Abstract
This essay aims to research the effect of okadaic acid (OA) on A549 cell multiplication, and cell apoptosis induced by OA was observed by cell morphology. MTT assay, trypan blue exclusion test (TBET), Giemsa staining method and acridine orange (AO) fluorescence staining assay were applied. The results of cell survival evaluated by TBET and colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) showed: The number of A549 cells was decreased in a dose-dependent manner. Cytomorphology observation of okadaic acid-treated cells showed that cells became shrinkage and turned round, some cells floated in the nutrient medium with nucleus agglutination broken, resulting in apoptotic bodies. Above-mentioned results indicated that OA exerted significantly inhibitory effect on A549 cell multiplication due to the apoptosis induced by OA.
Keywords: Okadaic acid, A549 cell line, cell multiplication, apoptosis
Introduction
In recent years, there has been a growing harmful red tides outbreak in China and other countries. Harmful red tides had caused damage to the stability of marine ecosystems and seriously red tide toxins have already threatened fisheries and human health [1]. Okadaic acid (OA) is known as one major component of diarrheic shellfish poisoning, the toxins responsible for the human intoxication. It is a hydrophobic fatty acid polyether and targets mainly the gastrointestinal tract in acute poisoning, causing diarrhea and vomit by enrichment of the food chain or consumption directly produced by marine dinoflagellates [2,3]. Previous work demonstrated that okadaic acid induced apoptosis and carcinogenesis in different cell lines [4]. It has already confirmed that okadaic acid treat can induce apoptosis of human hepatocellular cell line, colonic epithelial cell line and neuroblastoma cell line, etc [2,5]. Due to this characteristic, our study was then interesting to know whether OA induces apoptosis in A549 cells, a human lung adenocarcinoma cell line and non-target organ tumor cell line of okadaic acid. Okadaic acid induces apoptosis effects evaluated by growth increment determination and several cytomorphology observations. These results were analyzed to ascertain the facts whether okadaic acid has inhibitory effect on A549 cell multiplication and discuss the real cause of this inhibitory effect, then providing new drugs for the treatment of lung adenocarcinoma.
Materials and methods
Materials
RPMI-1640 was from Gibco (Grand Island, NY, USA). Okadaic acid was purchased from Sigma (St. Louis, MO, USA). Trypsin, MTT, and DMSO were purchased from Solarbio. Acridine Orange, trypan blue and Giemsa were purchased from Sinopharm Chemical Reagent Co., Ltd. Newborn calf serum was from Thermo.
Cell culture
A549 cells, a human lung adenocarcinoma cell line, were obtained from Academy of Military Medical Sciences (Beijing, China). The cells were grown as monolayer cultures in a humidified atmosphere of 95% air/5% CO2 in RPMI-1640 supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated newborn calf serum at 37°C. For cell counting and subculture, cells were dispersed with a solution of 0.05% trypsin and 0.02% EDTA. The attached cells were trypsinized after reaching optimum confluence, and cells in logarithmic growth phase were used for the following study.
MTT assay
Cells were seeded in 96-well microtitre plates at 3000 cells/well (100 μl), eight parallel samples of each group. Following an additional incubation of 24 h (37°C/5% CO2), the culture medium was removed. Cells grown in microtitre plates were treated with a series different concentration of OA (20, 30, 40, 50, 60, 70, 80, 90, 100 ng/ml) and incubated for different periods of time as described in results. MTT, 5 mg/ml, was added 4 h before the incubation period ended. After incubation for another 4 h, the culture medium was removed and 150 μl DMSO was added, and then oscillated in the dark at room temperature for 15-30 min. The sample absorbance was read in ELIASA at wavelengths of 570 nm. Cell viability was calculated as follows: Cell viability = (A-B)/ (C-B) × 100%.
A stand for OD value of the experimental group; B stand for OD value of the control group; C stand for OD value of the solvent control group.
Trypan blue exclusion test
Cells were seeded in 12-well plates at 2 × 104 cells/well (1 ml), three parallel samples of each group. Following 24 h, 48 h and 72 h exposure of cells to OA (34 and 68 ng/ml) when the culture medium was removed after incubated 24 h. The cells were trypsinized, collected and monitored with the membrane non-permeable dye trypan blue. Trypan blue exclusion test was used to record the number of viable cells of each group.
Giemsa staining method detect the apoptosis of A549 cell evoked by OA
In 6-well plates (2 × 104 cells/well, 2 ml/well), cells grown on coverslips were incubated for 24 h, three parallel samples of each group. Then cells were treated with 34 and 68 ng/ml OA for 48 h. After washing with PBS for twice, cells were fixed in Carnoy during 5-10 min. Finally, Giemsa staining fluid stained the cells, ddH2O wished twice. Cells were photographed under ordinary optics microscope.
Acridine orange (AO) fluorescence staining assay assessed nuclear morphology
Nuclear morphology was monitored with acridine orange, a live cell nucleic stain. Briefly, in 6-well plates (2.5 × 104 cells/well, 2 ml/well), cells grown on coverslips were incubated for 24 h, three parallel samples of each group. The cells were exposed to OA (34 and 68 ng/ml) at 37°C for 48 h after removing the culture medium. The cells were fixed in 95% ethanol for 10-20 min after washed with PBS (for twice). Nuclear condensation was observed by staining with acridine orange under laser scanning confocal microscope.
Statistical analysis
The data were expressed as means with standard deviations (SD) for at least three independent determinations in triplicate or for each experimental point. The statistical differences between treated cells and controls were determined by the Student t test and the Wilcoxon Rank Sum test and the levels of P < 0.05 [6].
Results
OA has significant inhibitory effect on A549 cells multiplication
Results of MTT assay showed OA exerted an increased inhibitory effect on A549 cell multiplication when cells were incubated with OA for 24 h, 48 h and 72 h in a time-dependent and dose-dependent manner (Figure 1). In the meanwhile, this assay has proved a 0.5% concentration of DMSO did not influence cells’ growth, consistent with pre-report [7] (Figure 2). In TBET assay, the number of living cells was obviously decreased in a dose-dependent manner, consistent with the results of MTT (Figure 3).
Figure 1.
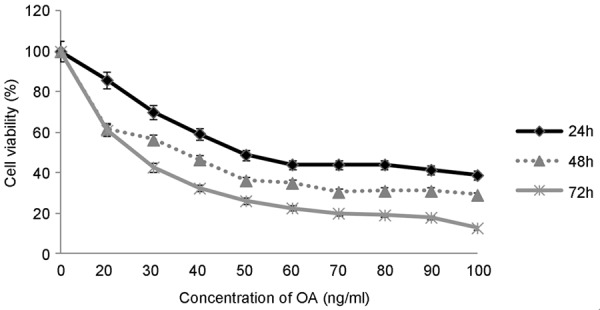
Cell survival rates of A549 cells after incubation with OA evaluated by MTT assay. Cell survival rates were decreased with OA’s concentration increase and time going on. The concentration of DMSO in solvent control group is equal to 100 ng/ml OA treated group.
Figure 2.
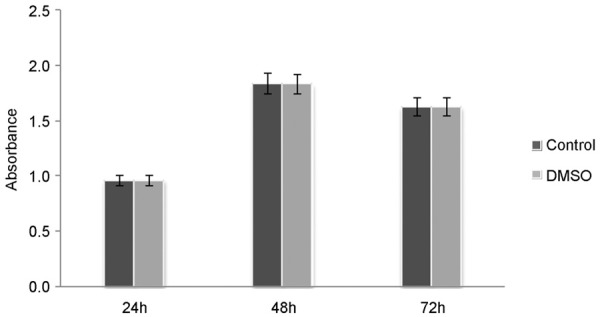
The effect of solvent for cells survival rates. P > 0.05, solvent control groups had no difference with respect to controls. Values are the mean ± SD of three experiments performed in triplicate. The concentration of DMSO in solvent control group is equal to 100 ng/ml OA treated group.
Figure 3.
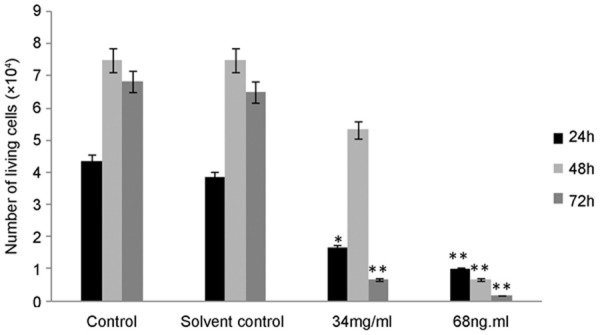
Trypan blue exclusion test detected the number of living cells. No obvious difference in control group and solvent control group. Treated cells were compared, each concentration to the controls. *Difference from the control were significant at P < 0.05; **difference from the control were highly significant at P < 0.01 (Student’ test). Values are the mean ± SD of three experiments performed in triplicate. 34 ng/ml is the 50% inhibited concentration (IC50), 68 ng/ml is 2 × IC50. The concentration of DMSO in solvent control group is equal to 68ng/ml OA treated group.
A549 cell apoptosis induced by OA
Staining method discovered solvent control group cells were similar to control group cells that cells’ body were normal, keeping in contact with the surrounding cells, with clear and full nuclei (Figure 4A, 4B). Cells number was evidently reduced and losing contact with surrounding cells, cells became round and budded around the cell membrane when incubated with 34 ng/ml OA for 48 h (Figure 4C). Exposing in 68 ng/ml OA, cells were more less and disconnecting with others, cells turned round, some floated in the nutrient medium, and significant apoptotic bodies were surrounding the cells (Figure 4D), with some broken (Figure 4D).
Figure 4.
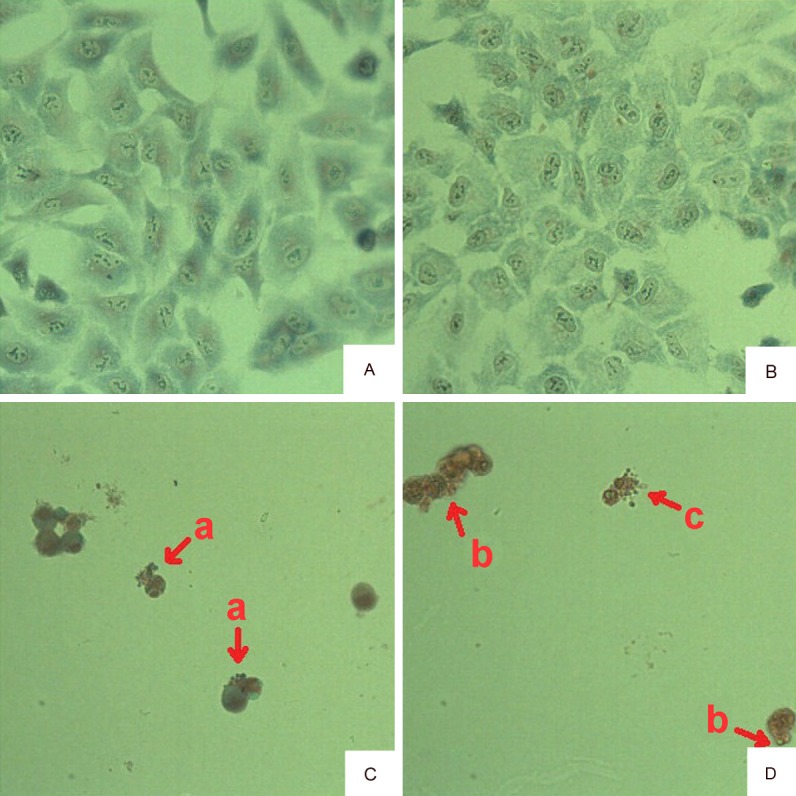
Giemsa staining method detected the apoptosis of A549 cell evoked by OA. A: Control; B: Solvent control; C: 34 ng/ml; D: 68 ng/ml. a, b: apoptotic body; c: broken cells. Values are the mean ± SD of three experiments performed in triplicate. The concentration of DMSO in solvent control group is equal to 68 ng/ml OA treated group.
A549 cells nuclear morphology was observed by staining with acridine orange under confocal laser scanning microscope. After A549 cells were stained by the AO, the RNA of cytoplasm and nucleolus turned yellowish red. The nucleus morphology in solvent control group cells was large and uniform, similar to control group cells (Figure 5A, 5B). After treatment with 34 ng/ml OA, karyopyknosis was found and a crescent cap structure distributed throughout the karyoplasms (Figure 5C). Chromatins were like that treated with 34 ng/ml OA, but fluorescence intensity was lower when cells were exposed in 68 ng/ml OA (Figure 5D).
Figure 5.
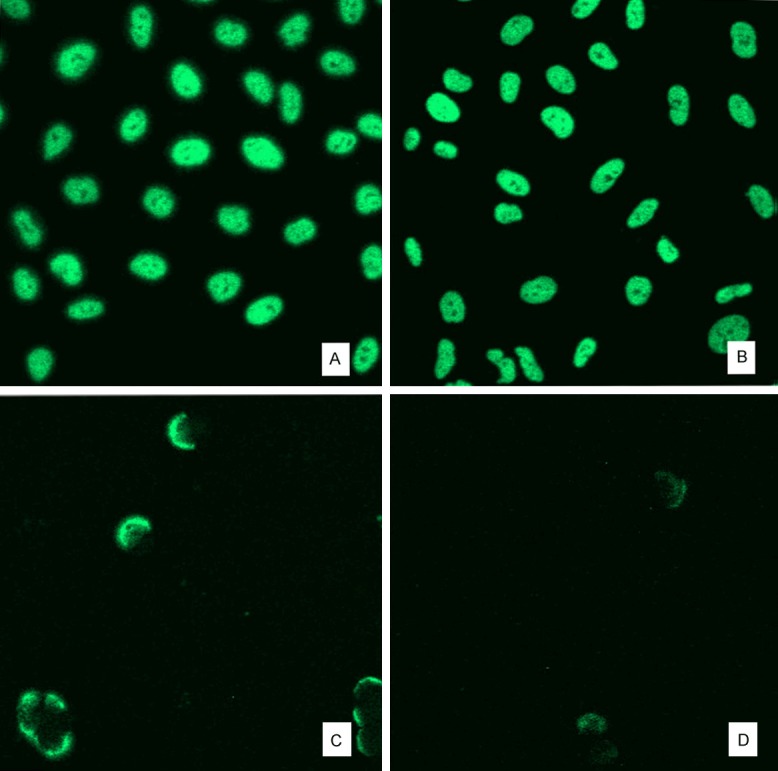
Acridine orange fluorescence staining assay assessed nuclear morphology. A: Control; B: Solvent control; C: 34 ng/ml; D: 68 ng/ml. Values are the mean ± SD of three experiments performed in triplicate. The concentration of DMSO in solvent control group is equal to 68 ng/ml OA treated group.
Discussion
MTT assay showed that A549 cells survival rates decreased with the increasing of OA’s concentration, presenting an evident time dependent and dose dependent manner. The IC50 calculated for the toxin was 34 ng/ml (42.23 nmol/ml). For recent years, the chemotherapy drug cisplatin has been the doctors’ first line of defense against tumors, especially for the lung. It often combined with other drugs due to drug resistance, and cisplatin-based combined therapy has achieved a significant effect [8]. The IC50 incubating A549 cells with cisplatin or curcumin alone for 48 h is separately 0.966 μg/ml and 18.4 μmol/ml, but the inhibition rate is 55.31% when cells exposed to 1 mg/L DDP in combination with 10 μmol/L CUR and 2 mg/L DDP in combination with 5 μmol/L CUR for 48 h, and a better lethal effect has been shown [9,10]. The IC50 that A549 cells incubated with erlotinib for 48 h is 200 μmol/L [11], and tetrandrine’s is 4.43 mg/ml [12]. The IC50s of above-mentioned toxins are higher than OA when treated A549 cells alone or in combination for the same time. So OA has a potential to develop into a new treatment for human lung adenocarcinoma due to it has a better lethal effect for A549 cell in a lower concentration.
Normal living cells’ plasma membrane keep intact, it can’t discover blue staining under the microscope by reason that trypan blue can not across cell membranes. When cell membrane penetrability is increasing, trypan blue can enter dying cells and showed blue stained under the microscope [13,14]. So trypan blue exclusion test was used to analyze cell viability. In our research, the results of TBET were consistent with the results of MTT. It’s further evident that OA has significant inhibitory effect on A549 cells multiplication.
Giemsa staining is a composite dye composed of the azure and eosin. Nuclei were stained into purple while cytoplasm and nucleolus were dyed into bluish violet when cells were tinted with Giemsa staining. In this study, we can see that cell number was evidently reduced and losing contact with surrounding cells, cells became round, cells dyed more deeply, and nuclei were marginalized and budded around the cell membrane when incubated with 34 ng/ml OA for 48 h. Exposed in 68 ng/ml OA, cells were more less and disconnecting with others, some floated in the nutrient medium, and significant apoptotic bodies were surrounding the cells, with some broken. There was no nuclear staining proved that nuclei were fragmented. This assay proved that A549 cells obviously died when exposed into 34 ng/ml or 68 ng/ml OA and the primary cause was OA could induce cell apoptosis.
Acridine Orange, a vital fluorescent dye, enters cells through the cytoplasmic membrane and intercalates into DNA and RNA, resulting in strong staining of complex, the complex produce kelly and yellowish red (not shown) separately [2,15,16]. Karyopyknosis and crescent cap structure distributed throughout the karyoplasms when treated with 34 ng/ml OA (the early period of apoptosis). After exposed in 68 ng/ml OA, chromatins were like that treated with 34 ng/ml OA, but fluorescence intensity was lower. Maybe because apoptotic bodies were phagocytosed and digested by surrounding cells, resulting in nuclear fragmentation and cytoclasis (the late period of apoptosis). The cells incubated with OA showed typical morphological change of apoptosis once again testified that OA could kill A549 cells due to apoptosis.
In conclusion, the results clearly demonstrate that OA has significantly inhibitory effect on A549 cell multiplication and cells had a high sensitivity to OA. Apoptosis is the main cause of this effect. This research was preliminarily proved that OA had the potential to be a new treatment of human lung adenocarcinoma. However, further studied are need to define the exact role of OA.
Acknowledgements
The study was supported by National Natural Science Foundation of China (NO.31200400).
Disclosure of conflict of interest
None.
References
- 1.Chen Y, Yan T, Yu RC, Zhou MJ. Okadaic Acid Inhibits Cell Proliferation and Induces Apoptosis of Human Hepatocyte Cell Line HL-7702 and Hepatocellular Cancer Cell Line Bel-7402. Acta Scie Natu Univ Suny. 2008;47:86–92. [Google Scholar]
- 2.Traore A, Baudrimont I, Ambaliou S, Dano SD, Creppy EE. DNA breaks and cell cycle arrest induced by okadaic acid in Caco-2 cells, a human colonic epithelial cell line. Arch Toxicol. 2001;75:110–117. doi: 10.1007/s002040000188. [DOI] [PubMed] [Google Scholar]
- 3.Zhou MJ, Li J, Yu RC, Yan T, Fu M. Advances in Research of Phycotoxins. Chin J Mari Drug. 1999:48–54. [Google Scholar]
- 4.Xing ML, Lou JL, Xv LH. DNA Damage and Alteration of Expression in Apoptosis Related Proteins Induced by Okadaic Acid in FL Cells. Acta Hydr Sini. 2007;31:661–665. [Google Scholar]
- 5.Cabado AG, Leira F, Vieytes MR, Vieites JM, Botana LM. Cytoskeletal disruption is the key factor that triggers apoptosis in okadaic acid-treated neuroblastoma cells. Arch Toxicol. 2004;78:74–85. doi: 10.1007/s00204-003-0505-4. [DOI] [PubMed] [Google Scholar]
- 6.Gad SC, Weil CS. Statistic for toxicologists. New York: Raven Press; 1982. [Google Scholar]
- 7.Zhang XX, Wang RJ, Zhao YF, Ding YP. Effects of Okadaic Acid on the Mouser 3T3 Embryonic Cells. J Qufu Norm Univ (Natu Scie) 2011;37:87–92. [Google Scholar]
- 8.Zhang MC, Hu CP. The molecular basis for cisplatin resistance in lung cancer. Int J Res P. 2006;26:152–155. [Google Scholar]
- 9.Cao H, Diao LM, Xia D. Effects of Curcumin Combined with Cisplatin on the Proliferation and Apoptosis of Human Lung Cancer Cell Line A549 in Vitro. Med J Wuhan Univ. 2008;29:213–217. [Google Scholar]
- 10.Cui JD, Hu YD. Study on the anti-proliferation effect of curcumin combined with cisplatin on the human lung cancer cell line A549 in vitro. Sichuan Med J. 2006;27:1–3. [Google Scholar]
- 11.Bai XY, Mu XY, Jiang SJ, Wang YL, Liu QL. Effects of Erlotinib on apoptosis in human pulmonary adenocarcinoma. Chin J Gero. 2010;30:1073–1076. [Google Scholar]
- 12.Jiang XH, Teng YZ, Huang KS, Zhou QX. Inhibitation of A549 cells induced by tetrandrine with MTT assay. SJFCD. 2012;2:18–20. [Google Scholar]
- 13.Peng B, Wu JJ, Li Y, Xu Q, Tang L, Wang BH. A Methodological Study of Trypan Blue Exclusion Test for Cultured Adherent Cells. Acta Lase Biol Sinica. 2011;20:269–273. [Google Scholar]
- 14.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3: Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 15.Huang LS, Wang Y, Liu LY, Huang WC, CHen GY. The clinical significance of acridine orange staining fluorescence in the diagnosis of gastric carcinoma. J Moder Onco. 2007;15:1794–1795. [Google Scholar]
- 16.Wang Y, Zhou T, Sun HY, Huang B. Study on the Relationship between Autophagy and Apoptpsis in A549 Cells Induced by Curcumin Analogue EF24. Chin J Cell Biol. 2012;34:590–596. [Google Scholar]


