Abstract
miR-21 shares a potential oncogenic function. The overexpression of miR-21 was common in glioblastoma, which is the most common lethal primary intracranial tumor. The study aimed at miR-21 effect on Glioblastoma cell line A172 of proliferation, apoptosis, and chemosensitivity and its definite mechanism of target gene FOXO1. Effect and mechanism were evaluated by colony forming cell assay, annexin V-FITC/PI apoptosis assay, TUNEL apoptosis assay, luciferase-reporter activities assay, RNA interference, western-blot and Real-Time PCR. The statistics revealed miR-21 promoted A172 cell proliferation and suppressed the chemosensitivity, and also showed that miR-21 could bind with FOXO1 mRNA and prevent FOXO1 translation via its 3’UTR to regulate the function. These findings suggest that miR-21 plays an important role in cell proliferation and chemosensitivity by inhibiting FOXO1, and show much more significance for exploring miR-21 inhibitor in A172 therapy.
Keywords: miR-21, A172, proliferation, chemosensitivity, FOXO1
Introduction
Glioma is the most common primary intracranial tumor, affecting approximately 1 case per 10,000 population annually, and most of which belongs to malignant tumor, which is among the most lethal of human cancers [1-3]. Notably, the rising tendency of the incidence rate is the most fearful [2,3]. However, the treatment of glioma do not yet make a breakthrough in the past 30 years, and the median survival time of which is about 1 year [1-3]. Recently, the miRNA expression studies come to the general consensus that miR-21 is overexpressed in glioma and glioma cell lines relative to normal tissue [4-7]. It is indicated that miR-21 can regulate glioma as a potential oncogenic factor [4,5]. However, the effect of miR-21 on glioblastoma cell line A172 cells of proliferation, apoptosis, and chemosensitivity and its definite mechanism has not been exploited [5,8,9]. Colony forming cell assay, annexin V-FITC/PI apoptosis assay and TUNEL apoptosis assay were developed and indicated that miR-21 promoted A172 cell proliferation and suppressed the chemosensitivity to cisplatin. On the other side, miR-21 inhibitor can suppress A172 cell proliferation, promote the cisplatin induced apoptosis and enhance the chemosensitivity of A172 cells.
Bioinformatics analysis was performed to predict the target gene, and FOXO1 was adopted. FOXO1 is a transcription factor and it can regulate some gene expression, such as P27, P21, Bim and FASL. Dual-luciferase-reporter activities assay, mutation assay and RNA interference were applied to illuminate the relationship between miR-21 and FOXO1 and its target sequence. Western-blot and qRT-PCR were performed to detect the expression of FOXO1 and its signaling pathway. And the result suggested miR-21 could bind with FOXO1 mRNA and prevent FOXO1 protein translation via its 3’UTR ranged from 1975th base site to 1996th base site to regulate the cell function.
Materials and methods
Cell culture and microRNA transfection
Cell line A172 which came from human glioblastoma multiforme (A172) tissue was conserved in Neurosurgery Laboratory of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, and was cultured in DMEM medium (High glucose) (Gibico, USA) containing 10% fetal calf serum (Hyclone, USA), 100 U/ml penicillin (Gibico, USA), and 100 μg/ml streptomycin (Gibco, USA), under humidified conditions in 95% air and 5% CO2 at 37°C. The cells were collected during the logarithmic growth phase. miR-21 mimics the extrinsic mature double-stranded micro RNA, which can imitate the endogenous miR-21. And miR-21 inhibitor is the extrinsic mature complementary single-stranded micro RNA, which can inhibit the endogenous miR-21. All the sequences including negative control of miR-21 have been respectively synthesized by Gene Pharma Co., Ltd in China. The sequences contained are as follows:
miR-21 mimic: 5’-UAGCUUAUCAGACUGAUGUUGA-3’, miR-21 inhibitor: 5’-UCAACAUCAGUCUGAUAAGCUA-3’, NC: 5’-UUGUACUACACAAAAGUAAUG-3’.
All the transfection was mediated by Lipofectamine 2000 (Invitrogen, USA) as recommended by the manufacturer. The cell models were divided into 3 groups, miR21 mimic cells, miR21 inhibitor cells, and NC cells.
Colony forming cell assay
Briefly, A172 cells with the logarithmic growth phase were respectively digested with 0.25% trypsin and dispersed into single-cell suspension, and then were suspended in DMEM medium containing 10% fetal calf serum. Dispersed cells were inoculated into 6-well plates, 200 cells per well, and incubated in 5% CO2 and saturated humidity incubator with 37°C for 24 hours. Subsequently, cells transfection with miR-21 mimic, inhibitor and NC was performed. Cell culture for 1 week was ended when visible clones appeared and the supernatant was discarded. A172 cells were washed with PBS for 2 times carefully, and then fixed with 4% paraformaldehyde for 15 minutes, subsequently washed with distilled water for 2 minutes, and finally stained with 0.1% crystal violet for 10 minutes. A172 cells in each group were photographed and counted respectively. The colonies that had 50 cells per colony were counted.
Annexin V-FITC/PI apoptosis assay
After transfection with microRNA, apoptosis was measured by Annexin V-FITC and PI double staining (Beyotime, China). Cells subjected to cisplatin (20 μM) were cultured for another 24 hours and then detached by 0.25% trypsin, washed twice with PBS, and stained by 5 μl of annexin V-FITC and 10 μl PI (10 μg/ml) for 10 min at room temperature according to the manufacturer’s instructions. Subsequently, flow cytometric analysis was performed and annexin V-positive/PI-negative (A+/P-) cells were apoptotic cells.
TUNEL apoptosis assay
Transfected cells subjected to cisplatin (20 μM) for 36 hours were performed to detect apoptosis using a TUNEL kit according to manufacturer’s instructions. The treated cells were first washed with PBS, subsequently fixed with 4% paraformaldehyde for 1 h, then washed with PBS once again, incubated with 0.1% Triton X-100 for 2 min on ice, and washed with PBS twice. After that, cells were stained with TUNEL detecting liquid for 1 h and PI (10 μg/ml) for 10 min shielded from light at 37°C. After being treated with antifade mounting medium, cells were detected by a confocal laser scanning microscope at 488 nm excitation and 530 nm emission.
Luciferase-reporter activities assay
Luciferase-reporters were successfully constructed using molecular cloning method. 3 sequences were inserted into pGL3-Basic vector (Promega, USA) respectively to obtain pGL3-IRS, pGL3-FOXO1-3’UTR and pGL3-FOXO1-3’UTR-mut.
pGL3-IRS contained the FOXO1 binding sequence (IRS-insulin-responsive sequence) as follows: 5’-GCAAAACAAACTTATTTTGAAGCAAAACAAACTTATTTTGAAGCAAAACAAACTTATTTTGAA-3’.
pGL3-FOXO1-3’UTR contained the miR-21 binding sequence (FOXO1-3’UTR sequence) as follows: AAGCCAGCUCUAUUGUAAGCUU.
pGL3-FOXO1-3’UTR-mut contained 2 mutated nucleotides in 3’UTR of FOXO1 (FOXO1-3’UTR-mut) for comparative analysis as follows: AAGCCAGCUCUAUUGUAAGGAU.
A172 cells were seeded in 24-well plates for 24 hours, then transfected with 1 μg of Luciferase-reporter plasmids per well using PEI Transfection Reagent. 50 ng of pRL-TK plasmids were co-transfected to normalize the transfection efficiency. 24 hours after transfection, A172 cells were transfected with microRNA. Then luciferase activities were measured using the dual-luciferase reporter gene assay kit (Promega, USA) according to the manufacturer’s instructions.
RNA interference
To downregulate FOXO1, RNA interference was developed in miR-21 inhibitor transfected A172 cells. In the exponential growth phase, cells in 6-well plate were transfected with gene-specific siRNA and negative control siRNA purchased from GenePharma (Shanghai, China) according to the manufacturer’s protocol. The siRNA sequence used is listed as: GCUCAAAUGCUAGUACUAUTT (sense), AUAGUACUAGCAUUUGAGCTA (antisense).
Western blot
A172 cells were collected and lysed for preparation of protein with RIPA containing PMSF. 20 μg of protein were separated by polyacrylamide gel electrophoresis and subsequently electrotransferred to PVDF membrane. After blocking the nonspecific antibody binding sites by incubating in 5% BSA, membranes were incubated with the following primary antibodies and dilutions: polyclonal rabbit anti-FOXO1 antibody diluted at 1:2500 (Abcam, USA), polyclonal rabbit anti-P27 antibody diluted at 1:1000 (Abcam, USA), polyclonal rabbit anti-P21 antibody diluted at 1:2500 (Abcam, USA), polyclonal rabbit anti-FASL antibody at 1:1000 (Abcam, USA), polyclonal rabbit anti-Bim antibody at 1:1000 (Abcam, USA), polyclonal rabbit anti-GAPDH antibody diluted at 1:2500 (Abcam, USA). After washing with PBS/T, membranes were incubated with peroxidase conjugated goat anti-rabbit antibody (Abcam, USA) for 1 h. Proteins were visualized using the enhanced chemiluminescence detection system (ECL, UK).
Real-Time PCR
Total RNA was extracted from the A172 cells using Trizol (TAKARA, Japan). Quantification of P27, P21, Bim and FASL mRNAs was performed using the Quantitect SYBR Green PCR kit (TAKARA, Japan) with ABI 7500 (TAKARA, Japan) quantitative PCR system according to the manufactures instructions. GAPDH mRNA was used as an internal control for determining the relative mRNA. All primers used are purchased from Gene Pharma (Shanghai, China). All experiments were performed in triplicate independently according to the manufacturer’s instructions.
Statistical analysis
All the former experiments were repeated at least three times. And data are considered to be objective and described as means ± standard error of the mean (SEM). Student’s t-test was developed to analysis the group means. P value < 0.05 was considered to be statistically significant, and two sided tests were essential for all statistics. All statistical analyses were performed with the Statistical Package of the Social Sciences software version 16.0.
Results
miR-21 promoted cell proliferation and inhibited apoptosis
The effects on cell proliferation in the former 3 groups were investigated using colony formation assay respectively. The overexpression of miR-21 significantly promoted not only the growth but also colony formation of A172 cells in 1 week (the group of miR-21 mimic relative to NC, P < 0.05), as shown in Figure 1A. On the contrary, the inhibitor of miR-21 significantly suppressed the growth and colony formation of A172 cells in 1 week (the group of 1miR-21 inhibitor relative to NC, P < 0.05), as shown in Figure 2A.
Figure 1.
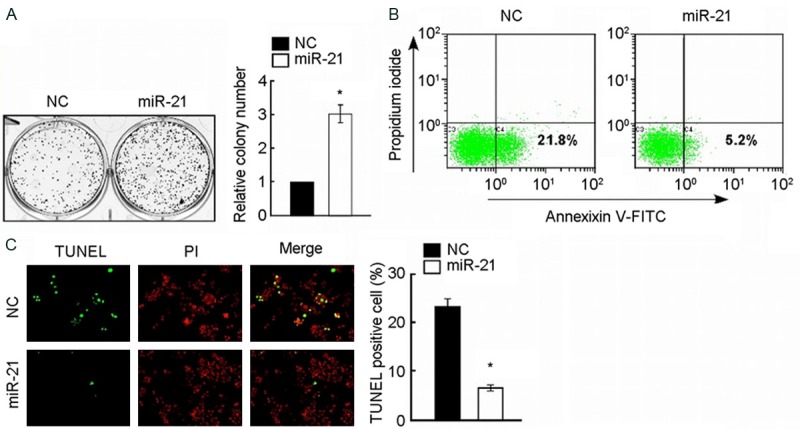
Upregulation of miR-21 promotes cell proliferation and enhances the chemosensitivity of glioma cell line A172 to cisplatin. A: Representative micrographs (left) and quantification (right) of crystal violet stained cell colonies, indicating that up regulation of miR-21 promoted cell growth of glioma cell line A172. B: Annexin V-FITC/PI staining of indicated A172 cells treated with cisplatin (20 μM) for 24 h. C: Representative micrographs (left) and quantification of TUNEL positive cells in indicated A172 cells treated with cisplatin (20 μM) for 36 h. *P < 0.05.
Figure 2.
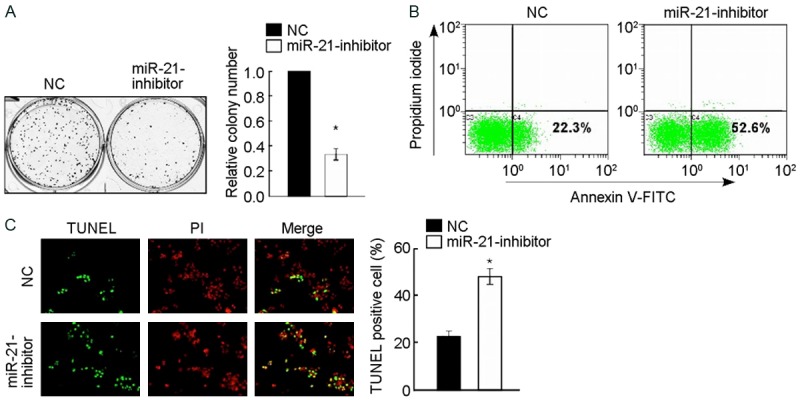
Inhibition of miR-21 suppresses cell proliferation and reduces the chemosensitivity of glioma cell line A172 cells to cisplatin. A: Representative micrographs (left) and quantification (right) of crystal violet stained cell colonies, indicating that inhibition of miR-21 induced apoptosis of glioma cell line A172. B: Annexin V-FITC/PI staining of indicated A172 cells treated with cisplatin (20 μM) for 24 h. C: Representative micrographs (left) and quantification of TUNEL positive cells in indicated A172 cells treated with cisplatin (20 μM) for 36 h. *P < 0.05.
miR-21 reduced the chemosensitivity of A172 cells to cisplatin
To determine whether miR-21 regulated the chemosensitivity of A172 cells to cisplatin, Annexin V-FITC/PI apoptosis assay and TUNEL apoptosis assay were performed. After being treated with cisplatin (20 μM) for all the former 3 groups in 24 hours, the percentage of apoptosis in miR-21 mimic group is lower than NC group, and NC group is lower than miR-21 inhibitor group using Annexin V-FITC/PI apoptosis assay with flow cytometry detection, the differences of which are significant (P < 0.05) (Figures 1B and 2B). According to the same thought, all groups were treated with cisplatin (20 μM) for 36 hours, following by TUNEL apoptosis assay with fluorescent microscope detection indicated that differences of the number of TUNEL positive cells are as follow: miR-21 inhibitor group was the most, NC group occupied the second place, and miR-21 mimic group was the least, the differences of which are significant (P < 0.05) (Figures 1B and 2B). The former tests showed that the increased expression of miR-21 significantly inhibited the cisplatin induced apoptosis of A172 cells, and inhibition of miR-21 promoted the process.
miR-21 inhibits FOXO1 signaling pathway via FOXO1 suppression
Based on the bioinformatics analysis between miR-21 and its target gene FOXO1, dual-luciferase-reporter activities assay was performed. PGL3-IRS vector containing FOXO1-binding insulin-responsive sequence was transfected into A172 cells for detecting the expression of FOXO1. Figure 3A shows the differences of FOXO1 luciferase-reporter activities among the 3 groups are significant: the luciferase-reporter activities in miR-21 inhibitor group was the most and miR-21 mimic group was the least, relative to NC, P < 0.05. The test indicated that miR-21 suppressed the expression of FOXO1, which resulted in the inhibition of the expression of pGL3-IRS. Downstream products in FOXO1 signaling pathway were detected by Real-Time PCR and Western-blot corresponding to mRNA level and protein level. Figure 3B and 3C illustrated the expression of downstream products in FOXO1 signaling pathway were significantly different. The expression of P27, P21, Bim and FASL reduced both in mRNA level and protein level in miR-21mimic group, but rose in miR-21 inhibitor group. The test suggested that miR-21 inhibits FOXO1 signaling pathway via FOXO1 suppression.
Figure 3.
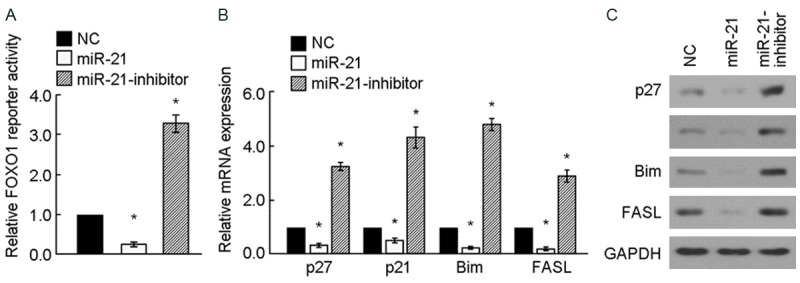
miR-21 inhibits FOXO1 signaling pathway. (A) FOXO1 luciferase-reporter activities were analyzed in miR-21-tranduced and miR-21-inhibited A172 cells. (B, C) Relative mRNA (B) and protein (C) expression of FOXO1-regulated genes in indicated A172 cells assessed by real-time PCR and western blotting. GAPDH was used as a loading control. *P < 0.05.
miR-21 suppresses FOXO1 expression via directly targeting FOXO1-3’UTR
FOXO1 expression that reduced in miR-21mimic group, but raised in MiR-21 inhibitor group were significantly different using Western blotting analysis, relative to GAPDH, P < 0.05. The test suggested that miR-21 inhibits FOXO1 expression. Bioinformatics analysis of FOXO1 target sequence indicated that the miR-21 binding sites in the FOXO1 mRNA sequence 3’UTR ranged from 1975th base site to 1996th base site. Wild oligonucleotide DNA for themiR-21 binding sequence (FOXO1-3’UTR sequence) and mutant Oligonucleotide DNA containing 2 mutated nucleotides in 3’UTR of FOXO1 (FOXO1-3’UTR-mut) were developed to detect the precise binding sites. Figure 4C reveals the differences of miR-21 luciferase-reporter activities of pGL3-FOXO1-3’UTR among the 3 groups are significant: the luciferase-reporter activities in miR-21mimic group was the most and miR-21 inhibitor group was the least, relative to NC, P < 0.05. The test indicated that miR-21 suppressed the expression of FOXO1-3’UTR via binding the target sequence. On the contrary, miR-21 luciferase-reporter activities of pGL3-FOXO1-3’UTR-mut among the 3 groups did not make significant difference. The test suggested that the prediction of miR-21 binding sequence is correct and miR-21 inhibited the effect of FOXO1 via this sequence. In order to explore the effect of miR-21 on proliferation of A172 cells via FOXO1, FOXO1 RNA interference acting on cell colony formation assay was developed. After FOXO1-siRNA transfecting MiR-21-inhibitor group, both the proliferation and colony formation of A172 cells were significantly promoted relative to the control of scramble transfection, P < 0.05. After being treated with cisplatin (200 μM) for miR-21-inhibitor group in 36 hours, the percentage of apoptosis in FOXO1-siRNA transfecting group is lower than scramble transfecting group using Annexin V-FITC/PI apoptosis assay with flow cytometry detection, the differences of which are significant (P < 0.05) (Figure 4F).
Figure 4.
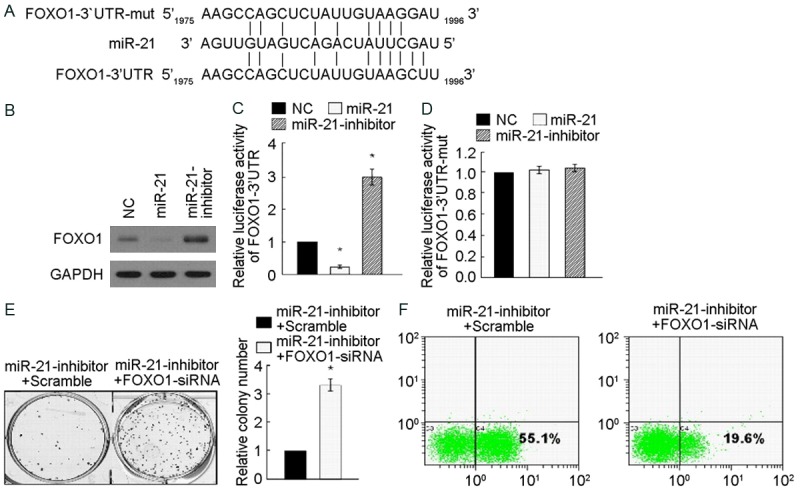
miR-21 Suppresses FOXO1 expression via directly targeting FOXO1-3’UTR. A: Predicted miR-21 target sequence in the 3’UTR of FOXO1 (FOXO1-3’UTR) and mutant containing two mutated nucleotides in 3’-UTR of FOXO1 (FOXO1-3’UTR-mut). B: Western blotting analysis of FOXO1 expression in miR-21-transduced or miR-21-inhibited A172 cells. GAPDH served as the loading control. C: Luciferase assays of pGL3-FOXO1-3’UTR reporter in miR-21-transduced or miR-21-inhibited A172 cells. D: Luciferase assays of pGL3-FOXO1-3’UTR-mut reporter in miR-21-transduced or miR-21-inhibited cells. E: Representative micrographs (left) and quantification (right) of crystal violet stained cell colonies, indicating that down regulation of FOXO1 rescued the inhibitory effect of miR-21 on cell growth. F: Representative micrographs (left) and quantification of TUNEL positive cells in indicated A172 cells treated with cisplatin (20 μM) for 36 h, indicating that down regulation of FOXO1 rescued the promotion effect of miR-21 on cell apoptosis. *P < 0.05.
Discussion
miR-21 has been identified as possessing a potential oncogenic function involved in cell proliferation, differentiation, apoptosis, and chemosensitivity. Some studies have reported that miR-21 overexpression was common in various cancers including glioma [4-6]. But the mechanism of miR-21 on cell proliferation, apoptosis, and chemosensitivity is still ill-informed. The investigation of miR-21 effect on primary culture A172 cells and its definite mechanism suggests much more significance for exploring new study hotspot and developing new A172 therapeutic target. Current Statistics further confirms that miR-21 plays an important role in cell proliferation, apoptosis, and chemosensitivity on A172 cells. And miR-21 inhibitor can suppress A172 cell proliferation and promote the cisplatin induced apoptosis, in other words, miR-21 inhibitor can enhance the chemosensitivity of A172 cells. The conclusion agreed with the reports of Wong [9] and Zhou [10].
miR-21 exists a potential oncogenic function, but it cannot function by itself in glioma, the target protein of which is essential. According to the function assay, Bioinformatics analysis of the target gene indicated miR-21 could bind with FOXO1 mRNA and prevent FOXO1 protein translation via its 3’UTR ranged from 1975th base site to 1996th base site to regulate the former function. Dual-luciferase-reporter activities assay, Real-Time PCR and Western-blot were performed to testify the hypothesis. As expected, miR-21 suppressed FOXO1 subsequently reduced its effect. The mutation and RNA interference of FOXO1 further confirmed the key role and key sequence of FOXO1. miR-21 is more likely to act as an oncogene via bind with FOXO1 mRNA and prevention FOXO1 protein translation. The mechanism can account for the relationship between poor prognosis of glioma and miR-21 overexpression, may also attribute to chemotherapy resistance in glioma [11].
As known, FOXO1 belongs to the fork head family of transcription factors that are characterized by a distinct fork head domain. The gene plays important roles in regulation of gluconeogenesis and glycogenolysis by insulin signaling, and is also central to the decision for a preadipocyte to commit to adipogenesis; however, recent study revealed FOXO1 could be a tumor-inhibiting factor [12,13]. And our statistics supported the conclusion. The study establishes a link between miR-21 and one of its targets FOXO1, which will explore new study hotspot and develop new A172 therapeutic target. miR-21 inhibitor can suppress A172 cell proliferation and enhance the chemosensitivity of A172 cells, which suggests that miR-21 inhibitor can be treated as a new target therapy in glioma.
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jovcevska I, Kocevar N, Komel R. Glioma and glioblastoma-how much do we (not) know? Mol Clin Oncol. 2013;1:935–941. doi: 10.3892/mco.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Yang YB, Zhang XH, Yu XL, Wang ZB, Cheng XC. MicroRNA-21 gene and cancer. Med Oncol. 2013;30:376. doi: 10.1007/s12032-012-0376-8. [DOI] [PubMed] [Google Scholar]
- 5.Orso F, Balzac F, Marino M, Lembo A, Retta SF, Taverna D. miR-21 coordinates tumor growth and modulates KRIT1 levels. Biochem Biophys Res Commun. 2013;438:90–96. doi: 10.1016/j.bbrc.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore LM, Zhang W. Targeting miR-21 in glioma: a small RNA with big potential. Expert Opin Ther Targets. 2010;14:1247–1257. doi: 10.1517/14728222.2010.527334. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Li G, Feng D, Qin H, Gong L, Zhang J, Zhang Z. MicroRNA-21 expression is associated with overall survival in patients with glioma. Diagn Pathol. 2013;8:200. doi: 10.1186/1746-1596-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong ST, Zhang XQ, Zhuang JT, Chan HL, Li CH, Leung GK. MicroRNA-21 inhibition enhances in vitro chemosensitivity of temozolomide-resistant glioblastoma cells. Anticancer Res. 2012;32:2835–2841. [PubMed] [Google Scholar]
- 10.Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi L, Liu N, Wang G, Pu P, You Y, Kang C. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol Rep. 2010;24:195–201. doi: 10.3892/or_00000846. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, Chen J, Yang J, Pan T, Zhang S, Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 13.Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]


