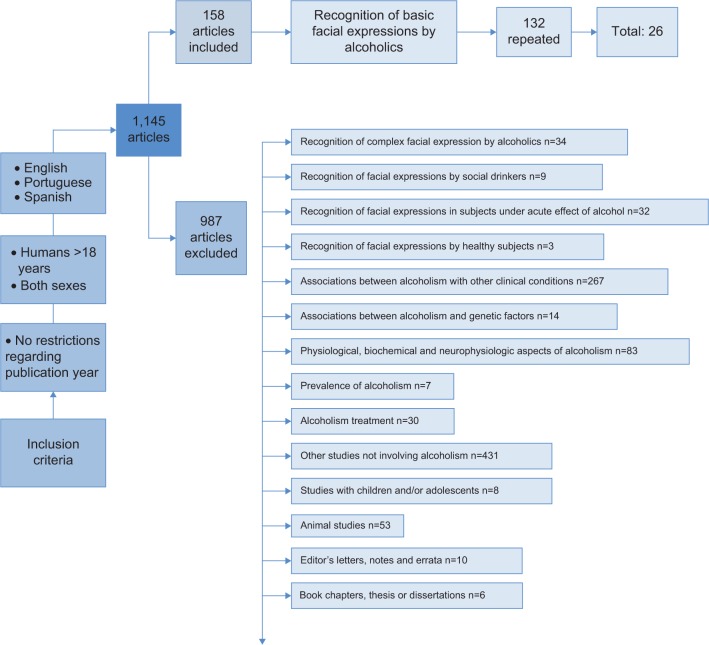
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement17 and followed the instructions in the Cochrane Handbook for Systematic Reviews of Interventions.18 A systematic search was performed in the PsycINFO, PubMed, and SciELO electronic databases using the following keywords: (alcohol OR alcoholism OR alcoholics) AND (faces OR facial) AND (recognition OR expression OR emotional). The cut-off date for the search was February 6, 2014. The main aspects of the article inclusion and exclusion process are shown in Figure 1. As observed in Figure 1, a total of 1,145 articles were found. Applying the inclusion and exclusion criteria, two researchers selected 26 articles for analysis in the current review.3,4,6,7,9–15,19–33
Figure 1.
Flowchart of the inclusion and exclusion process.
Results
Context and samples
In general, most of the studies were conducted in Belgium (57.7%, n=15), beginning in the early 2000s (96%). Only one study had a descriptive methodological design,30 with the remaining studies being of case-control design. The data are presented in Table 1, which also shows the main sociodemographic and clinical characteristics of the samples.
Table 1.
Sample composition and countries of origin in the analyzed studies
| Author | Country | Clinical group
|
Control group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, mean (SD) (years) | Education level (years) | PWC (weeks) | Consumption time (years) | Drinks per day (mean) | REC | n | Age mean (SD) (years) | Education level (years) | Type | ||
| Kornreich et al3 | Belgium | 9 M; 13 F | 38.1 (7.7) | 2.31 | 2–3 | 10.6 | 18.7 | IN | 9 M; 13 F | 37.2 (9.0) | 2.50 | HS |
| Maurage et al4 | Belgium | 9 M; 9 F | 50.7 (9.8) | 13.39 | 2 | – | 14.3 | IN | 9 M; 9 F | 48.0 (10.6) | 13.22 | SD |
| O’Daly et al6 | England | 17 M | 37.6 (9.6) | – | 2 | 16.3 | 39.1 | – | 31 M | 40.2 (8.7) | – | HS |
| Townshend and Duka7 | England | 8 M; 6 F | 47.9 (10.3) | – | 2 | – | 15.0 | IN | 8 M; 6 F | 42.1 (9.9) | – | SD |
| Philippot et al9 | Belgium | 18 M; 7 F | 43.0 (6.4) | 2.95 | 3 | – | – | IN | 18 M; 7 F | 43.8 (6.4) | 2.91 | HS |
| Frigerio et al10 | Italy | 13 M; 12 F | 46.7 (10.1) | 12.5 | 4 | – | – | OUT | 11 M; 12 F | 46.6 (8.8) | – | HS |
| Maurage et al11 | Belgium | 9 M; 1 F | 46.9 (11.3) | 13.4 | 2 | – | – | IN | 9 M; 1 F | 43.9 (9.5) | 13.5 | HS |
| Maurage et al12 | Belgium | 10 M; 5 F | 46.7 (12.0) | 13.46 | 2 | – | 15.4 | IN | 10 M; 5 F | 42.1 (14.2) | 14.47 | HS |
| Maurage et al13 | Belgium | 7 M; 8 F | 49.1 (8.0) | 13 | 2 | 19.5 | 16.7 | IN | 7 M; 8 F | 48.1 (7.2) | 13.5 | HS |
| Maurage et al14 | Belgium | 7 M; 5 F | 41.8 (6.6) | 12.67 | 2.5 | 9.4 | 27.3 | IN | 7 M; 5 F | 41.8 (9.1) | 12.92 | HS |
| Salloum et al15 | United States | 11 M | 35.7 (5.6) | – | 4 | – | 13.6 | IN | 11 M | 36.2 (5.8) | – | HS |
| Kornreich et al19 | Belgium | 18 M; 7 F | 43.0 (6.4) | 2.96 | 2–3 | – | – | IN | 18 M; 7 F | 43.8 (6.4) | 2.91 | HS |
| 18 M; 7 F | 44.6 (6.7) | 2.95 | 8–486 | – | – | |||||||
| Kornreich et al20 | Belgium | 20 M; 10 F | 44.1 (10.7) | 12.55 | 3–4 | 10–12 | 12.8 | IN | 20 M; 10 F | 42.1 (11.2) | 12.55 | HS |
| Kornreich et al21 | Belgium | 18 M; 12 F | 43.9 (9.8) | 13.06 | 3 | 13.5 | 19.7 | IN | 16 M; 14 F | 37.1 (10.4) | 14.38 | HS |
| Foisy et al22 | Belgium | 16 M; 9 F | 46.8 (9.3) | 13.04 | 3 | 16.8 | 18.8 | IN | 16 M; 10 F | 44.1 (9.5) | 13.48 | HS |
| Foisy et al23 | Belgium | 15 M; 12 F | 42.4 (8.1) | 12.64 | 4 | 13.0 | 18.0 | IN | 12 M; 10 F | 44.9 (9.3) | 14.9 | HS |
| 13 M; 9 F | 46.4 (7.9) | 13.27 | 8 | 13.0 | 14.7 | |||||||
| Maurage et al24 | Belgium | 17 M; 3 F | 44.6 (13.1) | 14.35 | 2–3 | 15.6 | 17.9 | IN | 17 M; 3 F | 44.6 (13.1) | 14.45 | HS |
| Sprah and Novak25 | Slovenia | 33 M | 45.0 (1.6) | 11.88 | 8 | 13.6 | – | IN | 36 M | 40.4 (11.2) | 11.72 | HS |
| Maurage et al26 | Belgium | 14 M; 4 F | 47.1 (10.3) | 13.17 | 2 | 9.6 | 26.7 | IN | 14 M; 4 F | 47.9 (11.9) | 13.78 | HS |
| Fein et al27 | United States | 28 M; 24 F | 45.5 (6.8) | 15.5 | 24–1,134 | – | – | NET | 25 M; 22 F | 45.5 (6.8) | 16.2 | HS |
| Acharya et al28 | England | 23 M | 39.3 (10.8) | – | 3 | – | – | IN | 26 M | 33.7 (10.7) | – | HS |
| Kumar et al29 | India | 30 M | 36.8 (5.5) | – | 2 | – | – | OUT | 30 M | 34.5 (4.8) | – | HS |
| Steinmet and Federspiel30 | Luxembourg | 5 M; 3 F | 60.8 (8.5) | – | 3.1 | 20.5 | – | RZP | NA | NA | NA | NA |
| Kornreich et al31 | Belgium | 17 M; 8 F | 42.1 (9.6) | 17.56 | 3 | 12.1 | 16.2 | IN | 17 M; 8 F | 43.5 (9.1) | 17.08 | HS |
| Charlet et al32 | Germany | 25 M; 8 F | 44.8 (9.8) | 15.7 | 1 | 11.6 | – | IN | 25 M; 8 F | 46.1 (9.8) | 15.8 | HS |
| Carmona-Perera et al33 | Spain | 31 M | 52.0 (6.5) | 13.7 | 2 | 26.5 | 18.8 | IN | 34 M | 48.7 (10.6) | 17.1 | HS |
Abbreviations: M, male; F, female; SD, standard deviation; PWC, period without consumption; HS, healthy subjects; SD, social drinkers; NA, not applicable; REC, recruitment source; RZP, rural zone population; NET, Internet; IN, hospitalized patients; OUT, outpatients.
The majority of the clinical subjects were alcohol-abstinent individuals who were undergoing treatment. Of these individuals, 80.8% were inpatients (n=21),3,4,7,9,11–15,19–26,28,31–33 and 7.7% were outpatients (n=2).10,29 The samples ranged from eight to 52 subjects (mean 22.9 and median 24), with mean ages between 35.7 and 60.8 years (median 44.7 years) and varying education levels. Prior consumption of alcohol ranged from 12.8 to 39.1 (mean 19) drinks per day. Periods without alcohol consumption generally ranged from 1–8 (median 3) weeks. In two of the studies, this variability was greater, reaching up to 21 years.19,27
In terms of the control group, 88.5% of the studies (n=23) selected healthy subjects with no history of alcohol use from the general population. Social drinkers were used as the control group in only two studies, and their alcohol consumption was 3.3–13 drinks per week.4,7 The control group sample sizes ranged from ten to 47 subjects (mean 23 and median 24), with ages ranging from 33.7 to 45.5 years (median 43.8 years) and variable education levels.
Most studies diagnosed alcohol abuse and/or dependence according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; n=25), with the exception of one study that used the DSM-III-R.9 All subjects abstained from alcohol. Furthermore, only one study utilized the Structured Clinical Interview for DSM-IV Axis I disorder.32 The controls were primarily healthy subjects from the general population without a prior history of alcohol dependence (n=23). Exclusion criteria for both groups were as follows: presence of comorbidity with any Axis I psychiatric disorder (n=17);3,4,9–12,14,20,22,25–28,30–33 presence of comorbidity with any Axis II psychiatric disorder (n=1);33 visual or hearing impairment (n=8);4,11–14,24–26 epilepsy (n=9);11–14,24–27,32 health problems in general (n=8);4,7,12,14,24,25,27,32 intellectual impairment (n=4);3,9,20,27 Wernicke-Korsakoff syndrome (n=2);26,27 and use of medications (n=2).21,32 Five studies did not present their exclusion criteria.6,15,23,28,29
Facial expression recognition tasks: stimuli and procedures
The main aspects related to types of stimuli and the procedures that the studies used to perform facial expression recognition tasks (FERT) are summarized in Table 2, which shows that the studies used a wide variety of stimuli and procedures. However, this diversity does not seem to have influenced the outcomes, as discussed below.
Table 2.
Main characteristics of stimuli and procedures used for FERT
| Studies (n) | References | |||
|---|---|---|---|---|
| FERT stimuli | ||||
| Standardized | ||||
| Yes | Ekman and Friesen | 12 | 4,6,7,11,12,14,24,25,26,30–32 | |
| Matsumoto and Ekman | 9 | 3,9,15,19–23,28 | ||
| Others | 3 | 13,29,33 | ||
| No | ––––––––– | 2 | 10,27 | |
| Number of stimuli | Mean 33, median 28 | ––––––––– | ––––––––– | |
| Evaluated emotions (*) | Happiness | 22 | 3,4,7,9–12,14,15,19–24,26–31,33 | |
| Sadness | 19 | 7,9–11,14,15,19–23,25–31,33 | ||
| Fear | 16 | 3,6,7,9,11,14,15,19,25,26,28–33 | ||
| Anger | 22 | 3,4,7,9,10,12,13,15,19–26,28–33 | ||
| Surprise | 8 | 7,25,28–33 | ||
| Disgust | 15 | 7,9,10,13,15,19–23,25,28–30,33 | ||
| Neutral | 7 | 6,11,12,14,24,27,29 | ||
| Actors | Number of actors | Mean 17.1, median 5 | ||
| Ethnicity | Caucasian | 8 | 3,4,9,19–23 | |
| Noninformed | 18 | 6,7,10,11–15,24–33 | ||
| Sex | Both | 18 | 3,6,9,10,12,13,15,19–21,23–25,27–29,31,32 | |
| Male | 4 | 7,11,14,26 | ||
| Noninformed | 4 | 4,20,22,33 | ||
| FERT procedures | ||||
| Standardized | ||||
| Yes | Hess and Blairy | 9 | 3,4,9,19–23,15 | |
| Others | 7 | 7,10,11,13,28,30,33 | ||
| No | ––––––––– | 10 | 6,12,14,24–27,29,31,32 | |
| Means of performing task | Computer | 21 | 3,4,6,9,13–15,19–32 | |
| Slide/card | 2 | 7,10 | ||
| Noninformed | 3 | 11,12,33 | ||
| Task type | Static | 12 | 3,7,15,20–23,26,27,29–31 | |
| Dynamic | 5 | 9,10,13,19,28 | ||
| Noninformed | 8 | 4,11,12,14,24,25,32,33 | ||
| Stimulus presentation | Randomized | 22 | 3,4,6,7,10,11,13,15,19–24,26–33 | |
| Noninformed | 4 | 9,12,14,25 | ||
| Emotional intensity | 30% and 70% | 6 | 3,15,20–23 | |
| 100% | 3 | 27,29,31 | ||
| Others | 4 | 6,7,14,32 | ||
| Noninformed | 8 | 4,11,12,20,25,26,32,33 | ||
| Response time | Controlled | 8 | 9,22,11,13,14,26,27,32 | |
| No time limit | 4 | 6,30,31,33 | ||
| Noninformed | 14 | 3,4,7,10,12,15,19–23,25,28,29 | ||
| Outcome variables (*) | Accuracy | 23 | 3,4,6,7,9,10,12–15,19–25,27,28,30–33 | |
| Intensity | 8 | 3,7,9,15,19,23,26,31 | ||
| Latency time | 11 | 4,11–15,22,24,29,31,32 | ||
Note:
Nonexclusive categories.
Abbreviation: FERT, facial expression recognition task.
Outcome variables
The main results according to the outcome variables analyzed in the studies are listed in Table 3. The results concerning accuracy were highly divergent. Of the 23 studies that analyzed accuracy, 17 (73.9%) found at least some difference between the groups, indicating impairment in alcoholic individuals with regard to correctly identifying disgust (26%) and sadness (26%). However, 16 studies found no differences between the groups, indicating no impairment in alcoholic individuals with regard to identifying emotions, especially anger (43.7%). In addition, ten studies (43.5%) that evaluated accuracy found differing results for the same sample group. Specifically, alcoholics showed greater impairment in recognizing some emotions but not others. The findings were generally contradictory.
Table 3.
Results according to the outcome variables analyzed
| Outcome variables (*) | Emotions | A > C
|
A < C
|
A = C
|
|||
|---|---|---|---|---|---|---|---|
| Studies (n) | References | Studies (n) | References | Studies (n) | References | ||
| Accuracy (N=23) | Happiness | – | – | 7 | 3,4,9,12,19,20,30 | 7 | 10,7,15,27,28,31,33 |
| Sadness | – | – | 6 | 9,10,19,20,28,30 | 5 | 7,15,27,31,33 | |
| Fear | – | – | 5 | 6,7,30,31,33 | 5 | 3,9,15,19,28 | |
| Anger | – | – | 6 | 3,12,19,20,28,30 | 7 | 7,9,10,13,15,31,33 | |
| Surprise | – | – | 3 | 4,28,30 | 4 | 7,15,31,33 | |
| Disgust | – | – | 6 | 9,13,19,20,30,33 | 4 | 10,7,15,28 | |
| Neutral | – | – | 2 | 12,31 | 2 | 6,27 | |
| Total score | – | – | 3 | 21,23,24 | 4 | 14,22,25,32 | |
| Intensity (N=8) | Happiness | 2a | 3,9 | – | – | 6b | 3,7,19,23,26,31 |
| Sadness | 3 | 9,26,31 | – | – | 3 | 19,7,23 | |
| Fear | 4 | 9,7,26,31 | – | – | 2 | 3,19 | |
| Anger | 3 | 9,7,23 | – | – | 1 | 19 | |
| Surprise | 4 | 9,7,23,26 | – | – | 3 | 3,19,31 | |
| Disgust | – | – | – | – | 1 | 7 | |
| Neutral | 2 | 26,31 | – | – | – | – | |
| Total score | – | – | – | – | 1 | 28 | |
| Latency time (N=1) | Happiness | 2 | 27,37 | 1 | 24 | 1 | 15 |
| Sadness | 2 | 27,29 | – | – | 1 | 15 | |
| Fear | – | – | – | – | 2 | 15,29 | |
| Anger | 1 | 13 | – | – | 2 | 15,29 | |
| Surprise | 2 | 24,29 | – | – | 2 | 15,13 | |
| Disgust | – | – | – | – | 1 | 29 | |
| Neutral | 2 | 27,29 | – | – | – | – | |
| Total score | 5 | 4,11,12,14,22 | – | – | 1 | 32 | |
Notes:
Nonexclusive categories
emotion at 30% intensity
emotion at 70% intensity.
Abbreviations: A, alcoholics; C, controls.
Of the eight studies that evaluated the emotional intensity needed for emotion recognition, at least six found a difference in specific emotions. The group of alcoholics required higher intensities for emotion recognition, particularly for fear (50%) and anger (37.5%). However, in seven of these studies this difference was not observed, especially with regard to happiness (75%).
Finally, of the eleven studies that evaluated latency time, five (45.5%) found that alcoholics required more time for emotion recognition. These results were more evident when the total score of emotions was evaluated. A small number of studies analyzed specific emotions, and the results were contradictory.
All of the results presented were also analyzed while taking into account the different variables associated with the stimuli and procedures used in FERT, ie, the methods chosen for data collection. Despite the great diversity of employed methodologies, none of them seemed to have clearly interfered with the results. In other words, no particular result was associated with any specific stimulus and/or procedure.
The influence of sociodemographic features, particularly the influence of male/female sex on FERT performance, was also evaluated. Of the four studies that analyzed this variable,9,10,23,27 only the study of Frigerio et al10 found significant differences. This study indicated that women needed less emotional intensity than men to recognize facial expressions, regardless of whether they had a history of alcoholism.
Only the study by Kornreich et al19 evaluated the influence of duration of alcohol abstinence on FERT performance. This study found that alcoholics who abstained from alcohol for a longer period of time required a lower intensity to judge the emotion compared with recently detoxified alcoholics; however, there was no improvement in accuracy. In addition, only the study by Foisy et al23 evaluated the difference between a group of abstinent subjects undergoing treatment and a group of subjects who discontinued alcohol detoxification due to relapses. This study showed that the latter had lower accuracy, but no difference was observed in the emotional intensity required for recognition.
Discussion
The present study aimed to systematize the literature on recognition of basic facial expressions by alcoholic subjects in terms of the following outcome variables: accuracy, emotional intensity, and latency time. One noteworthy finding is that these studies were conducted recently and were primarily developed by European researchers, with a group of researchers from Belgium being responsible for 57.7% of the studies (n=15).
Homogeneity in the group of researchers who have studied the topic and the significantly diverse methodologies used across studies could have considerably influenced the results. However, despite the great variability of stimuli and procedures in the reviewed studies, a qualitative initial analysis indicated that these variables did not have a direct influence on the studied outcomes. Specifically, different results did not display a direct relationship with any of these variables.
None of the studies included subjects in the clinical groups who were consuming alcohol at the time of measurement. Therefore, future studies in this population are necessary. It is also noteworthy that a small number of subjects were examined in both the clinical groups (8–52) and the control groups (10–47). Thus, the use of larger sample sizes is suggested for future studies.
The prior literature has suggested that respondent characteristics have a direct influence on FERT. Thus, the stimuli that compose FERT and respondent characteristics should be considered separately. Namely, the following variables stand out: male/female sex of the respondent, given that women recognize both positive and negative emotions more accurately and more rapidly than men;34 age of the respondent, given that adults above the age of 45 years tend to wrongly identify facial stimuli more often than do younger subjects;35 education level of the respondent, as individuals with lower education levels identify facial expressions of fear and disgust less accurately than individuals with higher education levels;36 and type of task used, because dynamic tasks have greater ecological validity than do static tasks, given that the former involve movement and are thus more similar to the actual stimuli.37–39
Only four studies used an intelligence test to screen the sample, and just one study considered the presence of comorbidity with Axis II personality disorders when assessing exclusion criteria. This observation warrants attention because the literature indicates that the presence of both cognitive deficits and a specific personality disorder can negatively impact FERT performance.40–45 These variables are particularly important when considering alcoholics, because cognitive deficits may be associated with degeneration of specific brain areas due to chronic and excessive alcohol use.40,41,46 The same approach is valid for personality disorders, as comorbidity rates (especially with antisocial and borderline personality disorders) are considerable, reaching levels greater than 50%.43–45
Some studies indicated alcoholics’ greater impairment in FERT, while others did not differentiate between their clinical and control groups. Several studies found differences in accuracy2,14,20,21,23,24,48 between alcoholics and controls. For sadness3,9,10,28,30 and disgust,3,9,13,20,30,33 which are considered negative emotions, there was a slight tendency towards impairment in the group of alcoholics. The same result was observed for emotional intensity, as some studies found that alcoholics were less sensitive in FERT3,9 and required greater emotional intensity to judge anger7,9,23 and fear.7,9,26,31
The justification given for such positive findings was that the chronic use of alcohol caused changes in the brain structures involved in processing visual-spatial information and the perception of, for example, nonverbal signals,9,20 especially with regard to negative and/or aversive emotions.19,28,30 In addition, it has been found that alcoholics show dysfunction in the right cerebral hemisphere, which is responsible for processing negative emotions.19,47 Thus, some authors support the hypothesis that impairments in social cognition are consequences of deficits in the central nervous system and that they even become a reason for maintaining the vicious cycle of drinking.2,3,9,13,20
Unlike the variables of accuracy and emotional intensity, studies have shown greater homogeneity for latency time. Specifically, alcoholics require more time to judge emotions as a whole.11,30,31,33–35,41 This difference suggests that chronic use of alcohol may impair cognitive functions, such as the speed of processing of emotional information and attention level while performing a task. Thus, alcoholics needed more time to focus on the task and complete it.4,12,14,27,29 Overall, most justifications for the presence of impairments are related to neurocognitive function.1,2
In contrast, the hypothesis used to explain the lack of differences in the three outcome variables (accuracy, intensity, and latency time) between the groups in terms of FERT is associated with the different methodological procedures used. According to Fein et al27 some paradigms may be more sensitive than others in detecting differences between groups. Studies that used simpler tasks, such as those in which the subject was offered the possibility of classifying emotions as high (70%) or low (30%) intensity or in which the subject answered “yes” or “no” as rapidly as possible after recognizing the emotion displayed,15,23 found no differences between groups. Conversely, studies that used more complex tasks, such as requesting the subject to classify the intensity of an emotion using a seven-point Likert scale or to choose one of four options of available emotions,10,19 found differences between groups. Thus, more complex tasks that require more effort from the subjects are able to demonstrate impairment, whereas simpler tasks can level all individuals and thus underestimate the possible deficits.
In addition, other hypotheses underpinning the lack of differences between groups suggest that impairments in the ability to recognize emotions may be independent of alcohol abuse. Such impairments may be associated with other factors, such as primary impairment, which is due to altered emotional intelligence21 or the genetic history of the individual. Further, this genetic history is capable of potentially leading to an increased predisposition to the development of abnormalities in the brain regions involved in emotional processing.9 As an example, in the study reported by Schandler et al48 preschool children from families with alcoholic individuals exhibited deficits in processing visual-spatial information.
Despite the inconclusive results, it is important to consider the judgment deficits mentioned because they may have a negative impact on the social life of the individual who abuses or is dependent on alcohol. This potential impact becomes even more relevant because the impairments related to social cognition are not restricted to the variables that were analyzed in the current study. It is also known that alcoholics have a tendency to overestimate emotions7 and show response biases toward negative emotions.11,12,22,23 Considering this set of impairments, alcoholics are more vulnerable to displaying inappropriate reactions in social situations. Thus, they may experience difficulties in interpersonal relationships and social isolation, and even become involved in fights and/or aggression, which can reduce their quality of life and reinforce their continued alcohol use.8,20
Conclusion
The presented data demonstrate divergent results across the relevant studies. Therefore, rather than reaching definite conclusions, only trends can be identified. The current study showed that this line of research is recent and restricted to small groups of researchers who do not use standard methodology. These inconsistencies further complicate direct comparison between studies because, as previously noted, the number of variations between the stimuli and procedures adopted is high. The systematization of a single procedure seems necessary and urgent. Researchers in this area of study should target their efforts in this direction. Most of the studies were performed in European countries, limiting potential generalizations of the findings to other sociocultural contexts. However, context is an important variable in the etiology of alcoholism.49,50 Attention must be paid to other critical points in the studies, such as: adoption of intellectual screening tests; ascertainment of comorbidity, particularly with personality disorders; the lack of samples arising from different sociocultural contexts; and the lack of samples comprising subjects who are currently consuming alcohol.
Future research can still delineate this field of knowledge, providing more accurate evidence on whether alcoholism influences social cognition. Nevertheless, some of the findings herein regarding the negative effect of alcohol use on the recognition of emotions have clinical implications. Such implications should be considered in both the treatment of alcoholism and the prevention of relapse, with the aim of minimizing the negative impacts of the distorted interpretation of feelings and/or emotions.19,27
Acknowledgments
This research was supported by Coordination for the Improvement of Higher Level Personnel and Agency of São Paulo Research Foundation (FAPESP- grant 2012/02260-7).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol. 2002;10(3):193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- 2.Vieira RMT, Pádua ASF. [Prejuízos neurocognitivos na dependência alcoólica: um estudo de caso] Neurocognitive impairments in alcohol dependence: a case report. Rev Psiquiatr Clin. 2007;34(5):246–250. Spanish. [Google Scholar]
- 3.Kornreich C, Blairy S, Philippot P, et al. Impaired emotional facial expression recognition in alcoholism compared with obsessive-compulsive disorder and normal controls. Psychiatr Res. 2001;102(3):235–248. doi: 10.1016/s0165-1781(01)00261-x. [DOI] [PubMed] [Google Scholar]
- 4.Maurage P, Campanella S, Philippot P, et al. Face processing in chronic alcoholism: a specific deficit for emotional features. Alcohol Clin Exp Res. 2008;32(4):600–606. doi: 10.1111/j.1530-0277.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 5.Uekermann J, Daum L. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103(5):726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Daly OG, Trick L, Scaife J, et al. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacol. 2012;37(10):2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townshend JM, Duka T. Mixed emotions: alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41(7):773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- 8.Marlatt GA, Kosturn CF, Lang AR. Provocation of anger and opportunity for retaliation as determinants of alcohol consumption in social drinkers. J Abnorm Psychol. 1975;84(6):652–659. [PubMed] [Google Scholar]
- 9.Philippot P, Kornreich C, Blairy S, et al. Alcoholics’ deficits in the decoding of emotional facial expression. Alcohol Clin Exp Res. 1999;23(6):1031–1038. [PubMed] [Google Scholar]
- 10.Frigerio E, Burt MD, Montagne B, et al. Facial affect perception in alcoholics. Psychiatr Res. 2002;113(1):161–171. doi: 10.1016/s0165-1781(02)00244-5. [DOI] [PubMed] [Google Scholar]
- 11.Maurage P, Philippot P, Verbanck P, et al. Is the P300 deficit in alcoholism associated with early visual impairments (P100, N170)? An oddball paradigm. Clin Neurophysiol. 2006;118(3):633–644. doi: 10.1016/j.clinph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Maurage P, Philippot P, Joassin F, et al. The auditory-visual integration of anger is impaired in alcoholism: an event-related potentials study. J Psychiatry Neurosci. 2007;33(2):111–122. [PMC free article] [PubMed] [Google Scholar]
- 13.Maurage P, Campanella S, Philippot P, et al. Electrophysiological correlates of the disrupted processing of anger in alcoholism. Int J Psychophysiol. 2008;70(1):50–62. doi: 10.1016/j.ijpsycho.2008.05.572. [DOI] [PubMed] [Google Scholar]
- 14.Maurage P, Campanella S, Philippot P, et al. Alcoholism leads to early perceptive alterations, independently of comorbid depressed state: an ERP study. Clin Neurophysiol. 2008;38(2):83–97. doi: 10.1016/j.neucli.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Salloum JB, Ramchandani VA, Boduka J, et al. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31(9):1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 16.Marinkovic K, Oscar-Berman M, Urban T, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33(11):1180–1192. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):1–6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. [Accessed July 31, 2014]. Available from: http://www.cochrane.org/handbook. [Google Scholar]
- 19.Kornreich C, Blairy S, Philippot P, et al. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. J Stud Alcohol. 2001;62(4):533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- 20.Kornreich C, Philippot P, Foisy ML, et al. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol. 2002;37(4):394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- 21.Kornreich C, Foisy ML, Philippot P, et al. Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatr Res. 2003;119(3):251–260. doi: 10.1016/s0165-1781(03)00130-6. [DOI] [PubMed] [Google Scholar]
- 22.Foisy ML, Kornreich C, Fobe A, et al. Impaired emotional facial expression recognition in alcohol dependence: do these deficits persist with midterm abstinence? Alcohol Clin Exp Res. 2005;31(3):404–410. doi: 10.1111/j.1530-0277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 23.Foisy ML, Kornreich C, Petiau C, et al. Impaired emotional facial expression recognition in alcoholics: are these deficits specific to emotional cues? Psychiatr Res. 2007;150(1):33–41. doi: 10.1016/j.psychres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Maurage P, Campanella S, Philippot P, et al. The crossmodal facilitation effect is disrupted in alcoholism: a study with emotional stimuli. Alcohol Alcohol. 2007;42(6):552–559. doi: 10.1093/alcalc/agm134. [DOI] [PubMed] [Google Scholar]
- 25.Sprah L, Novak T. Behavioural inhibition system (BIS) and behavioural activation system (BAS) as predictors of emotional and cognitive deficits observed in alcohol abstainers. Psychiatr Danub. 2008;20(2):184–193. [PubMed] [Google Scholar]
- 26.Maurage P, Campanella S, Philippot P, et al. Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol Alcohol. 2009;44(5):476–485. doi: 10.1093/alcalc/agp037. [DOI] [PubMed] [Google Scholar]
- 27.Fein G, Key K, Szymanski MD. ERP and RT delays in long-term abstinent alcoholics in processing of emotional facial expressions during gender and emotion categorization tasks. Alcohol Clin Exp Res. 2010;34(7):1127–1139. doi: 10.1111/j.1530-0277.2010.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acharya R, Dolan M. Impact of antisocial and psychopathic traits on emotional facial expression recognition in alcohol abusers. Personal Ment Health. 2001;6(2):126–137. [Google Scholar]
- 29.Kumar S, Khess CRJ, Singh AR. Facial emotion recognition in alcohol dependence syndrome: intensity effects and error pattern. Indian Journal of Community Psychology. 2011;7(1):20–25. [Google Scholar]
- 30.Steinmetz JP, Federspiel C. Alcohol-related cognitive and affective impairments in a sample of long-term care residents. GeroPsych. 2012;25(2):83–95. [Google Scholar]
- 31.Kornreich C, Brevers D, Canivet D, et al. Impaired processing of emotion in music, faces and voices supports a generalized emotional decoding deficit in alcoholism. Addict. 2012;108(1):80–88. doi: 10.1111/j.1360-0443.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 32.Charlet K, Schlagenhauf F, Richter A, et al. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol. 2014;19(3):439–451. doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- 33.Carmona-Perera M, Clark L, Young L, et al. Impaired decoding of fear and disgust predicts utilitarian moral judgment in alcohol dependent individuals. Alcohol Clin Exp Res. 2014;38(1):1–7. doi: 10.1111/acer.12245. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann H, Kessler H, Eppel T, et al. Expressions intensity, gender and facial emotion recognition: women recognize only subtle facial emotions better than men. Acta Psychol (Amst) 2010;135(3):278–283. doi: 10.1016/j.actpsy.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Horning SN, Cornwell E, Hasker P. The recognition of facial expressions: an investigation of the influence of age and cognition. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19(6):657–676. doi: 10.1080/13825585.2011.645011. [DOI] [PubMed] [Google Scholar]
- 36.Trauffer NM, Widen SC, Russell JA. Education and the attribution of emotion to facial expressions. Migr Teme. 2013;22(2):237–247. Croatian. [Google Scholar]
- 37.Alves NT. Recognition of static and dynamic facial expressions: a study review. Psicol Estud. 2013;18(1):125–130. Spanish. [Google Scholar]
- 38.Ambadar Z, Schooler J, Cohn JF. Deciphering the enigmatic face: the importance of facial dynamics to interpreting subtle facial expressions. Psychol Sci. 2005;16(5):403–410. doi: 10.1111/j.0956-7976.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- 39.Fiorentini C, Viviani P. Is there a dynamic advantage for facial expressions? J Vis. 2011;11(3):1–15. doi: 10.1167/11.3.17. [DOI] [PubMed] [Google Scholar]
- 40.Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain grey and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16(6):1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen AC, Porjesz B, Rangaswamy M, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31(1):156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 42.Adams D, Oliver C. The expression and assessment of emotions and internal states in individuals with severe or profound intellectual disabilities. Clin Psychol Rev. 2011;31(3):293–306. doi: 10.1016/j.cpr.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Echeburua E, Bravo De Medina R, Aizpiri J. Alcoholism and personality disorders: an exploratory study. Alcohol Alcohol. 2005;40(4):323–326. doi: 10.1093/alcalc/agh158. [DOI] [PubMed] [Google Scholar]
- 44.Echeburua E, Bravo De Medina R, Aizpiri J. Comorbidity of alcohol dependence and personality disorders: a comparative study. Alcohol Alcohol. 2007;42(6):618–622. doi: 10.1093/alcalc/agm050. [DOI] [PubMed] [Google Scholar]
- 45.March AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosc Biobehav Rev. 2008;32(3):454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jermingan TL, Butters N, DiTraglia G, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15(3):418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 47.Hutner N, Osacar-Berman M. Visual laterality for the perception of emotional words in alcoholic and aging individuals. J Stud Alcohol. 1996;57(2):144–154. doi: 10.15288/jsa.1996.57.144. [DOI] [PubMed] [Google Scholar]
- 48.Schandler SL, Thomas CS, Cohen MJ. Spatial learning deficits in preschool children of alcoholics. Alcohol Clin Exp Res. 1995;19(4):1067–1072. doi: 10.1111/j.1530-0277.1995.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 49.Popova S, Rehm J, Patra J, et al. Comparing alcohol consumption in central and eastern Europe to other European countries. Alcohol Alcohol. 2007;42(5):465–473. doi: 10.1093/alcalc/agl124. [DOI] [PubMed] [Google Scholar]
- 50.Laranjeira R, Pinsky I, Sanches M, et al. Alcohol use patterns among Brazilian adults. Rev Bras Psiquiatr. 2010;32(3):231–232. doi: 10.1590/s1516-44462009005000012. [DOI] [PubMed] [Google Scholar]