Abstract
Metadherin (MTDH) is highly expressed in many tumors and is involved in the proliferation, metastasis and drug resistance of tumor cells by regulating multiple signaling pathways. Our previous studies demonstrated that MTDH is overexpressed in diffuse large B cell lymphoma (DLBCL) and involved in apoptosis resistance, in part, via Wnt signaling. Here, we investigated the role of MTDH in the chemo-sensitivity of DLBCL. The study was performed in the DLBCL cell line LY8 to investigate the relationship between MTDH expression and doxorubicin (DOX) sensitivity in DLBCL. A MTDH interference model was developed in LY8 cells by transfected with lentivirus which is carrying MTDH interference sequence. Western blot was used to detect the protein expression. A CCK-8 assay was used to evaluate cell proliferation. The results showed that DOX treatment had no effect on the intracellular MTDH expression of LY8 cells. The proliferation of LY8 cells was inhibited after MTDH interference. MTDH interference increased the DOX sensitivity in the LY8 cell lines. The results suggested that MTDH is a potential therapeutic target in DLBCL, and it cooperates with DOX in treatment of DLBCL.
Keywords: Diffuse large B cell lymphoma, metadherin, doxorubicin, chemo-resistance, therapeutic
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin’s lymphoma (NHL) in adults, accounting for 30%-40% of NHL [1,2]. DLBCL can be divided into three subtypes, which include germinal center B-like DLBCL (GC-like DLBCL), activated B-cell-like DLBCL (ABC-like DLBCL) and the third type [3]. Currently, the standard treatments for DLBCL include the anthracycline-based combinatorial chemotherapy regimens R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone); when used as the first-line treatment regimen, these compounds have an approximately 60% cure rate [4]. Despite the improvements induced by the successful therapeutic effects of R-CHOP, patients who experience relapse and a refractory disease remain a challenge in the treatment of this disease [5,6]. Gene-expression analyses have revealed many different pathways that resulted in drug resistance in DLBCL. Further elaborating the molecular pathways and the associated resistance genes may contribute to the discovery of new therapeutic targets and would ultimately benefit the prognosis of DLBCL patients [7,8].
Metadherin (MTDH) was first found in astrocyte cells, which were treated with tumor necrosis factor (TNF)-α or infected with human immunodeficiency virus (HIV) [9,10]. MTDH was expressed in nearly all tumor cells and promoted tumors via a variety of mechanisms involved in tumor cell proliferation, survival and metastasis activity. Recently, MTDH was associated with the chemo-sensitivity and chemo-resistance of tumor cells by regulating series genes [11-15]. This effect resulted from the activation of phosphatidylinositol 3-kinase (PI3K)/AKT and nuclear factor (NF)-κB and increased multi-drug resistance (MDR1) translation by MTDH [13,16,17].
Our previous study demonstrated that MTDH is overexpressed in DLBCL primary cells and the DLBCL cell line LY8, which was associated with the apoptosis deregulation of DLBCL [18]. Here, we investigated its effect on proliferation in DLBCL and its role in DOX treatment in DLBCL using the LY8 cell line.
Materials and methods
Cell line culture and DOX treatment
The human DLBCL cell line LY8 was maintained in Iscove’s Modified Dulbecco’s Medium (IMDM; Hyclone, Logan, UT, USA) supplemented with 10% fetal calf serum (FBS, Hyclone, Logan, UT, USA) at 37°C and 5% carbon dioxide.
Western blot analyses
The proteins were lysed by RIPA containing 1% phenylmethanesulfonyl fluoride (PMSF) (Shenergy Biocolor, Shanghai, China). A BCA assay was used to detect the protein concentration. Forty μg of protein underwent 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were electrophoresed on a subsequent transfer membrane for 90 min under a voltage of 100 V. This was followed blocking by 5% milk at room temperature for 1 hour and then incubated with antibodies at 4°C for overnight. The concentration of the antibodies used in this study was as follows: anti-MTDH 1:250 (Invitrogen, Frederick, MD), anti-β-actin 1:2000 (Zhongshan Goldenbridge, Beijing, China). The next day, the samples and the secondary antibody were incubated for 30 min after the membrane was washed three times and developed using Multi Gauge Ver.4.0 software for analysis. The experiment was repeated three times.
Small interference RNAs
siRNAs that targeted MTDH and a control scrambled siRNA were synthesized by Shanghai Genechem Co. The sequencing was designed according to other reports, and the efficacy was detected by our previous studies [12,18,19]. The methods were as followed: 104 LY8 cells were plated into 96-wells culture plates and co-cultured with lentivirus with sequences of siRNA that interfered with the MTDH gene or a negative control in the total culture volume of 100 μl for 10 hours. The multiplicity of infection (MOI) was 100. The interfering efficiency in LY8 was determined by fluorescence microscope and flow cytometry after three days of transfection.
Proliferation assays
Seven days after transfection, 5 × 103 cells from different sources of subgroup species were plated in 96-well plates. Each sample was repeated three times. The culture system was 100 μl. After 24 hours in culture, 10 μl of CCK-8 reagent were added to the system for the detection of proliferation. After 4 hours of co-culture with CCK-8, the absorbances were detected at 450 nm wavelength.
Statistical methods
SPSS18.0 software for windows was used for data analysis. All data were analyzed by 2-tailed Student’s t tests, P<0.05.
Results
DOX inhibited the proliferation of LY8 and had no effect on MTDH expression
DOX is a basic therapeutic agent in DLBCL treatment. According to the literature, 0.1 ng/ml was chosen as the terminal concentration of DOX [20]. After treatment with DOX for 24 hours, a CCK-8 assay was used to detect the proliferation activity of the LY8 cell line. The results showed that the proliferation of LY8 was inhibited by approximately 30% (P<0.05) (Figure 1).
Figure 1.
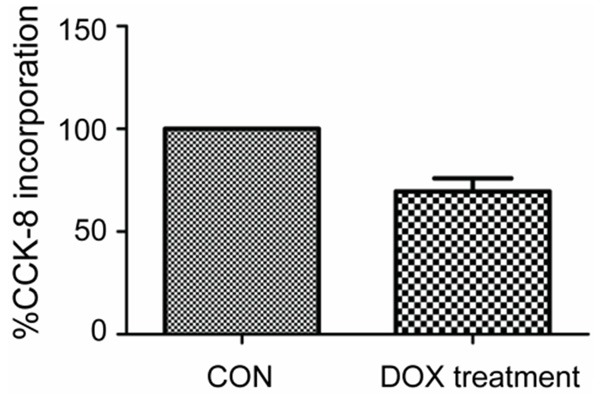
Proliferation change in LY8 after DOX treatment for 24 h. Compared to the CON without DOX treatment, proliferation in LY8 cells with DOX (0.1 ng/ml) treatment for 24 h was inhibited by approximately 30%.
Then, we investigated whether DOX treatment reduced the expression of MTDH in protein levels. The results showed after 24 hours in cultures with DOX, the expression of MTDH in LY8 was not significantly changed (P>0.05) (Figure 2).
Figure 2.
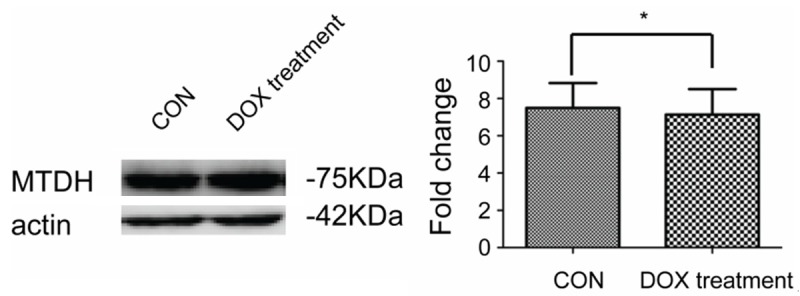
MTDH protein levels after treatment with DOX. After treatment with DOX for 24 h, there was no obvious change in the MTDH protein level. The studies were repeated three times, *P<0.05.
MTDH interference inhibited proliferation of LY8
The CCK-8 method was used to evaluate the proliferation change in the following studies. Lentivirus that contained a MTDH interference sequence or a negative control sequence was used to infect the LY8 cell lines. The transfection efficiency of the cell lines in this part of the study was evaluated by fluorescence microscopy and flow cytometry. The transfection efficiency was approximately 78% (Figure 3). Furthermore, the transfection efficiency was consistent with our previous results [18]. In this condition, the MTDH protein level in the MTDH interference LY8 cells was downregulated approximately 60% [18]. The CCK-8 method was then used to detect the proliferation changes in LY8 cells. After MTDH interference, the proliferation of LY8 cells was inhibited about 34% (Figure 4).
Figure 3.
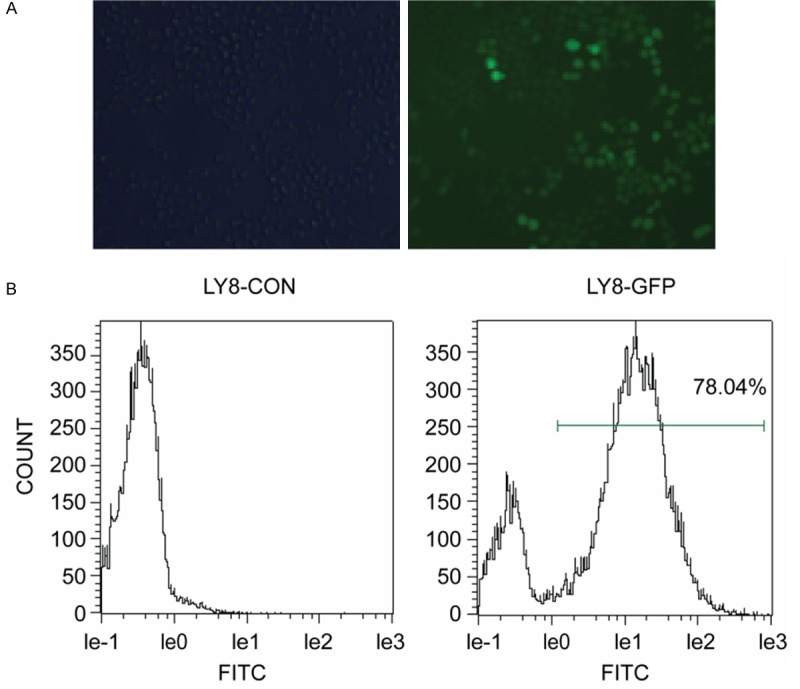
Transfection efficiency of LY8 cells. A. Fluorescence microscopy images showing lentivirus-infected cells. B. GFP expression in LY8 cells infected with lentivirus as determined by flow cytometry.
Figure 4.
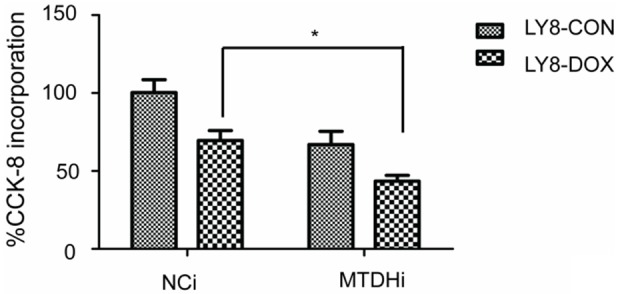
MTDH interference enhanced the chemo-sensitivity of DOX in LY8 cells. The proliferation rate in LY8 cells infected with lentivirus that contained the MTDH interference sequence (mtdhi) or a negative control sequence (nci) was 69.55±6.335 vs 43.46±3.694, respectively, *P<0.05.
MTDH interference enhanced DOX sensitivity
Then, we investigated whether MTDH interference enhanced the sensitivity of DOX in the LY8 cells. The CCK-8 method was used to evaluate the change in proliferation. The results showed that MTDH interference further enhanced the sensitivity of LY8 to DOX treatment, and the proliferation rate was further downregulated by approximately 26% (Figure 4).
Discussion
Our results showed that MTDH can promote the proliferation activity in DLBCL. Furthermore, MTDH can mediate the chemo-sensitivity of DOX in the DLBCL cell line LY8.
DOX was treated as a basic therapeutic agent in DLBCL. Clinically, clear differences exist in the prognosis of GCB-type and ABC-type DLBCL [3]. The traditional opinion insists that the activity of NF-κB signaling pathway is one of the most important features of ABC-type DLBCL. Hoverer, in the GCB-type DLBCL cell line LY8, the activity of the NF-κB pathway was upregulated [20,21]. Furthermore, the ABC-type DLBCL cell lines showed increased sensitivity to DOX treatment compared with the GCB-type in vitro, which is different from the result of patients with DLBCL after therapy in a clinical trial [20]. Thus, we chose the LY8 cell line, which belongs to the GCB-type DLBCL and has an intermediate sensitivity to DOX treatment, to evaluate the effects of MTDH in DOX chemo-sensitive cells.
Our data show that DOX at a concentration of 0.1 ng/ml inhibited 30% proliferation of LY8 after 24 h of treatment. There was no effect on MTDH expression during DOX treatment. In our previous study, we demonstrated that MTDH interference induced apoptosis of the LY8 cell line [18]. Here, we further evaluated the proliferation changes in the LY8 cell line. The results showed that MTDH interference inhibited the proliferation of LY8 cells. In MTDH interference cells, DOX had greater inhibitor activity in proliferation of LY8 cells. This finding indicated that MTDH interference is beneficial to DOX treatment in DLBCL.
DOX leads to DNA double strand breaks, which affects the cell cycle control, DNA repair and cell death. It is broadly used in the chemotherapeutic regimen of many malignancies, such as small cell lung cancer, breast cancer and lymphoma [22-24]. In other tumors, such as ovarian cancer and hepatic cancer, the inhibition activity of the PI3K or NF-κB pathways can enhance the sensitivity of the chemotherapy of DOX [25-27]. MTDH has been shown to be involved in promoting chemo-sensitivity and reversing the state of multidrug resistance of chemo-resistance to DOX in hepatic cancer, breast cancer and neuroblastoma via PI3K and NF-κB [28-30]. DLBCL has also been demonstrated to protect tumors cells from death induced by chemotherapy, in part, through the activation of the PI3K or NF-κB pathways [20,31,32]. This may be the downstream mechanism of MTDH, which promotes the sensitivity of DOX in DLBCL.
Therefore, our study results elucidate the functional significance of MTDH in the biology of DLBCL, and its role in regulating the chemo-sensitivity of DOX. Further research should focus on the concise mechanism of MTDH that enhances chemo-sensitivity. The relapse and refractory models should also be used to further investigate whether MTDH is involved in the chemo-resistance of DLBCL. These results may elucidate novel therapeutic targets in DLBCL and a potential therapeutic strategy to overcome drug resistance.
Acknowledgements
This work was supported by the National Natural Science Foundation (No. 81270598 and No. 31340009), the Natural Science Foun-dations of Shandong Province (No. Y2007C053, No. 2009ZRB14176, No. ZR2012HZ003 and No. ZR2011HQ009), the Technology Development Projects of Shandong Province (No. 2007GG10, No. 2010GSF10250 and 2012GSF11848), the National Public Health Grand Research Foundation (No. 201202017), the Program of Shandong Medical Leading Talent and the Taishan Scholar Foundation of Shandong Province.
Disclosure of conflict of interest
None.
References
- 1.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, Trichopoulos D. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 3.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 4.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, Chan WC, Iqbal J, Meyer PN, Lenz G, Wright G, Rimsza LM, Valentino C, Brunhoeber P, Grogan TM, Braziel RM, Cook JR, Tubbs RR, Weisenburger DD, Campo E, Rosenwald A, Ott G, Delabie J, Holcroft C, Jaffe ES, Staudt LM, Gascoyne RD. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. 2009;109:2989–3011. doi: 10.1021/cr9000226. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Ilizaliturri FJ, Czuczman MS. Therapeutic options in relapsed or refractory diffuse large B-cell lymphoma. Part 2. Novel therapeutic strategies. Oncology (Williston Park) 2009;23:610–615. [PubMed] [Google Scholar]
- 7.Li C, Thompson MA, Tamayo AT, Zuo Z, Lee J, Vega F, Ford RJ, Pham LV. Over-expression of Thioredoxin-1 mediates growth, survival, and chemoresistance and is a druggable target in diffuse large B-cell lymphoma. Oncotarget. 2012;3:314–326. doi: 10.18632/oncotarget.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell SA, Mousavi-Fard S. Non-Hodgkin’s B-cell lymphoma: advances in molecular strategies targeting drug resistance. Exp Biol Med (Maywood) 2013;238:971–990. doi: 10.1177/1535370213498985. [DOI] [PubMed] [Google Scholar]
- 9.Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 10.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, Leslie KK. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6:e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, Fisher PB, Sarkar D. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X, Thiel KW, Leslie KK. Drug resistance mediated by AEG-1/MTDH/LYRIC. Adv Cancer Res. 2013;120:135–157. doi: 10.1016/B978-0-12-401676-7.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y, Tong L, Chen X, Ji Y, Shang Q, Xu B, Chu M, Wei L. Metadherin confers chemoresistance of cervical cancer cells by inducing autophagy and activating ERK/NF-kappaB pathway. Tumour Biol. 2013;34:2433–2440. doi: 10.1007/s13277-013-0794-z. [DOI] [PubMed] [Google Scholar]
- 16.Vatsyayan R, Chaudhary P, Lelsani PC, Singhal P, Awasthi YC, Awasthi S, Singhal SS. Role of RLIP76 in doxorubicin resistance in lung cancer. Int J Oncol. 2009;34:1505–1511. doi: 10.3892/ijo_00000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emdad L, Das SK, Dasgupta S, Hu B, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv Cancer Res. 2013;120:75–111. doi: 10.1016/B978-0-12-401676-7.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge X, Lv X, Feng L, Liu X, Gao J, Chen N, Wang X. Metadherin contributes to the pathogenesis of diffuse large B-cell lymphoma. PLoS One. 2012;7:e39449. doi: 10.1371/journal.pone.0039449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 20.Houldsworth J, Petlakh M, Olshen AB, Chaganti RS. Pathway activation in large B-cell non-Hodgkin lymphoma cell lines by doxorubicin reveals prognostic markers of in vivo response. Leuk Lymphoma. 2008;49:2170–2180. doi: 10.1080/10428190802428369. [DOI] [PubMed] [Google Scholar]
- 21.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Song W, Tang Z, Lv S, Lin L, Sun H, Li Q, Yang Y, Hong H, Chen X. Nanoscaled poly(L-glutamic acid)/doxorubicin-amphiphile complex as pH-responsive drug delivery system for effective treatment of nonsmall cell lung cancer. ACS Appl Mater Interfaces. 2013;5:1781–1792. doi: 10.1021/am303073u. [DOI] [PubMed] [Google Scholar]
- 23.Fabbri A, Cencini E, Alterini R, Rubegni P, Rigacci L, Delfino C, Puccini B, Fimiani M, Bosi A, Bocchia M, Pimpinelli N. Rituximab plus liposomal pegylated doxorubicin in the treatment of primary cutaneous b-cell lymphomas. Eur J Haematol. 2014;93:129–36. doi: 10.1111/ejh.12315. [DOI] [PubMed] [Google Scholar]
- 24.Bourdeanu L, Dee V. Assessment of chemotherapy-induced nausea and vomiting in women with breast cancer: a Neuman systems model framework. Res Theory Nurs Pract. 2013;27:296–304. doi: 10.1891/1541-6577.27.4.296. [DOI] [PubMed] [Google Scholar]
- 25.Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 26.Gao AM, Ke ZP, Shi F, Sun GC, Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol Interact. 2013;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Bezler M, Hengstler JG, Ullrich A. Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances apoptosis of ovarian cancer cells. Mol Oncol. 2012;6:516–529. doi: 10.1016/j.molonc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian BJ, Yan F, Li N, Liu QL, Lin YH, Liu CM, Luo YP, Guo F, Li HZ. MTDH/AEG-1-based DNA vaccine suppresses lung metastasis and enhances chemosensitivity to doxorubicin in breast cancer. Cancer Immunol Immunother. 2011;60:883–893. doi: 10.1007/s00262-011-0997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J Exp Clin Cancer Res. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, Fisher PB, Sarkar D. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarosiek KA, Nechushtan H, Lu X, Rosenblatt JD, Lossos IS. Interleukin-4 distinctively modifies responses of germinal centre-like and activated B-cell-like diffuse large B-cell lymphomas to immuno-chemotherapy. Br J Haematol. 2009;147:308–318. doi: 10.1111/j.1365-2141.2009.07851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavan A, Spina M, Canzonieri V, Sansonno S, Toffoli G, De Re V. Recent prognostic factors in diffuse large B-cell lymphoma indicate NF-kappaB pathway as a target for new therapeutic strategies. Leuk Lymphoma. 2008;49:2048–2058. doi: 10.1080/10428190802444176. [DOI] [PubMed] [Google Scholar]


