Abstract
Objective: To investigate the joint protective effect of exogenous neuroglobin (Ngb) and hemin on ischemic brain tissue in rats and its possible mechanisms. Methods: Sprague Dawley rats (n = 175) were randomly divided into five groups (n = 35): sham, ischemia, hemin intervention, Ngb plasmid intervention, and hemin and plasmid joint intervention. The classic MCAO rat model of focal cerebral ischemia was used. After recovery from anaesthesia, neurobehavioral testing was performed. The following factors were measured 24 hours post-surgery: brain water content, infarct volume ratio, neuron apoptosis detected by in situ cell apoptosis technology (TUNEL), Bcl-2 protein expression detected by immunofluorescence, and Ngb and Bcl-2 protein expression analyzed by western blot. Results: In the Ngb plasmid and hemin joint intervention group, there were significant reductions (i.e., improvements) in neurobehavioral scores, brain water content, and infarct volume ratio. The reduction of the number of apoptotic neurons and the increase in Ngb protein and Bcl-2 protein expression in this group were both significantly different from the sham group (P < 0.05). Conclusion: In the event of focal cerebral ischemia in rats, the joint action of exogenous Ngb and hemin could strengthen the inhibition of cell apoptosis, which achieves its protection effect on ischemic brain tissues, possibly by up-regulating Bcl-2 protein expression.
Keywords: Neuroglobin, hemin, focal cerebral ischemia, neuroprotection, apoptosis
Introduction
Neuroglobin (Ngb), discovered in 2000, is an oxygen-carrying globulin mainly present in the central nervous system [1,2]. Research has confirmed that reduction in Ngb protein expression during brain ischemia could lead to hypoxic damage to neurons. Overexpression of Ngb globulin can improve neuronal survival under hypoxic conditions such as ischemic-hypoxic brain injuries [3-5]. Hemin induces significant increases in Ngb protein expression in vitro, an effect that is dose- and time-dependent. Hemin intervention in rats with focal cerebral ischemia induces endogenous Ngb protein expression, strengthening the protection of neurons. Using plasmid as carrier for exogenous Ngb protein up-regulates Bcl-2 gene expression and inhibits neuronal apoptosis, yielding a protective effect in focal cerebral ischemia. Therefore, this experiment demonstrates the joint protective effect of exogenous Ngb protein carried by plasmid and hemin on rat focal ischemic brain tissues and its possible mechanism.
Materials and methods
Drugs, reagents, and plasmid
Hemin was purchased from Sigma-Aldrich (preparation: Hemin was first dissolved with NaOH; phosphate buffer was then used to adjust its pH to 7.14, with a final concentration of 12.5 g/L). Triphenyltetrazolium chloride (TTC) was purchased from Sigma-Aldrich. TUNEL kit was purchased from Roche, and the DAB chromogenic reagent was purchased from DAKO Corporation. The Bcl-2 immunofluorescence kit was purchased from Jackson. Bovine serum albumin, SDS, glycine, acrylamide and TEMED were purchased from Sigma-Aldrich, and the PVDF membrane was purchased from Millipore. Ngb plasmid (containing green fluorescent EGFP) was prepared by Shanghai Genechem Corporation. Plasmid extraction kit was purchased from Omega with an extract concentration of (800 ± 50) ng/μL.
Animals and grouping
Healthy adult male SD rats (n = 175) weighing 250-300 g were provided by the Experimental Animal Center of Chongqing Medical University. They were divided into five groups of 35: sham (B), ischemia (M), hemin intervention (H), plasmid intervention (P), hemin and plasmid joint intervention (H + P). Sham group rats received an incision on the neck to separate the neck blood vessels and their branches, without artery ligation or the line-embolism blocking operation. The ischemia group was used in a rat focal cerebral ischemia model with MCAO. The hemin intervention group received intraperitoneal injection (concentration 12.5 g/L, 50 mg/kg) 12 hours before modeling of MCAO. The plasmid intervention group received intracranial injection of 3.3 μL recombinant plasmid at 0.25 μL/min using sterotactic technique 24 hours before modeling, and the hemin and plasmid group was treated with both interventions. Each group of rats was divided again into five groups of seven. Chloral hydrate (10%, 300 mg/kg) was injected intraperitoneally into each rat. Heating units were used during surgery to maintain body temperature. Breathing and heart rate were also monitored during surgery. Neurobehavioral testing was performed after mice recovered from anaesthesia. Brain water content, infarct volume ratio, TUNEL staining, immunofluorescence staining, and western blot analysis were done 24 hours post-surgery.
MCAO model
The Longa method was used [6] to insert line embolism at the bifurcation of the right common carotid artery to block the right middle cerebral artery. The animals were euthanized by decapitation 24 hours after modeling.
Neurobehavioral score
Rats were scored when they awakened after anesthesia using the Longa 5-point scale [6] as follows: 4 points: being unable to walk spontaneously with disturbance to consciousness; 3 points: swaying to the left when walking; 2 points: circling to the left; 1 point: being unable to fully extend the left front paw; 0 point: no neurological deficits. Rats with scores 1-3 were included in experimental groups, whereas those with scores 0 or 4 were eliminated.
Brain water content detection
The wet and dry weight method was used. Fresh brain tissue, immediately after being weighed for its wet weight, was placed in an oven for 24 hours until its weight became constant (dry weight). Brain water content = (wet weight - dry weight)/wet weight × 100%.
Infarct volume ratio calculation
Seven rats from each group were decapitated 24 hours after surgery and the brains extracted. Olfactory bulb, cerebellum, and lower brain stem were removed. Coronal slices (2 mm spacing line) were incubated in a 2% TTC solution in a dark incubator at 37°C for 30 min. Normal brain tissue is a dark red color, whereas infarct tissue is pale white. The brain slices were captured and edited by Photoshop software to calculate infarct volume versus total brain volume and subsequently determine the infarct volume ratio: infarct volume/whole brain volume %.
In situ apoptosis detection (TUNEL)
Paraffin-embedded sections of rat brain tissue underwent dewaxing, membrane rupture repair by antigen, endogenous peroxidase activity blocking, DAB staining, re-staining, hydrochloric acid alcohol differentiation, back blue, and dehydration. Their pictures were taken and analyzed after being mounted with neutral gum.
Immunofluorescence staining
Rat brain tissue slices underwent dewaxing, antigen heat-revival, overnight primary antigen incubation, secondary antibody incubation, DAPI staining, and were eventually mounted with fluorescence-quenching resistant reagent. Observation and image acquisition were carried out using an inverted fluorescence microscope.
Western blot analysis
Using protein quantification, electrophoresis, electric transfer, hybridization and detection, quantitative analysis was performed on Ngb and Bcl-2 protein. Optical density scanning was performed on each sample strip to determine and statistically analyze the optical density value of each group.
Statistical analysis
SPSS statistical software was used for analysis; normal-distribution data was presented as mean ± standard deviation (x ± s). The experimental groups were compared using one-way ANOVA, and inter-group paired comparisons were examined by LSD.
Results
In a rat model of cerebral ischemia, a dual regimen of exogenous neuroglobin and hemin is more beneficial for neuroprotection than either treatment alone
Because they are simple operations and give satisfactory results, rat MCAO models are widely used in the study of focal cerebral ischemia. When the rats awaken from a successful operation, there will be a series of specific behaviors, such as extending left front paw, circling to the left, swaying to the left, circling to the side when lifting by the tail, or right Horner’s syndrome. Grading the severity of the behavior indirectly assesses the severity of focal cerebral ischemia. When focal cerebral ischemia occurs, a large number of neurons are damaged and edema follows, so that the brain shows a corresponding increase in water content. By measuring the water content of the brain, we can estimate the degree of severity of the brain infarct. TTC staining, which directly shows cerebral infarct volume, provides us with a very intuitive understanding of the severity of cerebral infarction. Finally, when we compare the severity of cerebral infarction between groups given different interventions, we can assess the strength of the neuroprotection offered by different drugs.
After the operation rats from groups except the group sham presented with an inability to extend the left front paw, circling to the left, swaying to the left, circling to the side when lifting by the tail, or right Horner’s syndrome [6]. The sham group showed no neurological defects.
Compared with the sham group, the four remaining groups of rats had significantly higher neurobehavioral scores, with statistically significant differences (P < 0.05) (Table 1). The three intervention groups all received lower scores than the ischemia group, with statistically significant differences (P < 0.05). The hemin and plasmid joint intervention group (H + P) received lower scores than the single-factor intervention groups, with statistically significant differences (P < 0.05). The scores the two single-factor intervention groups received were not statistically different from each another (P > 0.05).
Table 1.
Comparison of neurological function scores in different groups of rats
| Group | Neurological score (point) |
|---|---|
| Group B | 0# |
| Group M | 2.71 ± 0.45 |
| Group H | 2.00 ± 0.53*,# |
| Group P | 1.86 ± 0.35*,# |
| Group H + P | 1.28 ± 0.45* |
Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P). n = 7, x ± s,
P < 0.05 vs. Group M;
P < 0.05 vs. Group H + P.
Compared with the sham group, the other groups of rats had significantly higher infarct volume, with statistically significant differences (P < 0.05) (Figure 1 and Table 2). The three intervention groups had lower infarct volume ratio than the ischemic group, with statistically significant differences (P < 0.05). The H + P group showed statistically significantly lower infarct volume ratio than the single-factor intervention groups (P < 0.05).
Figure 1.
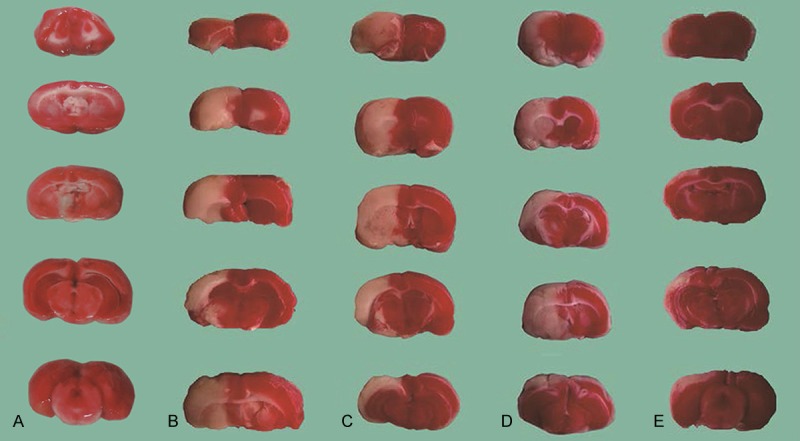
Rats’ brain TTC staining results of different treatment groups. Every rat from each group was decapitated 24 hours after surgery. 2-mm spacing line coronal brain slices were incubated in a 2% TTC solution in the dark at 37°C for 30 min. Normal brain tissue carries a dark red color, whereas infarct brain tissue is pale white. Sham group (A), ischemia group (B), hemin intervention group (C), plasmid intervention group (D), hemin and plasmid joint intervention group (E).
Table 2.
Comparison of infarct volume ratio in different groups of rats
| Group | Brain infarct volume (%) |
|---|---|
| Group B | 0# |
| Group M | 47.36 ± 1.75 |
| Group H | 36.51 ± 1.66*,# |
| Group P | 35.93 ± 1.99*,# |
| Group H + P | 22.90 ± 1.73* |
Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P). n = 7, x ± s,
P < 0.05 vs. Group M;
P < 0.05 vs. Group H + P.
Compared with the sham group, the four other groups of rats had significantly higher brain water content, with statistically significant differences (P < 0.05) (Table 3). The three intervention groups had lower brain water content than the ischemia group, with the differences reaching statistical significance (P < 0.05). The H + P group had lower brain water content than the single-factor intervention groups, also reaching statistical significance (P < 0.05). However, the differences in brain water content between the two single-factor intervention groups were not statistically significant (P > 0.05).
Table 3.
Comparison of brain water content in different groups of rats
| Group | Brain water content (%) |
|---|---|
| Group B | 76.00 ± 0.51# |
| Group M | 80.88 ± 0.23 |
| Group H | 79.55 ± 0.31*,# |
| Group P | 79.65 ± 0.27*,# |
| Group H + P | 78.44 ± 0.20* |
Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P). n = 7, x ± s,
P < 0.05 vs. Group M;
P < 0.05 vs. Group H + P.
Additive effects of exogenous neuroglobin and hemin in reducing the numbers of TUNEL-positive cells
Ischemic central neurons undergo apoptosis after acute cerebral ischemia. Neurons in the ischemic penumbra still maintain basic cell activity, although electrophysiological activity temporarily disappears [7]. If timely and effective treatments are given during the acute ischemic period, the apoptotic process can be reversed and nerve function restored [8-11]. Therefore, after ischemia it is crucial to restore the normal physiological function of neurons as much as possible by inhibiting neuronal apoptosis. In order to assess the protective effect of exogenous neuroglobin and/or hemin in reducing neuronal apoptosis, rat brain tissue slices were examined by in situ cell apoptosis-detection technology. Compared with the sham group, rats in the other groups showed a significant increase in apoptotic neurons (P < 0.05). The two single-factor intervention groups were not significantly different in terms of the number of apoptotic neurons (P > 0.05), but were significantly different from the ischemic group (P < 0.05). The H + P group showed further reduction in the number of apoptotic neurons compared with the two single-factor intervention groups (P < 0.05) (Figure 2).
Figure 2.
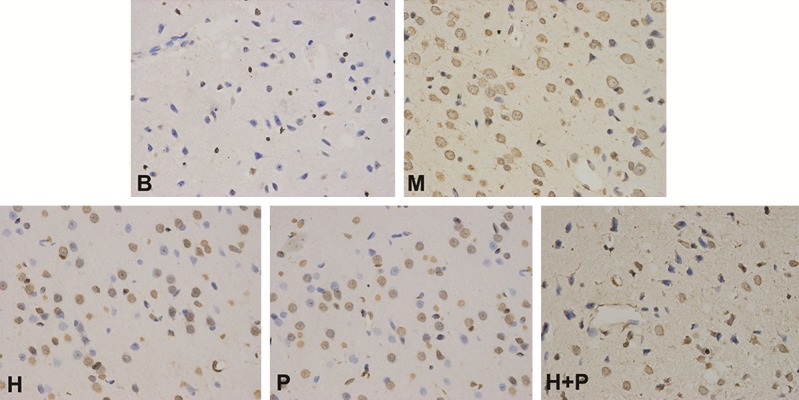
Rats’ cortex TUNEL staining results of different treatment groups. Apoptotic neuronal nuclei show brown staining granules; negative control shows no staining. Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P).
Combinational therapy with exogenous neuroglobin and hemin significantly increases the expression of anti-apoptotic protein Bcl-2
Apoptosis, a mode of programmed cell death under cerebral ischemia, is subject to biological processes influenced by some genes in cells and extracellular factors, including the death receptor-mediated apoptosis pathway and the mitochondrial-mediated pathway [12,13]. The latter is regulated by anti-apoptotic protein Bcl-2 and other Bcl-2 family members [14]: firstly, Bcl-2 family member proteins (e.g. Bcl-2, Bax, Bcl-xL) specifically change the permeability of the mitochondrial membrane; secondly, Bcl-2 family proteins induce mitochondrial permeability transition pore opening. In this study, immunofluorescence staining was used to evaluate the expression of neuronal Bcl-2 protein so that we could gain a better understanding of the relationship among neuroglobin, hemin, and neuronal apoptosis. Immunohistochemical staining of rat brain tissue slices showed lower Bcl-2 protein expression in the ischemia group compared with the other groups of rats (P < 0.05). The two single-factor intervention groups showed no significant difference in Bcl-2 protein expression (P > 0.05), but both showed increased expression compared with the ischemia group (P < 0.05). Finally, the hemin and plasmid joint intervention group showed higher Bcl-2 protein expression compared with the two single-factor intervention groups (P < 0.05) (Figure 3).
Figure 3.
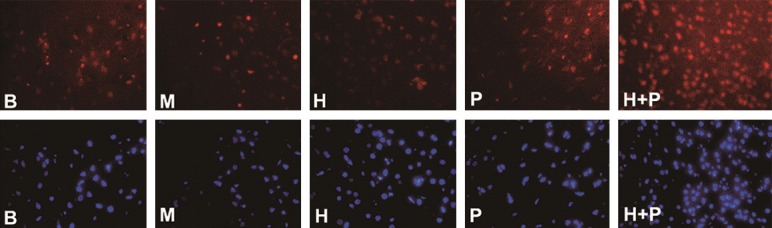
Immunofluorescence staining of rat cortical Bcl-2 protein in different treatment groups. Bcl-2 immunoreactive cells are shown as red particles. Cell nuclei are stained blue and are located in the cytoplasm of neurons. Negative control showed no staining. Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P).
Western blot analysis was used to gain a better understanding of the relationship among neuroglobin, hemin, and Bcl-2 protein. Bcl-2 protein expression levels showed the following: No significant difference between the ischemia and sham groups (P > 0.05). The two single-factor intervention groups showed significantly higher levels than the ischemia group (P < 0.05), but were not significantly from each other (P > 0.05); and the hemin and plasmid joint intervention group was significantly higher than the two single-factor intervention groups (P < 0.05).
Western blot analysis was also used to make sense of the expression of the endogenous neuroglobin. The sham group had significantly lower expression than any treatment group (P < 0.05). The two single-factor intervention groups were significantly higher than the ischemia group (P < 0.05), but were not significantly different from each other (P > 0.05). Finally, the hemin and plasmid joint intervention group had significantly higher levels of Ngb expression than either of the two single-factor intervention groups (P < 0.05) (Figures 4, 5).
Figure 4.
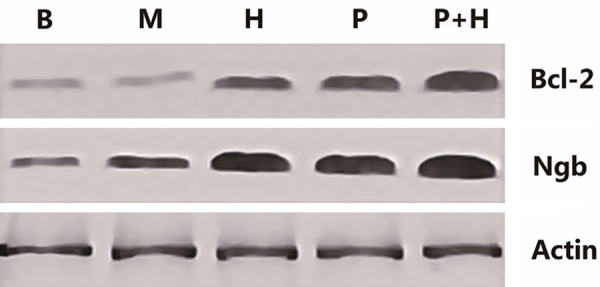
Ngb and Bcl-2 protein expression levels in rat cortex of each treatment group. β-actin is a loading control. Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P).
Figure 5.
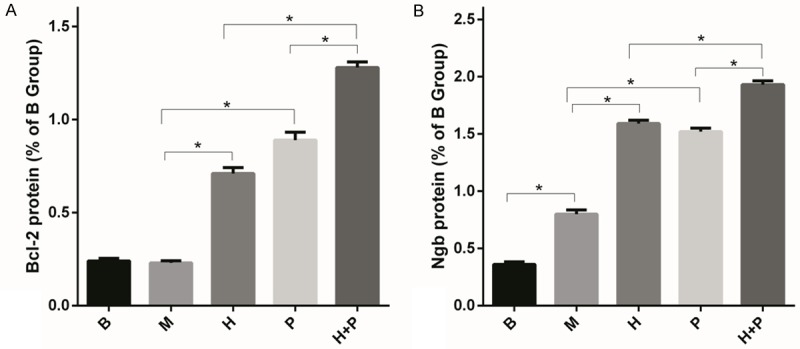
Bcl-2 and Ngb protein expression levels in rat cortex of each treatment group. Figure A shows Bcl-2 protein expression. Figure B shows Ngb protein expression. Sham group (B), ischemia group (M), hemin intervention group (H), plasmid intervention group (P), hemin and plasmid joint intervention group (H + P) (n = 7, *P < 0.05).
Discussion
Ischemic cerebrovascular disease is a serious threat to human health. With the dietary changes already under way, an aging population, and improvement of diagnostic techniques, the incidence and detection rate will both increase substantially. Due to the complexity of cerebral ischemia pathogenesis and the presence of the blood-brain barrier, single-drug therapy often yields unsatisfactory treatment outcomes and problems such as toxicity [15]. Therefore, it is important to discover drugs for prevention and treatment of ischemic cerebrovascular disease and multi-drug joint treatment methods.
Ngb is an oxygen-carrying globulin present mainly in the central nervous system of vertebrate animals [1,2,16]. Similar to myoglobin and hemoglobin, it has high affinity for and reversibly binds to oxygen. Though Ngb levels are relatively low in the brain under normal physiological conditions, in cerebral hypoxic-ischemic events, Ngb levels increase at both the mRNA and protein levels [17]. Using gene overexpression carried by viral vectors for rats with focal cerebral ischemia showed significantly reduced infarct volume compared with the control group [5]. Reduction of Ngb expression using antisense oligonucleotides resulted in significantly higher brain infarct volume compared with the control group [18,19]. All these findings indicate that Ngb plays an important protective role for neurons against hypoxic-ischemic brain damage [20]. Therefore, the effective increase of Ngb content in the central nervous system after acute ischemia seems to be the key to satisfactory protective effects on neurons.
Experimental overexpression of genes commonly makes use of carriers such as viruses and plasmids. However, although viral transfection is efficient, it can easily induce gene mutations, cytotoxicity, can cause inflammation, and has low exogenous gene content. Therefore, it is not suitable for clinical use. Plasmid vectors attracted a lot of research attention because they are non-immunogenic, safe, and have high exogenous gene content [21,22]. Experiments confirmed that plasmid-mediated Ngb gene transfer into the brain could rapidly produce a protein concentration in nerve cells capable of protecting them from or reversing hypoxic-ischemic damage. In addition, Ngb inhibits focal ischemic apoptosis in neurons through up-regulation of Bcl-2 gene expression, protecting focal ischemic tissues [23] and achieving fast and direct protection from hypoxic-ischemic brain injury [24].
Hemin is a product of heme oxidation that is involved in oxygen transportation and storage, energy production, and regulation of oxidative damage. Experiments confirmed that hemin induces significant in vitro enhancement of Ngb expression, in a dose- and time-dependent manner [25]. Experiments also confirmed that hemin induces increased exogenous expression of Ngb [26].
This study showed that in rat focal ischemic events, the combined effects of exogenous Ngb-carrying plasmid and hemin significantly improved neurobehavioral scores, brain water content, infarct volume ratio, number of apoptotic neurons, and Ngb and Bcl-2 protein expression compared with no intervention or single-factor intervention. This suggests that the joint effect of exogenous neuroglobin-carrying plasmid and hemin also induced an increase in Bcl-2 protein expression, possibly through enhancing Ngb protein expression. This inhibited neuronal apoptosis and eventually significantly reduced the number of apoptotic neuronal cells, infarct volume ratio, brain water content, and neurobehavioral scores, ultimately protecting focal ischemic brain tissues. Through novel interventions, different drug-delivery routes, and administration of drug combinations, a more timely, faster, more effective, and safer treatment for ischemia disease was created. This lays the foundation for further clinical applications. The neural interactions between Ngb and hemin merit further study.
Acknowledgements
This work was supported by funds from Chongqing Natural Science Project Fund (2010JJ0254 to J. Z.), National Clinical Key Specialty Construction Project (to J. Z.), and the Bill & Melinda Gates Foundation (to X. W.).
Disclosure of conflict of interest
None.
References
- 1.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 2.Moens L, Dewilde S. Globins in the brain. Nature. 2000;407:461–462. doi: 10.1038/35035181. [DOI] [PubMed] [Google Scholar]
- 3.Guo ZD, Sun XC, Zhang JH. Mechanisms of early brain injury after SAH: matrix metalloproteinase 9. Acta Neurochir Suppl. 2011;110:63–65. doi: 10.1007/978-3-7091-0353-1_11. [DOI] [PubMed] [Google Scholar]
- 4.Sun YJ, Jin KL, Mao XO, Zhu YH, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun YJ, Jin KL, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K. Pathophysiology of brain injury and targets of treatment in acute ischemic stroke. Rinsho Shinkeigaku. 2013;53:1159–1162. doi: 10.5692/clinicalneurol.53.1159. [DOI] [PubMed] [Google Scholar]
- 8.Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61:321–330. doi: 10.1159/000210544. [DOI] [PubMed] [Google Scholar]
- 9.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Wang J, Jiang J, Stavrovskaya IG, Li M, Li W, Wu Q, Zhang X, Luo C, Zhou S, Sirianni AC, Sarkar S, Kristal BS, Friedlander RM, Wang X. N-Acetyl-Serotonin Offers Neuroprotection through Inhibiting Mitochondrial Death Pathways and Autophagic Activation in Experimental Models of Ischemic Injury. J Neurosci. 2014;34:2967–2978. doi: 10.1523/JNEUROSCI.1948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S, Day AL, Kristal BS, Friedlander RM. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40:1877–1885. doi: 10.1161/STROKEAHA.108.540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 13.Wang X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther. 2009;15:345–357. doi: 10.1111/j.1755-5949.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang X, Baranov SV, Zhu S, Huang Z, Fellows-Mayle W, Jiang J, Day AL, Kristal BS, Friedlander RM. Dipyrone inhibits neuronal cell death and diminishes hypoxic/ischemic brain injury. Neurosurgery. 2011;69:942–956. doi: 10.1227/NEU.0b013e318222afb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 2009;14:228–235. doi: 10.1179/135100009X12525712409931. [DOI] [PubMed] [Google Scholar]
- 16.Simon R. Neuroglobin: neuroprotection and neurogenesis. Neurosci Lett. 2013;549:1–2. doi: 10.1016/j.neulet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li RC, Guo SZ, Lee SK, Gozal D. Neuroglobin protects neurons against oxidative stress in global ischemia. J Cereb Blood Flow Metab. 2010;30:1874–1882. doi: 10.1038/jcbfm.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiocchetti M, De Marinis E, Ascenzi P, Marino M. Neuroglobin and neuronal cell survival. Biochim Biophys Acta. 2013;1834:1744–1749. doi: 10.1016/j.bbapap.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Cao YJ, Shibata T, Rainov NG. Hypoxia-inducible transgene expression in differentiated human NT2N neurons--a cell culture model for gene therapy of postischemic neuronal loss. Gene Ther. 2001;8:1357–1362. doi: 10.1038/sj.gt.3301536. [DOI] [PubMed] [Google Scholar]
- 22.Dokka S, Malanga CJ, Shi X, Chen F, Castranova V, Rojanasakul Y. Inhibition of endotoxin-induced lung inflammation by interleukin-10 gene transfer in mice. Am J Physiol Lung Cell Mol Physiol. 2000;279:L872–877. doi: 10.1152/ajplung.2000.279.5.L872. [DOI] [PubMed] [Google Scholar]
- 23.Cho BB, Toledo-Pereyra LH. Caspase-independent programmed cell death following ischemic stroke. J Invest Surg. 2008;21:141–147. doi: 10.1080/08941930802029945. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Tang WY. Cerebroprotection with recombinant neuroglobin plasmid in a rat model of focal cerebral ischemia. Neural Regeneration Research. 2010;5:52–57. [Google Scholar]
- 25.Zhu Y, Sun Y, Jin K, Greenberg DA. Hemin induces neuroglobin expression in neural cells. Blood. 2002;100:2494–2498. doi: 10.1182/blood-2002-01-0280. [DOI] [PubMed] [Google Scholar]
- 26.Song X, Xu R, Xie F, Zhu HY, Zhu J, Wang X. Hemin offers neuroprotection through inducing exogenous neuroglobin in focal cerebral hypoxic-ischemia in rats. Int J Clin Exp Pathol. 2014;7:2163–71. [PMC free article] [PubMed] [Google Scholar]


