Abstract
Objective: To research and reveal Tetrahydroxystilbene glucoside (TSG) anti-thinning effect of dermas on skin of ageing mice. Methods: The dermal layer thickness was measured with hematoxylin-eosin (HE) staining; the levels of collagen and elastic fibers were measured with immunohistochemical staining; the levels of insulin, insulin-like growth factor-1 (IGF-1), and two receptors of insulin and IGF-1 were measured with Elisa kits; the levels of Ca2+ and P were measured with ELISA kits. Results: TSG and Polygonum multiflorum extract (PME) made thicken dermal layer thickness (P<0.01, vs. control group); promoted collagen fiber expression (P<0.01, vs. control group, 22.94% and 28.26% vs. 20.41%); reduced the levels of insulin (P<0.01, vs. control group, 2.50 ng/ml and 2.69 ng/ml vs. 3.04 ng/ml), insulin like growth factor 1 (IGF-1, P<0.01, vs. control group, 154.75 ng/ml and 155.60 ng/ml vs. 209.28 ng/ml), receptors of insulin (P<0.01, vs. control group, 0.423 ng/ml and 0.426 ng/ml vs. 0.648 ng/ml) and IGF-1 (P<0.01, vs. control group, 71.96 ng/ml and 81.68 ng/ml vs. 87.02 ng/ml) in aging mice skin; raised the levels of Ca2+ and P in serum in mice (P<0.01, vs. control group, 1.24 mol/ml and 1.30 mol/ml vs. 1.08 mol/ml; P<0.01, vs. control group, 2.00 mol/ml and 2.03 mol/ml vs. 1.197 mol/ml). Conclusion: TSG and PME showed their protections to skin aging in mice challenged with control groups. It ensured the anti-thinning effect of dermas of TSG and provided two potential factors (insulin/IGF-1 signal pathway and the level of Ca2+) related to skin senescence of aging mice.
Keywords: Tetrahydroxystilbene glucoside, dermal layer thickness, collagen fiber, insulin/IGF-1 signal pathway, Ca2+
Introduction
Skin senescence was characterized as a classical performance of aging mammals or human. The degree of skin senescence depends on high content collagen fiber, natural elastic fiber, and slowly thinning dermal layer that are inflicted on skin senescence symptoms (wrinkle, loose skin, etc). One of the most effective factor combatants in this battle to maintain skin younger is collagen fiber, which locates undesirable senescence symptoms bases in skin’s dermal layer and then heals the ditches made by the bases with increasing age, initiating the “time return” process of skin [1-3]. Strong support force of collagen fiber is essential for prevention of senescence arising from collagen protein loss in skin, and for growing thinner dermal layer during skin aging [4,5]. It has been reported that collagen loss and decreased biosynthesis are accompanied with low level of IGF-1 in serum in connective tissue cell [6,7], and insulin administration in vitro can reverse decreased collagen in diabetic rats [8]. In addition to aforementioned information, more research would be needed in aging related factors IGF-1, insulin, especially the signal pathway they made in while they have so much influence in aging process in other areas on aging mentioned below. Weaken signals of insulin/IGF-1 pathway extended the lifespan of diverse living organisms [9,10], reduced the toxic effects induced by excessive ROS [11].
Anti-aging effect related to insulin/IGF-1 signal pathway tetrahydroxystilbene glucoside (TSG) has been proved in vitro in our prior work [12], and its other benefits: a strong antioxidant in vitro [13-15], a modifier of sirtuin family [16] and a useful treatment for brain injury of mice in vitro in aging progress [17-19] were also reported, so we wondered the effect of TSG against skin aging in mice.
If collagen loss, elastic degeneration, dermal thinner, and the signals of insulin/IGF-1 signal pathway response to skin aging are affected by TSG, medication of TSG should ensure these beneficial effects. We ensured the effect on Klotho protein of TSG in aging mice induced by d-galactose and the effect on both Klotho protein and insulin/IGF-1 signal pathway in aging mice, which proved the TSG and PME’s dosage used in this study. In this study, we tested the hypothesis aforementioned by assessing the effects of TSG on skin aging of mice.
Materials and methods
Animals
Male Kunming mice aged 18 months were purchased from the Center of Experimental Animals of Fourth Military Medical University. All of the animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical University. Animal Certificate No. SXCK Jun 2007-007; experimental environment level: SPF.
Reagent
PME (TSG≥57%, and the remaining portion is anthraquinone, No. 2012030302) and TSG (≥95%, No. 2012030502) were provided by Department of Natural Medicine, School of Pharmacy, and Fourth Military Medical University. The purities of reagents abovementioned were determined by HPLC. Trizol (Dalian TaKaRa Biotechnology Co. Ltd.), Mice ELISA Kits (Shanghai Bogoo Biotechnology Co. Ltd.).
Treatment protocol
24 aging male mice were divided into three groups randomly: control group treated with saline, TSG group treated with 180 mg·kg-1 (according to preliminary experiment’s data) TSG and PME group treated with 280 mg·kg-1 PME by gastric lavage for 8 weeks after the body weight recording at eight every morning. The animals were sacrificed on the 57th day after blood donation under anesthesia.
Sample collection
Aging skin of mice was resected 2×2 cm, and parts of them were fixed with paraformaldehyde and prepared for paraffin. Skins used for making tissue homogenate were kept in paraformaldehydes which had been added with Trizol. They were ground into powder using liquid nitrogen; 200 μL phosphate buffer saline (PBS) was added and the solution was first homogenized and then centrifuged (3000 rpm for 15 minutes). Supernatant was discarded and a lysate containing proteinase inhibitor (Shanghai Bogoo Biotechnology Co. Ltd., Shanghai, China) was added to the pellet and it was stored at 4°C for 30 minutes. Supernatant was collected after centrifuging at 15000 rpm for 45 mins at 4°C.
ELISA kits analysis
The levels of insulin, IGF-1, and their receptors were measured by sandwich ELISA with the Mice ELISA Kit (Shanghai Bogoo Biotechnology Co. Ltd., Shanghai, China) and according to the requirements of the specification of operation.
Immunohistochemical analysis
Immunohistochemical analysis was performed using the Enhance Labeled Polymer System (ELPS). Portions of organs were embedded in paraffin for immunohistochemistry. The paraffin sections were deparaffinized and washed thrice with PBS (0.01 moL/L pH 7.4) for 3 mins each, repaired with thermal induction (pH 6.0 0.01 M, CB 20 mins), cooled at ambient temperature, washed with PBS. Endogenous peroxidase was inhibited by treating with 0.3% H2O2 for 20 mins at ambient temperature. Then the samples were then washed with PBS, incubated with 20% serum of sheep for 30 mins at room temperature without washing, incubated with SKL 1:100 at 37°C for 2 h, washed with PBS (3×3 mins), treated with EnVision agent (HRP-M) at 37°C for 30 min, washed again with PBS (3×3 mins), stained with DAB (50 mg of 3’-3-diaminobenzidine added to 100 ml of TBS, agitated, and added to 0.03% H2O2) for 10 mins, and with hematoxylin blue in hot water. The samples were then blow-dried, mounted with balsam, and observed under microscope, then analyzed the photo with MIQAS (medical image quantitative analysis system) and the software (Medical image quantitative analysis software).
Statistical analysis
The SPSS10.0 analysis statistics software was used. Results are expressed as means ± S.E. Statistical differences between each two groups were tested by one way ANOVA, p<0.05 was considered statistically significant.
Results
TSG protects the dermal layer from getting thinner in skin
The thickness of dermal layer in TSG group was significantly thicker than the other groups (p<0.01, vs. control group). The results are shown in Figure 1A and 1B.
Figure 1.
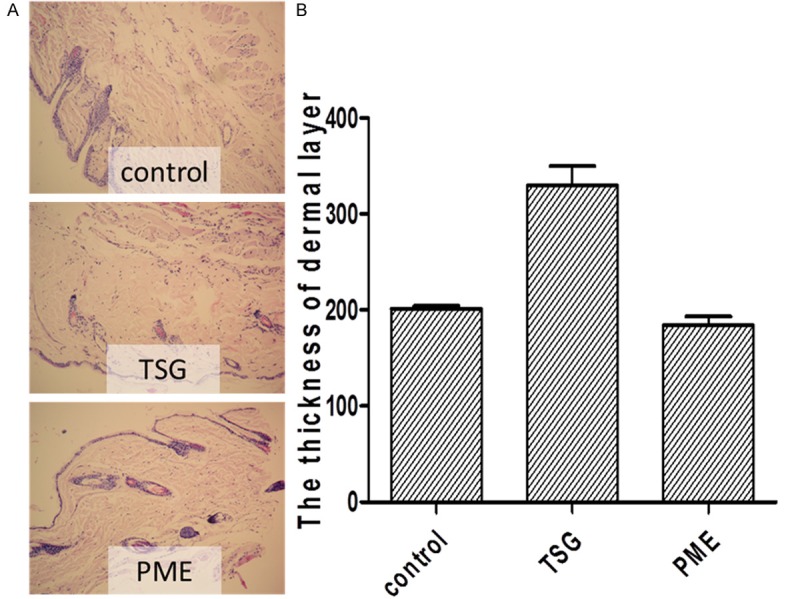
A. The thickness of dermal layer was much thicker in TSG group than the other two groups (×200). And TSG group had more flattered edge than the control group and PME group. B. The result of image scan showed that the thickness of dermal layer in TSG group was significantly thicker than control group and PME group (p<0.01, vs. Control group, p<0.01, vs. PME group).
TSG improves the collagen content in skin
The collagen content of TSG group and PME group were significantly increased than the control group compared with control group (p<0.01, vs. control group). The results are shown in Figure 2A and 2B.
Figure 2.
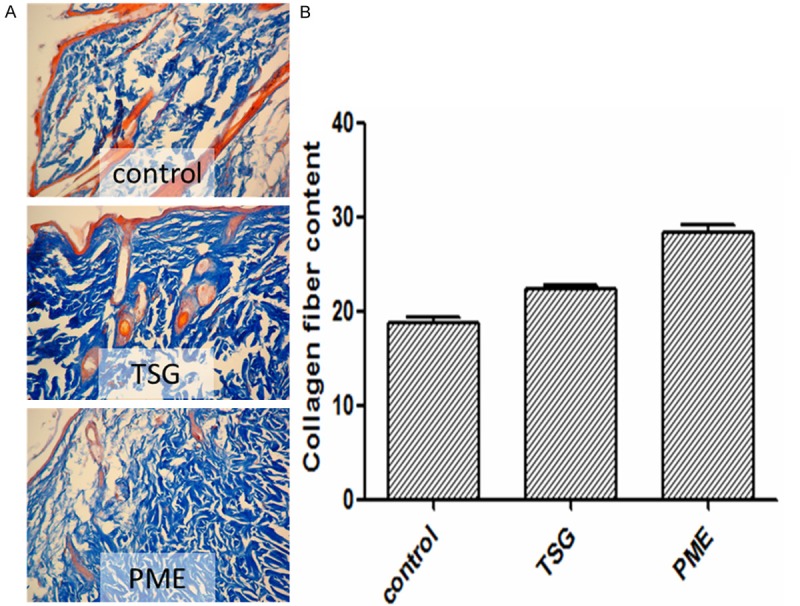
A. TSG and PME increased the thickness of dermal layer (×200). Collagen fiber was shown by blue color in three pictures which proved that TSG and PME groups had larger distrubution and more orderly than the control group. B. The result of image scan showed more cleared that the contant of collagen fiber in TSG and PME groups were significantly more than control group (p<0.01, vs. Control group).
PME increases the elastic fiber in skin
There was no difference in levels of elastic fiber between TSG group and control group. PME increased the level of elastic fiber (P<0.05, vs. control group). The result is shown in Figure 3A and 3B.
Figure 3.
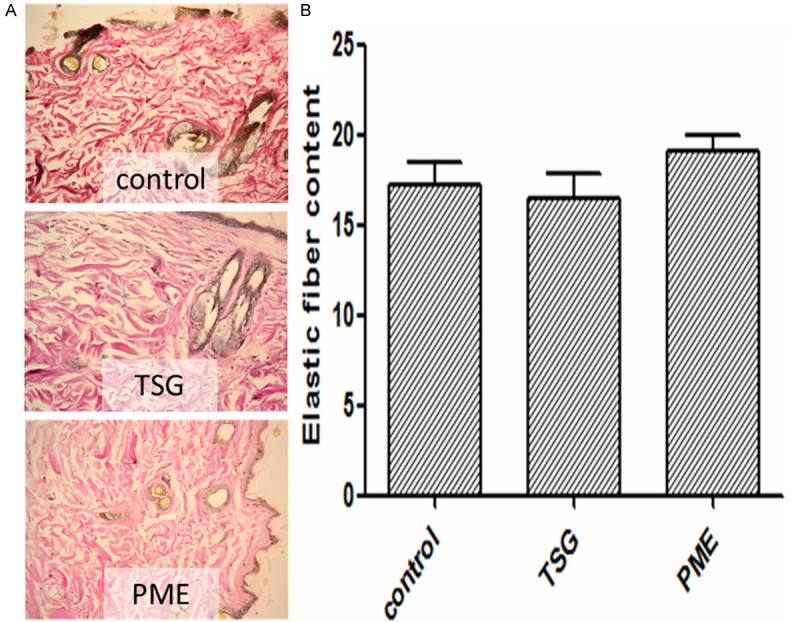
A. PME increased the thickness of dermal layer (×200). Elastic fiber was shown by red color in three pictures which proved that PME groups had larger distrubution than the other two groups, but TSG group had more orderly elastic fibers and flatter edge than the control group and PME group. B. The result of image scan showed that the content of elastic fiber in PME group was higher than control group (p<0.05, vs. Control group).
TSG reduces the levels of insulin, IGF-1 and their receptors in skin
The levels of insulin, the receptor of insulin (insulin-R), IGF-1, and the receptor of IGF-1 (IGF-1R) were significantly decreased than control group (p<0.01, vs. control group). The results are shown in Figure 4A-D.
Figure 4.
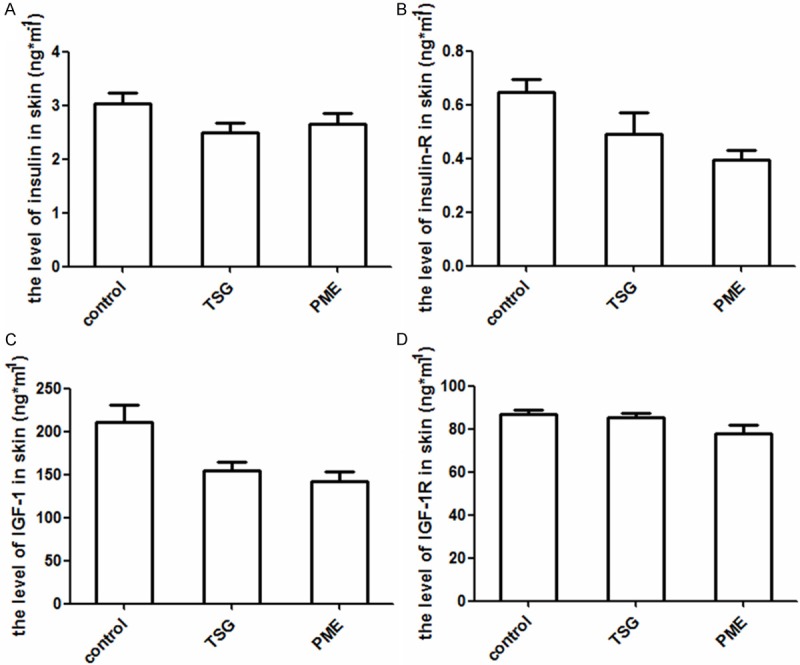
TSG decreased the levels of insulin, IGF-1, and the receptors of insulin and IGF-1 in skin of mice. A. Showed significant differences in the level of insulin between TSG, PME groups, and control group (P<0.01, vs. Control group). B. Showed the reduction in the level of insulin-R made by TSG and PME (P<0.01, vs. Control group). C. Showed that TSG and PME decreased the levels of IGF-1 (P<0.01, vs. Control group). D. Showed the level of IGF-1R was lower in TSG and PME groups than control group (P<0.01, vs. control group).
TSG raised the levels of Ca2+, P in serum
The levels of Ca2+ was significantly increased in TSG and PME groups than control group (p<0.01, vs. control group), and the levels of P also raised accompanied with increased Ca2+ level. The results are shown in Figure 5A and 5B.
Figure 5.
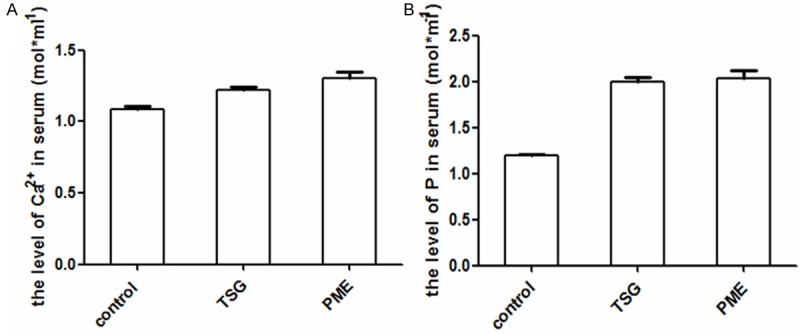
TSG increased the levels of Ca2+, P, in serum. A. Showed significant differences in the level of Ca2+ between TSG, PME groups, and control group (P<0.01, vs. Control group). B. Showed the increase in the level of P made by TSG and PME (P<0.01, vs. Control group).
Discussion
This study was performed to determine the role of collagen fiber, insulin/IGF-1 signal pathway and Ca2+ in the anti-aging effects of TSG, and compare with PME on against skin senescence effect in mice. Because the collagen fiber is the central point of convergence for cues related to aging and insulin/IGF-1 signal pathway play critical roles in this task [8,20-22], we employed these two factors with dermal layer to assess the role of TSG in mediating downstream beneficial effects of skin aging. We also invited Ca2+ and P in because the Ca2+ props skin structure [23] and P has a balance with Ca2+ [24]. Massive loss of collagen fibers are the main characteristic of skin aging, and the anti-skin-aging effect of TSG may be exerted via suppressing signals levels of insulin/IGF-1 signal pathway and thickening the dermal layer. The current findings further suggest that TSG is integral in mediating at least some of the beneficial effects associated with skin aging, and that collagen fiber, dermal layer and insulin/IGF-1 signal pathway are key components in response to its anti-skin-aging effect.
The role of TSG’s anti-skin-aging effect is not yet well understood, but TSG distributed in extending the lifespans of mice, and has been shown to be repressive to radicals in vitro and in vitro [13,25]. The current data appear to support the repressive role for TSG in aging [12,26]. Moreover, aging progression in aged mice skin corroborates the involvement of the collagen fiber, dermal layer [27] and insulin/IGF-1 signal pathway [28-30]. Given that the aged mice are not only deficient in collagen fiber but also in other factors involved in skin aging metabolism, further studies are needed to determine the extent of involvement of other factors besides insulin/IGF-1 signal pathway in aging process under TSG. It should be noted that while our data indicate reduced signals of the insulin/IGF-1 signal pathway and increased collagen fiber can exert a significant effect on TSG’s ability to mitigate aging-induced skin injury, and the extent of insulin/IGF-1 signal pathway involved in other factors of skin aging related also warrants further study.
Our study indicates that TSG exerted beneficial effects on skin senescence via increasing collagen fiber and suppressing the insulin/IGF-1 signal pathway. Another critical finding of this study was the anti-skin-aging effect of TSG is better than PME. While TSG led to increase the collagen fiber, as would be expected, TSG lead to show thicker dermal layer in aged skin of mice. While it has been shown that aged skin of mice have reduced collagen, thinner dermal layer, some findings that collagen reduced was reversed by insulin [8] and companied with increased IGF-1 [7] suggests that skin aging may be related with the insulin/IGF-1 signal pathway.
The decrease of IGF-1 inhibited the collagen synthesis in chick embryo chondrocytes and adult human skin fibroblasts, and the function of insulin on collagen mechanism in diabetic animals, which are the two major factors of insulin/IGF-1 signal pathway, further support the hypothesis that the insulin/IGF-1 signal pathway signaling impacts degradations of the physiological functions because anti-skin-aging effects are characterized by less collagen loss [6]. It has been hypothesized that the insulin/IGF-1 signal pathway involved in skin aging process through its action on collagen loss, which is consistent with the results of this study. In normal modals, thinner dermal layer during skin aging process would aggravate skin senescence. While TSG makes thicker dermal layer in these age mice skin, it exerts effect on mouse skin aging because increased collagen and thicker dermal layer are characteristic signals to delay skin aging coming. In ageing modals, weaken signals of the insulin/IGF-1 signal pathway during aging process would ease mammals aging symptoms to extending their lifespans. Given that TSG has been proposed to contribute to anti-skin-aging, the effect of TSG on aging mice proved in our previous work strengthens this assertion [12].
It is important to note that the aforementioned changes occurred despite equivalent anti-skin-aging effects in TSG group compared with control group. Furthermore, the TSG group had significant reductions in signals of the insulin/IGF-1 signal pathway, increase in collagen fiber and thicker dermal layer in mouse skin. Regarding functional implications of aforementioned factors in the skin aging, the TSG-treated mice did show reverse to skin aging process. These results enhanced that TSG was sufficient to suppress skin aging and the regulation on collagen fiber, dermal layer, and the insulin/IGF-1 signal pathway may be one important mechanism for its anti-skin-aging effects.
Taken together, the current results show some of the protective effects associated with TSG are contingent upon the collagen fiber response to the insulin/IGF-1 signal pathway, and in particular where other unknown factors are concerned. It is of further interest to elucidate the mechanisms behind the association between TSG, collagen fiber, dermal layer, and the insulin/IGF-1 signal pathway onset skin aging. The extent of involvement of TSG actions and contributions from related aforementioned factors like the insulin/IGF-1 signal pathway in skin aging also warrant additional studies. Regardless, the regulation of TSG to the insulin/IGF-1 signal pathway is a promising target in the search against aging.
The hypothesis aforementioned is ensured by increased collagen fiber, thicker dermal layer, and lower levels of insulin, IGF-1 and their receptors’ expression in aging skin of mice, and increasing collagen fiber and suppressed insulin/IGF-1 signal pathway may be important mechanisms to exert anti-skin-aging effect of TSG.
Acknowledgements
This study was supported by The Project of Shaanxi Medicinal Plant Effect Components Research and New Drug Create Engineering Technology Research Center (No. 2010ZDGC-19).
References
- 1.Takema Y, Hattori M, Aizawa K. The relationship between quantitative changes in collagen and formation of wrinkles on hairless mouse skin after chronic UV irradiation. J Dermatol Sci. 1996;12:56–63. doi: 10.1016/0923-1811(95)00467-x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Obayashi K, Okano Y, Satoh Y, Masaki H, Funasaka Y. Efficacy of thermal stimulation on wrinkle removal via the enhancement of collagen synthesis. J Dermatol Sci. 2006:S39–S49. [Google Scholar]
- 3.Narins RS, Baumann L, Brandt FS, Fagien S, Glazer S, Lowe NJ, Monheit GD, Rendon MI, Rohrich RJ, Werschler WP. A randomized study of the efficacy and safety of injectable poly-L-lactic acid versus human-based collagen implant in the treatment of nasolabial fold wrinkles. J Am Acad Dermatol. 2010;62:448–462. doi: 10.1016/j.jaad.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Haifei S, Xingang W, Shoucheng W, Zhengwei M, Chuangang Y, Chunmao H. The effect of collagen-chitosan porous scaffold thickness on dermal regeneration in a one-stage grafting procedure. J Mech Behav Biomed Mater. 2014;29:114–125. doi: 10.1016/j.jmbbm.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Helary C, Bataille I, Abed A, Illoul C, Anglo A, Louedec L, Letourneur D, Meddahi-Pelle A, Giraud-Guille MM. Concentrated collagen hydrogels as dermal substitutes. Biomaterials. 2010;31:481–490. doi: 10.1016/j.biomaterials.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 6.Oyamada I, Palka J, Schalk EM, Takeda K, Peterkofsky B. Scorbutic and fasted guinea pig sera contain an insulin-like growth factor I-reversible inhibitor of proteoglycan and collagen synthesis in chick embryo chondrocytes and adult human skin fibroblasts. Arch Biochem Biophys. 1990;276:85–93. doi: 10.1016/0003-9861(90)90013-o. [DOI] [PubMed] [Google Scholar]
- 7.Feld SM, Hirschberg R, Artishevsky A, Nast C, Adler SG. Insulin-like growth factor I induces mesangial proliferation and increases mRNA and secretion of collagen. Kidney Int. 1995;48:45–51. doi: 10.1038/ki.1995.265. [DOI] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Zlatev T, Spanheimer RG. Correction of altered collagen metabolism in diabetic animals with insulin therapy. Matrix. 1989;9:336–342. doi: 10.1016/s0934-8832(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 9.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 10.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 11.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 12.Zhou XX, Yang Q, Xie YH, Sun JY, Qiu PC, Cao W, Wang SW. Protective effect of tetrahydroxystilbene glucoside against D-galactose induced aging process in mice. Phytochemistry Letters. 2013;6:372–378. [Google Scholar]
- 13.Jiang Z, Xu J, Long M, Tu Z, Yang G, He G. 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009;85:345–350. doi: 10.1016/j.lfs.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Wang M, Rosen RT, Ho CT. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J Agric Food Chem. 1999;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- 15.Liu HC, Chen WS. Polygonum multiflorum water-soluble components of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D glucoside in vitro antioxidant effects. 2000;18:232–234. [Google Scholar]
- 16.Li C, Cai F, Yang Y, Zhao X, Wang C, Li J, Jia Y, Tang J, Liu Q. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: involvement of SIRT1 and TGF-beta1 pathway. Eur J Pharmacol. 2010;649:382–389. doi: 10.1016/j.ejphar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Yang QD, Ma ZJ. Polygonum learning and memory in rats on Alzheimer’s disease and the role of acetylcholinesterase. China Pharmacist. 2004;2004:394–396. [Google Scholar]
- 18.Zhang L, Xing Y, Ye CF, Ai HX, Wei HF, Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer’s disease and intervention by using tetrahydroxystilbene glucoside. Behav Brain Res. 2006;173:246–254. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Yang YJ, Wu PF, Wang W, Hu ZL, Long LH, Xie N, Fu H, Wang F, Chen JG. Tetrahydroxystilbene glucoside, a plant-derived cognitive enhancer, promotes hippocampal synaptic plasticity. Eur J Pharmacol. 2011;650:206–214. doi: 10.1016/j.ejphar.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Shim JH, Shin DW, Lee TR, Kang HH, Jin SH, Noh M. The retinoic acid-induced up-regulation of insulin-like growth factor 1 and 2 is associated with prolidase-dependent collagen synthesis in UVA-irradiated human dermal equivalents. J Dermatol Sci. 2012;66:51–59. doi: 10.1016/j.jdermsci.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Claassen H, Schluter M, Schunke M, Kurz B. Influence of 17beta-estradiol and insulin on type II collagen and protein synthesis of articular chondrocytes. Bone. 2006;39:310–317. doi: 10.1016/j.bone.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee PK, Maity N, Nema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Youm JK, Jo H, Hong JH, Shin DM, Kwon MJ, Jeong SK, Park BD, Choi EH, Lee SH. K6PC-5, a sphingosine kinase activator, induces anti-aging effects in intrinsically aged skin through intracellular Ca2+ signaling. J Dermatol Sci. 2008;51:89–102. doi: 10.1016/j.jdermsci.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs CS. Fetal Control of Calcium and Phosphate Homeostasis - Lessons from Mouse Models. London: Academic Press Inc; 2013. [Google Scholar]
- 25.Han X, Ling S, Gan W, Sun L, Duan J, Xu JW. 2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucoside ameliorates vascular senescence and improves blood flow involving a mechanism of p53 deacetylation. Atherosclerosis. 2012;225:76–82. doi: 10.1016/j.atherosclerosis.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Pan J, Chen SD. Kinases and kinase signaling pathways: potential therapeutic targets in Parkinson’s disease. Prog Neurobiol. 2012;98:207–221. doi: 10.1016/j.pneurobio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Calleja-Agius J, Brincat M, Borg M. Skin connective tissue and ageing. Best Pract Res Clin Obstet Gynaecol. 2013;27:727–740. doi: 10.1016/j.bpobgyn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87:201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghdam SY, Eming SA, Willenborg S, Neuhaus B, Niessen CM, Partridge L, Krieg T, Bruning JC. Vascular endothelial insulin/IGF-1 signaling controls skin wound vascularization. Biochem Biophys Res Commun. 2012;421:197–202. doi: 10.1016/j.bbrc.2012.03.134. [DOI] [PubMed] [Google Scholar]
- 30.Cechowska-Pasko M, Palka J. Expression of IGF-binding protein-1 phosphoisoforms in fasted rat skin and its role in regulation of collagen biosynthesis. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:703–711. doi: 10.1016/s1096-4959(03)00028-9. [DOI] [PubMed] [Google Scholar]


