Abstract
This study is to explore the association of adverse cardiovascular events with B vitamins supplementation. Rev.Man 5.1 and Stata 11.0 software were applied for the meta-analysis. The number of cardiovascular events was collected and calculated using indicates of odds ratio and 95% confidence intervals in a fixed-effects or a random-effects model when appropriate. The study includes 15 studies which consists of 37,358 study objects (experimental group: 19,601; control group: 17,757). This study showed that the pooled ORs was 1.01 (95% CI = 0.96~1.06, P > 0.05) for objects with Experimental group (B vitamins supplementation) vs. Control group (placebo or regular treatment), which suggests no significant differences were found in the overall effect of the number of cardiovascular events between the two groups. Further stratification of subgroup analysis indicates no significant differences were found between the two groups as well. There were also no publication bias existing by the Egger’s linear regression test (P > 0.05). Our result indicates that the number of cardiovascular events in experimental group using B vitamins supplementation during the treatment is equal to placebo or regular treatment group thus further studies is necessary.
Keywords: B vitamins supplementation, folic acid, cardiovascular events, meta-analysis
Introduction
Cardiovascular disease is one of the main risks of death around the world [1,2], the annual mortality is higher than any other diseases. It has been shown to increase with symptomatic disease locations as well [3]. Cardiovascular disease recurrences after acute coronary syndrome remain high [4], patients with cardiovascular disease risk is difficult to distinguished [5]. Study showed that high plasma homocysteine level is a risk factor which influences mortality [6] and cardiovascular disease [7]. Study also suggested folic acid, vitamins B6 and vitamins B12 may lower homocysteine level after intake from diet [8]. B vitamins supplementation can lower plasma total homocysteine level [9]. Cardiovascular diseases rates would be higher with elevated plasma homocysteine levels [10], but whether elevated plasma homocysteine levels were associated with cardiovascular disease is uncertain, and it was indicated that the association between them was causal [11]. Current studies showed that the supplementation of vitamin B reduces the risk of hyperhomocysteinemia, but it is uncertain if vitamin B supplementation reduces the rate of cardiovascular diseases [12]. Furthermore, recent studies with vascular diseases failed to prove the association between B-vitamin supplementation and cardiovascular diseases [13].
The association between B vitamins supplementation and adverse cardiovascular events for objects in the studies is controversial [8,10,11,13-17]. In order to achieve a comprehensive understanding regarding the effect of the difference between B vitamins supplementation and placebo of the objects in the study, we consider to perform a meta-analysis of the published studies.
Materials and methods
Source of material
We retrieved the relevant trials up to September 2013 from several public databases, including PubMed, Medline, Springer, Elsevier Science Direct, Cochrane Library and Google scholar. The key words “B vitamins supplementation”, “cardiovascular events”, “folic acid”, “complication”, “stroke”, “coronary heart disease”, “study” and “trial” were used for to retrieve data. At the same time, references from searched studies were verified for additional reports. We collected information from all full-published English papers. Meeting or conference abstract papers are excluded.
In the assumption of differences may occur (such as the included literature was not consistent with another investigator), a third investigator (C) will make additional assessment. If the third investigator’ assessment is consistent with one of them, then the discussion should be made for the final decision of the included literatures.
Inclusion and exclusion standards of studies
The comparison between experimental group (B vitamins supplementation) and control group (placebo or regular treatment) were provided in the papers, the study design was randomized controlled trial (RCT), the effect size of the number of cardiovascular events was odds ratio (OR), and sample size or range of age were not limited. Studies which not described cardiovascular events data in the review or report, reduplicated studies or records, and not comparing experimental group vs control group were excluded.
Evaluation of quality and extraction of data
We evaluated the quality of the study using JADAD score. We developed and extracted the data after all investigators received prior training. Data items including study details (e.g., the first author’s name, research year of the study, year publication, location of participants, design of studies.), and participants characteristics (e.g., age, gender and sample size). Two investigators (A and D) extracted the data independently using the standard protocol, and the third investigator (E) reviewed their results of studies. Discrepancies were resolved by discussing within our research team or contacting the original investigators via e-mail. We recorded the first author’s name, year of publication, sample size, country, mean duration of follow up, and folic acid food reinforcement in experimental group vs. control group.
Meta-analysis methods
The meta-analysis combined the OR of adverse cardiovascular events in the objects of study between experimental group and control group, and then studies were stratified by sample size, mean duration of follow-up and folic acid food reinforcement, using subgroup analysis.
The overall or pooled estimation of the Odds ratio (OR) was obtained using Mantel-Haenszel method in the fixed effect model [18] or using DerSimonian and Laid method in the random effect model [19]. We assessed the within- and between-study variation or heterogeneity by testing Cochran’s Q-statistic [20]. We also quantified the effect of heterogeneity using I2 = 100% × (Q-df)/Q [21]. A significant Q-statistic (P < 0.10) or I2-statistic (I2 > 50%) indicates heterogeneity across the studies, and then the random effects model was used for meta-analysis. The fixed effects model was used as alternative.
Evaluation of publication bias
We evaluated the publication bias using Egger’s linear regression test [22], which measures funnel plot asymmetry by the natural logarithm scale of the effect size.
Statistical analysis
The meta-analysis was performed using the Review Manager 5.1 software (Cochrane Collaboration, http://ims.cochrane.org/revman), and the publication bias of the included studies were calculated using the STATA package v.11.0 (Stata Corporation, College Station, TX, USA). All the P-values were two-sided. P value less than 0.05 considered statistically significant, while P value less than 0.1 considered statistically significant in heterogeneity analysis.
Results
Characteristics of eligible studies
Overall, 1,062 papers potentially relevant to the search terms (PubMed: 352; Medline: 108; Springer: 153; Elsevier Science Direct: 99; Cochrane Library: 31; Google Scholar: 319). Figure 1 illustrates the study selection process. After removing duplicates or irrelevant studies, 96 studies were potentially relevant. During abstracts screening, 49 articles were excluded (22 were review articles; 37 not report the cardiovascular events). Forty seven studies remained for full publication review, 32 of which were excluded (15 only reports B vitamin supplementation data but no comparison; 17 provides no available data).
Figure 1.
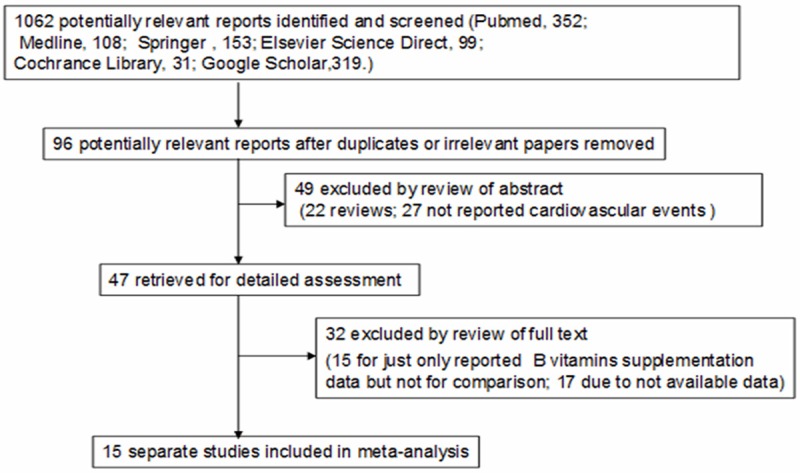
Flow diagram for the selection of studies.
Table 1 showed 15 studies [6-17,23-25] in the meta-analysis, and the characteristics of included studies were presented. The included studies were published between 2002 and 2010. A total of 37,358 objects of study (experimental group: 19,601; control group: 17,757) were considered in the meta-analysis. The studies’ sample size was between 81 and 12,064, and the average age was between 56.0 and 72.2 years old. Six studies reached 5 in Jadad score, six studies reached 4, and the rest of three studies were 3 (Table 2).
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Country | Sample size | Mean duration of follow-up, months | Folic acid food reinforcement | Experimental group (B vitamins supplementation) | Control group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Sample size | Age, years (mean) | Therapeutic schedule | Male (%) | Sample size | Age, years (mean) | Therapeutic schedule | Male (%) | |||||
| Schnyder, 2002. [23] | Switzerland | 553 | 6 | No | 272 | 63.4±10.6 | Folic acid (1 mg/d), vitamin B12 (cyanocobalamin, 0.4 mg/d), and vitamin B6 (pyridoxine hydrochloride, 10 mg/d) | 79 | 281 | 61.8±11.0 | Placebo | 82 |
| Righetti, 2003. [14] | Italy | 81 | 12 | No | 51 | 64±2 | Folic acid (5 or 15 mg/d) | 45 | 30 | 64±3 | Regular treatment | 43 |
| Toole, 2004. [24] | USA | 3680 | 24 | Yes | 1827 | 66.4±10.8 | Folic acid (2.5 mg/d), vitamin B12 (0.4 mg/d), and vitamin B6 (25 mg/d) | 62 | 1853 | 66.2±10.8 | Folic acid (0.02 mg/d), vitamin B12 (6 micro g/d), and vitamin B6 (0.2 mg/d) | 62 |
| Wrone, 2004. [25] | USA | 510 | 24 | Yes | 342 | 59.5±15.4 | Folic acid (5 or 15 mg/d) | 50 | 168 | 61.3±14.6 | Folic acid (1 mg/d), | 50 |
| Liem, 2005. [15] | The Netherlands | 593 | 42 | No | 300 | NA | Folic acid (0.5 mg/d) | NA | 293 | NA | Regular treatment | NA |
| Zoungas, 2006. [16] | Australia | 315 | 43 | Yes | 156 | 56±13 | Folic acid (15 mg/d) | 73 | 159 | 56±14 | Placebo | 63 |
| Bonaa, 2006. [7] | Norway | 2815 | 36 | No | 1872 | 63.2±11.7 | Folic acid (0.8 mg/d), vitamin B12 (0.4 mg/d), and vitamin B6 (40 mg/d) | 73 | 943 | 62.6±11.4 | Placebo | 75 |
| Lonn, 2006. [8] | Canada | 5522 | 60 | Yes | 2758 | 68.8±7.1 | Folic acid (2.5 mg/d), vitamin B12 (1 mg/d), and vitamin B6 (50 mg/d) | 71 | 2764 | 68.9±6.8 | Placebo | 71 |
| Righetti, 2006. [12] | Italy | 88 | 29 | No | 37 | 63.9±1.6 | Folic acid (5 mg/d) | 65 | 51 | 65.1±1.9 | Regular treatment | 49 |
| Jamison, 2007. [6] | USA | 2056 | 38 | Yes | 1032 | 65.4±12.0 | Folic acid (40 mg/d), vitamin B12 (2 mg/d), and vitamin B6 (100 mg/d) | 98 | 1024 | 66.2±11.5 | Placebo | 98 |
| Heijer, 2007. [17] | The Netherlands | 701 | 36 | No | 353 | NA | Folic acid (5 mg/d), vitamin B12 (0.4 mg/d), and vitamin B6 (50 mg/d) | 52 | 348 | NA | Placebo | 51 |
| Albert, 2008. [13] | USA | 5442 | 87 | Yes | 2721 | 62.8±8.8 | Folic acid (2.5 mg/d), vitamin B12 (1 mg/d), and vitamin B6 (50 mg/d) | NA | 2721 | 62.8±8.8 | Placebo | NA |
| Mann, 2008. [10] | Canada | 619 | 60 | Yes | 307 | 72.2±6.6 | Folic acid (2.5 mg/d), vitamin B12 (1 mg/d), and vitamin B6 (50 mg/d) | 67 | 312 | 72.2±6.5 | Placebo | 66 |
| Ebbing, 2008. [9] | Norway | 2319 | 38 | No | 1540 | 61.4±10.1 | Folic acid (0.8 mg/d), vitamin B12 (0.4 mg/d), and vitamin B6 (40 mg/d) | 80 | 779 | 62.0±9.9 | Placebo | 77 |
| Armitage, 2010. [11] | The United Kingdom | 12064 | 80 | No | 6033 | NA | Folic acid (2 mg/d), vitamin B12 (1 mg/d) | 83 | 6031 | NA | Placebo | 83 |
Table 2.
Jadad scoring items of each eligible study for meta-analysis
| Study | Was the study randomized? | Was the randomization method described and appropriate? | Was the study described as double-blind? | Was the method of blinding described and appropriate? | Was there a description of withdrawals and dropouts? | Jadad scores |
|---|---|---|---|---|---|---|
| Schnyder, 2002. | Yes | NA | Yes | Yes | Yes | 4 |
| Righetti, 2003. | Yes | Yes | No | No | Yes | 3 |
| Toole, 2004. | Yes | Yes | Yes | Yes | Yes | 5 |
| Wrone, 2004. | Yes | Yes | Yes | Yes | Yes | 5 |
| Liem, 2005. | Yes | Yes | No | No | Yes | 3 |
| Zoungas, 2006. | Yes | NA | Yes | Yes | Yes | 4 |
| Bonaa, 2006. | Yes | Yes | Yes | Yes | Yes | 5 |
| Lonn, 2006. | Yes | Yes | Yes | Yes | Yes | 5 |
| Righetti, 2006. | Yes | Yes | No | No | Yes | 3 |
| Jamison, 2007. | Yes | NA | Yes | Yes | Yes | 4 |
| Heijer, 2007 | Yes | Yes | Yes | NA | Yes | 4 |
| Albert, 2008. | Yes | Yes | Yes | Yes | Yes | 5 |
| Mann, 2008. | Yes | Yes | Yes | NA | Yes | 4 |
| Ebbing, 2008. | Yes | Yes | Yes | NA | Yes | 4 |
| Armitage, 2010. | Yes | Yes | Yes | Yes | Yes | 5 |
Overall effects of the number of cardiovascular events
The summary of the meta-analysis for the number of cardiovascular events between experimental group and control group is shown in Table 3 and Figure 2. There were 15 separate studies consisting of 37358 objects of study (experimental group: 19601; control group: 17757) been analyzed in the meta-analysis. No heterogeneities were found between studies (Q2 = 16.71, I2 = 16.4%, P > 0.1) were found, so we used the fixed effects model to compare the number of cardiovascular events between this two groups. The overall meta-analysis showed that there were significant differences (OR = 1.01, 95% CI = 0.96 to 1.06, P > 0.05) between the two groups, suggesting that the number of cardiovascular events of study objects in experimental group may be higher than control group.
Table 3.
Pooled odds ratios for experimental group versus control group in meta-analyses
| Subgroups | No. of studies | Random model | Test of heterogeneity | Egger’s test for publication bias | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| OR (95% CI) | Z | P value | Q | P value | I2 (%) | t | P value | ||
| Overall effects | 15 | 1.01 (0.96, 1.06) | 0.28 | 0.78 | 16.74 | 0.27 | 16.4 | -1.80 | 0.09 |
| Sample size | |||||||||
| More than 1000 | 7 | 1.02 (0.97, 1.08) | 0.80 | 0.42 | 3.78 | 0.71 | 0.0 | 0.33 | 0.75 |
| Less than or equal to 1000 | 8 | 0.87 (0.73, 1.03) | 1.62 | 0.11 | 9.79 | 0.20 | 28.5 | -1.11 | 0.31 |
| Mean duration of follow-up | |||||||||
| More than 36 months | 8 | 1.02 (0.96, 1.08) | 0.58 | 0.56 | 5.7 | 0.58 | 0.0 | -0.44 | 0.68 |
| Less than or equal to 36 months | 7 | 0.97 (0.86, 1.09) | 0.58 | 0.56 | 10.45 | 0.11 | 42.6 | -1.69 | 0.15 |
| Folic acid food reinforcement | |||||||||
| Yes | 7 | 0.99 (0.92, 1.07) | 0.21 | 0.83 | 3.88 | 0.69 | 0.0 | 0.93 | 0.39 |
| No | 8 | 1.02 (0.95, 1.09) | 0.58 | 0.56 | 12.55 | 0.08 | 44.2 | -2.44 | 0.05 |
Figure 2.
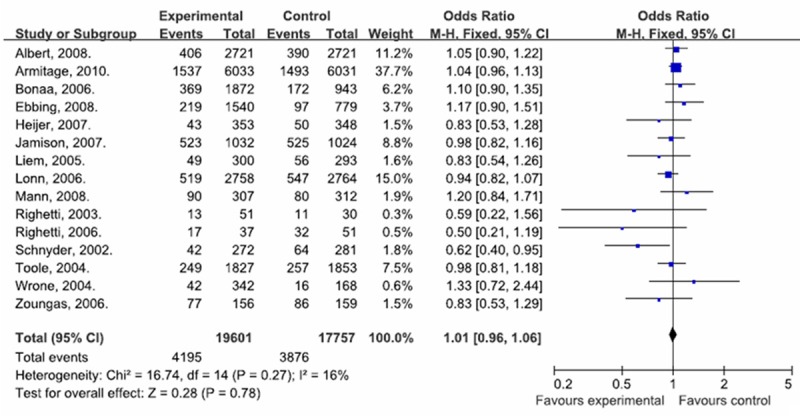
Forest plot of cardiovascular events with experimental group vs. control group.
Subgroup analyses of the number of cardiovascular events
We performed subgroup analyses stratified by sample size, mean duration of follow-up and folic acid food reinforcement. As shown in Table 3, the further stratification of subgroup analysis indicates that for patients with experimental group vs. control group the pooled OR was 1.02 (95% CI = 0.97~1.08, P > 0.05) in studies with sample size more than 1,000; 0.87 (95% CI = 0.73~1.03, P > 0.05) in studies with sample size less than or equal to 1000; 1.02 (95% CI = 0.96~1.08, P > 0.05) in studies with mean duration of follow-up more than 36 months; 0.97 (95% CI = 0.86~1.09, P > 0.05) in studies with mean duration of follow-up less than or equal to 36 months; 0.99 (95% CI = 0.92~1.07, P > 0.05) in studies with folic acid food reinforcement; and 1.02 (95% CI = 0.95~1.09, P > 0.05) in studies without folic acid food reinforcement, respectively.
Evaluation of publication bias analysis
The Egger’s linear regression test (Table 3) showed no publication bias existing in our study (P > 0.05).
Discussion
Many studies [8,10-12,16,17] reported the comparison of effect between B vitamins supplementation and adverse cardiovascular events. Nevertheless, the studies indicate different consequences due to low statistical power or small sample size. To explore and signify the association, we performed a meta-analysis of 15 studies which includes 37,358 objects of study (experimental group: 19601; control group: 17757). The meta-analysis showed the pooled OR was 1.01 (95% CI = 0.96~1.06, P > 0.05) for objects with B vitamins supplementation vs. Placebo, which suggested no significant differences were found in the overall effect of the number of cardiovascular events between the two groups. Further stratification of subgroup analysis indicates no significant differences between the two groups as well.
Observational studies have demonstrated that the concentration of total homocysteine in blood is associated with risk of coronary artery disease (CAD) and stroke [26]. Clinicians and trialists have difficulty with identifying which patients is highest risk for cardiovascular events [5]. Study suggested that serum homocysteine level would be associated with cardiovascular events, which predicts that patients would happen adverse cardiac events with the serum homocysteine level [23], thus high serum total homocysteine in the body should be considered as a risk factor for cardiovascular events in human [25]. Because plasma total homocysteine levels can be easily lowered by oral administration of folic acid, trials to investigate whether cardiovascular disease could be prevented by such homocysteine-lowering therapy were called for [26].
Several limitations of this study should be discussed. Firstly, the sample size of some recruited studies were small [15], more high-quality studies is needed to test and verify the association of adverse cardiovascular events with B vitamins supplementation. Therefore, we performed earnest study for all-published reports and used explicit means for research selection, data extraction and analysis.
Conclusions
Our study indicates that the number of cardiovascular events in experimental group with B vitamins supplementation during the treatment is equal to placebo or regular treatment group. Thus, further studies are necessary.
Acknowledgements
We would like to thank all respondents of the study and all the people who give the help for this study.
Disclosure of conflict of interest
The authors have declared that no competing interests exist.
References
- 1.Purwanto , Eswaran C, Logeswaran R. Prediction models for early risk detection of cardiovascular event. J Med Syst. 2012;36:521–31. doi: 10.1007/s10916-010-9497-9. [DOI] [PubMed] [Google Scholar]
- 2.Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, van der Post JA, Porath M, Ponjee G, Tamsma JT, Mol BW, de Groot CJ. 10-Year cardiovascular event risks for women who experienced hypertensive disorders in late pregnancy: the HyRAS study. BMC Pregnancy Childbirth. 2010;10:28. doi: 10.1186/1471-2393-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suarez C, Zeymer U, Limbourg T, Baumgartner I, Cacoub P, Poldermans D, Röther J, Bhatt DL, Steg PG REACH Registry Investigators. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc Med. 2010;15:259–65. doi: 10.1177/1358863X10373299. [DOI] [PubMed] [Google Scholar]
- 4.Carballo D, Auer R, Carballo S, Windecker S, Matter C, Luscher T, Vogt P, Perneger T, Mach F, Rodondi N, Keller PF SPUM-ACS. [A Swiss multicentric project to improve the prevention of cardiovascular event recurrence after acute coronary syndromes] . Rev Med Suisse. 2010;6:518, 520–2, 524. [PubMed] [Google Scholar]
- 5.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–7. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 6.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM Veterans Affairs Site Investigators. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–70. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 7.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 8.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Jr Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 9.Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygård O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 10.Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, Yusuf S, Lonn EM HOPE-2 investigators. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease--results of the renal Hope-2 study. Nephrol Dial Transplant. 2008;23:645–53. doi: 10.1093/ndt/gfm485. [DOI] [PubMed] [Google Scholar]
- 11.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–94. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 12.Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24:379–86. doi: 10.1159/000093680. [DOI] [PubMed] [Google Scholar]
- 13.Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–36. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Righetti M, Ferrario GM, Milani S, Serbelloni P, La Rosa L, Uccellini M, Sessa A. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003;9:PI19–24. [PubMed] [Google Scholar]
- 15.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart. 2005;91:1213–4. doi: 10.1136/hrt.2004.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–16. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 17.den Heijer M, Willems HP, Blom HJ, Gerrits WB, Cattaneo M, Eichinger S, Rosendaal FR, Bos GM. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood. 2007;109:139–44. doi: 10.1182/blood-2006-04-014654. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. BMJ Books; 2005. pp. 285–312. [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288:973–9. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 24.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 25.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–6. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 26.Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygård O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]


