Abstract
Findings from epidemiologic studies concerning red and processed meat intake and bladder cancer risk remain conflicting. Thus, we conducted this meta-analysis to examine the associations of red and processed meat intake with bladder cancer. Eligible studies published up to May 2014 were retrieved via both computer searches and review of references. Finally, we identified 14 studies on red meat (involving 9,084 cases) and 11 studies on processed meat (7,562 cases) involving up to 1,558,848 individuals. Random-effects models were used to estimate summary relative risk estimates (SRRE) based on high vs. low intake, and heterogeneity between study results was explored through stratified analyses on the basis of red/processed meat category, gender, study design and geographical region. Overall, the SRRE for all studies regarding red meat intake was 1.15 (95% CI: 0.97-1.36). Significant positive association was observed between processed meat consumption and bladder cancer (SRRE = 1.22; 95% CI: 1.04-1.43). Interestingly, increased by 25% and 33% risk of bladder cancer were observed for red meat and processed meat intake respectively in populations from the American continent. In conclusion, our fi ndings showed that there was an absence of an association between red meat intake and bladder cancer, but suggested that high consumption of processed meat probably correlated with rising risk of bladder cancer. In addition, positive relationships were observed regarding people intake of red and processed meat in the American continent. These findings need to be confirmed in future research.
Keywords: Bladder cancer, meat, meta-analysis, epidemiology, nutrition
Introduction
Bladder cancer is the fourth most common cancer in men and the ninth most common incident in women, with the incidence ranked fifth in the United States [1]. It presents a substantial challenge to public health. More than 50,000 men and 16,000 women are diagnosed with bladder cancer each year in the United States [2]. An increasing trend in incidence and mortality rates has been observed for bladder cancer in the past 30 years.
The past few years have seen growing much attention in the etiology of bladder cancer and have identified many factors as being causally associated with the risk of this malignancy, with most factors being lifestyle or genetic in nature. Smoking, occupational exposures to aromatic amines and Schistosoma haematobium infestation were well-known risk factors [3].
Many epidemiologic studies have investigated the link between red or processed meat intake and bladder cancer, however, their findings varied between studies, with some positive associations observed in some studies [4,5] and other studies observing no association [6,7]. A previous meta-analysis summarized several epidemiologic studies and reported that greater consumption of red and processed meat significantly elevated by 17% (95% CI: 1.02-1.34) and 10% (95% CI: 1.00-1.21) risk of bladder cancer, respectively, when comparing the highest category of red or processed meat intake with the lowest category [8].
Of note, the primary outcome of the cases defined in Wang et al. [8] analysis involved bladder cancer and other urinary cancer as well, thus providing the results not entirely accurate. It would be valuable if taking into account this remark and then providing a new accurate estimation. With recently accumulating evidence [6,9], our purpose was to estimate the summary relative risk of bladder cancer only at level of high compared to low intake of red or processed meat and examine potential sources of heterogeneity across studies by conducting a meta-analysis of epidemiological studies published up to May 2014.
Materials and methods
Literature search
We performed this meta-analysis in accordance with Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [10]. A PubMed database search through May 2014, was conducted to identify eligible studies that estimated the association between red or processed meat intake and bladder cancer risk. The primary search string included the following terms: (“bladder” and “carcinoma” OR “cancer” OR “tumor” OR “neoplasms”) AND (meat OR beef OR pork OR lamb). The search focused on human studies, without restriction on language. Additionally, we performed hand searches via the references of all retrieved publications again in an effort to identify all available literature that may not have been identified by the PubMed search.
Inclusion criteria
The study would be included in our analysis if it met the following criteria: (1) The study is an original study in human beings; (2) The study has reported the association between red and processed meat intake and bladder cancer risk between 1980 and February, 2012; (3) The study was required to report a point estimate (i.e., relative risks RR or odds ratio OR) and its variability (i.e., 95% confidence intervals CI) for the highest category of red or processed meat intake compared with the lowest category or information sufficient to calculate them. If there were multiple publications of the same or overlapping study population, only the studies published the most recently were included. The studies that reported only data for a broad classification of meat or total meat were excluded. They may include poultry or fish. The other exclusion criteria were (1) duplicates; (2) irrelevant data reported; (3) cross-sectional, experimental and mechanistic analyses.
Red meat is commonly defined as beef, pork, lamb, or a combination thereof, and processed meat is generally defined as meat made largely from beef, pork, or poultry that undergoes methods of preservation, such as salted, smoking or drying [11]. The definitions of red meat and processed meat varied across studies. Most studies reported data for variables labeled as ‘red meat’, ‘processed’ or ‘preserved’ meat, although some studies reported data for single meat items, such as beef, pork, lamb, or hamburger.
Data extraction
We extracted the information in a standardized data collection form from each included study. The information was extracted including: the first author, the year of publication, the country, study design, sample size, methods of dietary exposure ascertainment, red meat and processed meat dietary variables, the analytical comparison, the number of exposed cases, the RR or OR, 95% confidence intervals, and the factors adjusted in the analyses. Considering that bladder cancer is a rare disease, the RR was assumed approximately the same as OR. In case of multiple data sets, we used the results from the main multivariable model that included most adjusted confounders. The discrepancy in data extraction was resolved by repeating the study review and discussion.
Statistical analysis
Random effects models were used to calculate summary relative risk estimates (SRRE) for the highest compared with the lowest category of red or processed meat intake, 95% confidence intervals, and corresponding P-values for heterogeneity. Forest plots were applied to assess the relation between red or processed meat and bladder cancer. When data for single food items were reported separately in the original study (e.g. data for beef and data for pork), we first combined them using a fixed effects model, then this weighted average was used in our random effects meta-analysis models.
To explore the sources of heterogeneity across studies, we created meta-analysis models of studies that reported associations for a variable labeled as ‘red/processed meat’, while excluding single red/processed meat items, or for the variable ‘single red/processed meat items’ with excluding associations for red/processed meat. In addition, Subgroup analyses were also performed based on gender, study design, and geographic region. Furthermore, a further sensitivity analysis was performed to explore sources of heterogeneity. Each study was omitted in turn to assess robustness of the results.
We used the tests of Egger and Begg to assess publication bias [12]. Considering both tests have low power to detect potential bias, we set P = 0.1 as our statistical penalty. All statistical analyses were done with Stata Statistical Software, version 11.0. A P value of less than 0.05 indicates significance except where specifically noted.
Results
We identified 61 articles that examined the risk of bladder cancer with red and/or processed meat consumption published until 2014 May. Upon closer examination, 46 articles were excluded, as they did not provide sufficient information to estimate a summary odds ratio and its 95% confidence intervals or reported a broad classification of meat [13,14], or the studies that the primary outcome of the cases involved other urinary tract cancer besides bladder cancer were excluded [15-17]. At last, 14 studies on red meat (involving 9,084 cases) and 11 studies on processed meat (7,562 cases) with a total study population up to 1,558,848 individuals were included in our analysis [4-7,9,18-27]. The characteristics of the 15 articles included in this meta-analysis are presented in Table 1. Six large cohort studies [6,7,18-21] with 1,532,823 participants, and nine case-control studies [4,5,9,22-27] with 26,025 participants, were identified that evaluated the association of red and/or processed meat and bladder cancer, and reported data that could be meta-analyzed. Food frequency questionnaires (FFQs) was utilized in the majority of studies to ascertain dietary information pertaining to meat consumption. Seven of these studies were conducted in Europe [6,18,22-24,26,27], while four were in the United States [7,9,19,20], two in Uruguay [4,24], one in Canada [5] and one in Japan [21]. Four articles reported associations between consumption of specific red meat (pork) [23,24] or processed meat (ham or sausage) and the risk of bladder cancer [21,25]. The included studies were published between 1980 and May 2014.
Table 1.
Characteristics of studies that analyzed red meat or processed meat consumption and bladder cancer
| Author and year | Study location | Casesa/Subjiects | Exposure Variable (Definition) | Casesb | Analytical comparison (high vs. low intake) | Adjustments |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| Jakszyn 2011 [6] | Europe | 1,001/481,419 | Red meat (fresh and processed meat) | 355 | Quarter of intake Q4 vs. Q1 | Total energy intake, smoking, educational level and BMI |
| Ferrucci 2010 [7] | USA | 854/300,933 | Red meat (bacon, beef, cold cuts, liver, ham, hot dogs, pork, sausage, and steak) | 226 | Quintiles of intake Q5 vs. Q1 | Age, sex, smoking, vegetables beverages fruit and total energy |
| Processed meat (bacon, ham, hotdogs sausage and luncheon meats) | 200 | Q5 vs. Q1 | ||||
| Larsson 2009 [18] | Sweden | 485/82,002 | Red meat (beef, pork, meatballs hamburger, veal and kidney or liver) | 164 | ≥ 5 servings/week vs 0-3 servings/month | Age, sex, smoking, education and total energy intake |
| Processed meat (ham, sausage and salami or cold cuts) | 215 | ≥ 5 servings/week vs. 0-3 servings/month | ||||
| Beef, pork, or veal | 21 | ≥ 5 servings/week vs. 0-3 servings/month | ||||
| Hamburger | 39 | ≥ 5 servings/week vs. 0-3 servings/month | ||||
| Sausage (fried, grilled, or boiled) | 41 | ≥ 5 servings/week vs. 0-3 servings/month | ||||
| Cross 2007 [19] | USA | 1,666/494,036 | Red meat (beef, pork and lamb; bacon, beef, cold cuts, ham, hamburger, hot dogs, liver, pork, sausage, and steak) | Age, sex, smoking, education, marital status, family history of cancer, race, BMI, frequency of vigorous physical activity, total energy intake, and fruit and vegetable consumption | ||
| Processed meat (bacon, red and poultry meat sausage, luncheon meats, cold cuts, ham, regular hot dogs) | ||||||
| Michaud 2006 [20] | USA | 808/135,893 | Beef, pork, or lamb | 72 | ≥ 5 servings/week vs. 0 | Age, smoking, caloric, geographic region and total fluid intake |
| Processed meat (bacon; hamburger) | 39 | ≥ 5 servings/week vs. 0 | ||||
| Hamburger | 242 | 2-4 servings/week vs. 0 | ||||
| Nagano 2000 [21] | Japan | 114/38,540 | Ham/sausage | 26 | 2+/week vs. 0/week | Age, gender, smoking, radiation exposure, educational, BMI and calendar time |
| Case-control studies | ||||||
| Lin 2012 [9] | USA | 884/1,762 | Red meat (beef, veal, lamb, pork, game) | 319 | Quartiles of intake Q4 vs. Q1 | Age, sex, smoking, BMI, energy intake, ethnicity, total vegetable intake, total fruit intake |
| Processed meat (hot dogs or sausage) | 268 | Q4 vs. Q1 | ||||
| Aune 2009 [4] | Uruguay | 254/5,571 | Red meat (beef and lamb) | 47 | Tertile of intake T3 vs. T1 | Age, sex, smoking, BMI, energy residence, education, milk, fish, income, fruits, interviewer, alcohol, grains, fatty foods, poultry and vegetables |
| Beef | 39 | T3 vs. T1 | ||||
| Lamb | 18 | T3 vs. T1 | ||||
| Processed meat | 77 | T3 vs. T1 | ||||
| Hu 2008 [5] | Canada | 1,029/6,068 | Red meat (beef, pork, or lamb as a main or mixed dish and hamburger) | NR | Quartiles of intake Q4 vs. Q1 | Age, sex, smoking, BMI, energy intake, province, education, alcohol, total of vegetable and fruit intake |
| Processed meat (bacon, smoked meat, hotdogs, corned beef and sausage) | NR | Q4 vs. Q1 | ||||
| García 2007 [22] | Spain | 912/1,785 | Red meat (beef, veal, pork and lamb) | 324 | Quintiles of intake Q5 vs. Q1 | Age, gender, smoking, region and quintiles of fruit and vegetable intake |
| Processed red meat | 368 | Q5 vs. Q1 | ||||
| Baena 2006 [23] | Spain | 74/163 | Pork | 68 | ≥ 3 servings/week vs. 0-3 servings/month | Age, smoking and fluid intake |
| Radosavljević 2005 [24] | Serbia | 130/260 | Pork | 48 | Tertile of intake T3 vs. T1 | Multivariable analysis |
| Balbi 2001 [25] | Uruguay | 124/744 | Red meat (beef and lamb) | NR | Tertile of intake T3 vs. T1 | Age, sex, smoking, BMI, total calories, education, residence, urban/rural status and ‘mate’ drinking |
| Salted meat | NR | T3 vs. T1 | ||||
| Barbecue | NR | T3 vs. T1 | ||||
| Tavani 2000 [26] | Italy | 431/8,451 | Red meat (beef, veal and pork) | 127 | ≥ 6 portions/week vs. 0-3 portions/week | Age, sex, smoking, education, residence, alcohol, milk, fruits and vegetables |
| Riboli 1991 [27] | Spain | 432/1,221 | Red meat (beef, pork and lamb) | NR | Quarter of intake Q4 vs. Q1 | Smoking and energy intake |
| Preserved meat | NR | Q4 vs. Q1 |
BMI: body mass index; NR: not reported.
Number of bladder cancer in study.
Number of cases in highest intake category.
The meta-analysis results for red meat and processed meat consumption and bladder cancer are summarized in Table 2. Five cohort studies [6,7,18-20] and nine case-control studies [4,5,9,22-27] were included in the meta-analysis model of total red meat. No association was observed between consumption (high vs. low intake) of red meat and bladder cancer risk (SRRE = 1.15, 95% CI: 0.97-1.36), and substantial heterogeneity was detected (P-value for heterogeneity < 0.0001) (Table 2; Figure 1). Of these 14 studies, 12 studies reported results for ‘red meat’ (as a category) while single red meat items (i.e., beef, pork, and/or lamb) were reported in the other two studies. The summary association was similar after excluding reported data for single red meat items only (SRRE = 1.15, 95% CI: 0.97-1.35; P-value for heterogeneity < 0.0001) (Table 2). The summarized association restricted to prospective cohort data only was also approximately null (SRRE = 1.08, 95% CI: 0.97-1.20) without any variability (P-value for heterogeneity = 0.236). Whereas, the summary association for the nine case-control studies was stronger in magnitude (SRRE = 1.23, 95% CI: 0.91-1.67), and the individual study point estimates were more variable (P-value for heterogeneity < 0.0001). Summary associations were similar in the subgroups stratified by gender (Male, SRRE = 1.04, 95% CI: 0.64-1.69; Female, SRRE = 0.97, 95% CI: 0.75-1.27).
Table 2.
Summary of meta-analysis results for red meat or processed meat consumption (high intake vs. low intakea) and bladder cancer
| Analysis specifications | Studies | Total cases | Total population | SRRE (95% CI) | P-Heterogeneity |
|---|---|---|---|---|---|
| Red meat | |||||
| Total red meat | 14 | 9,084 | 1,520,308 | 1.15 (0.97-1.36) | 0.000 |
| Red meat category only | 12 | 8,880 | 1,519,885 | 1.15 (0.97-1.35) | 0.000 |
| Single red meat items | 2 | 204 | 423 | 1.22 (0.21-7.03) | 0.008 |
| Cohort studies | 5 | 4,814 | 1,494,283 | 1.08 (0.97-1.20) | 0.236 |
| Case-control studies | 9 | 4,270 | 26,025 | 1.23 (0.91-1.67) | 0.000 |
| Men | 5 | 3,199 | 620,458 | 1.04 (0.64-1.69) | 0.000 |
| Women | 3 | 2,693 | 619,074 | 0.97 (0.75-1.27) | 0.009 |
| Europe | 7 | 3,464 | 575,301 | 1.03 (0.77-1.39) | 0.000 |
| America | 7 | 5,620 | 945,007 | 1.25 (1.02-1.54) | 0.001 |
| Processed meat | |||||
| Total processed meat variables | 11 | 7,562 | 1,068,555 | 1.22 (1.04-1.43) | 0.002 |
| Processed meat category | 9 | 7,324 | 1,029,271 | 1.25 (1.09-1.43) | 0.031 |
| Single processed meat items | 2 | 238 | 39,284 | 1.71 (0.32-9.16) | 0.000 |
| Cohort studies | 5 | 3,927 | 1,051,404 | 1.08 (0.96-1.20) | 0.553 |
| Case-control studies | 6 | 3,635 | 171,51 | 1.46 (1.10-1.95) | 0.002 |
| Europe | 3 | 1,829 | 850,08 | 1.10 (0.93-1.30) | 0.608 |
| America | 7 | 5,619 | 945,007 | 1.33 (1.06-1.67) | 0.001 |
The intake contrast (i.e., exposure vs. referent group) for each study is reported in Table 1.
Figure 1.
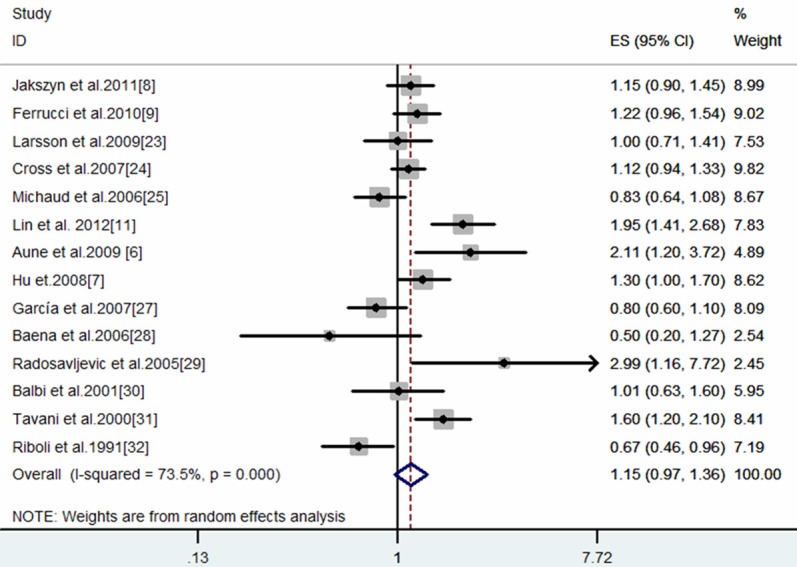
Meta-analysis of red meat intake and bladder cancer (high vs. low intake).
In the subgroups according to geographical region, a significant positive association was detected between red meat intake and bladder cancer in the American continent (U.S., Canada and Uruguay) [4,5,7,9,19,20,25]. The bladder cancer risk remarkably increased by 25% on the basis of comparisons between the highest and lowest quartiles of red meat intake (SRRE = 1.25, 95% CI: 1.02-1.54; P-value for heterogeneity = 0.001) (Table 2; Figure 3A). In contrast, no statistically significant association was founded in Europe (SRRE = 1.03, 95% CI: 0.77-1.39; P-value for heterogeneity < 0.0001) [6,18,22-24,26,27]. The Begg (P = 0.91) and Egger (P = 0.83) tests, as well as visual inspection of the funnel plot (not shown), did not suggest a publication bias.
Figure 3.
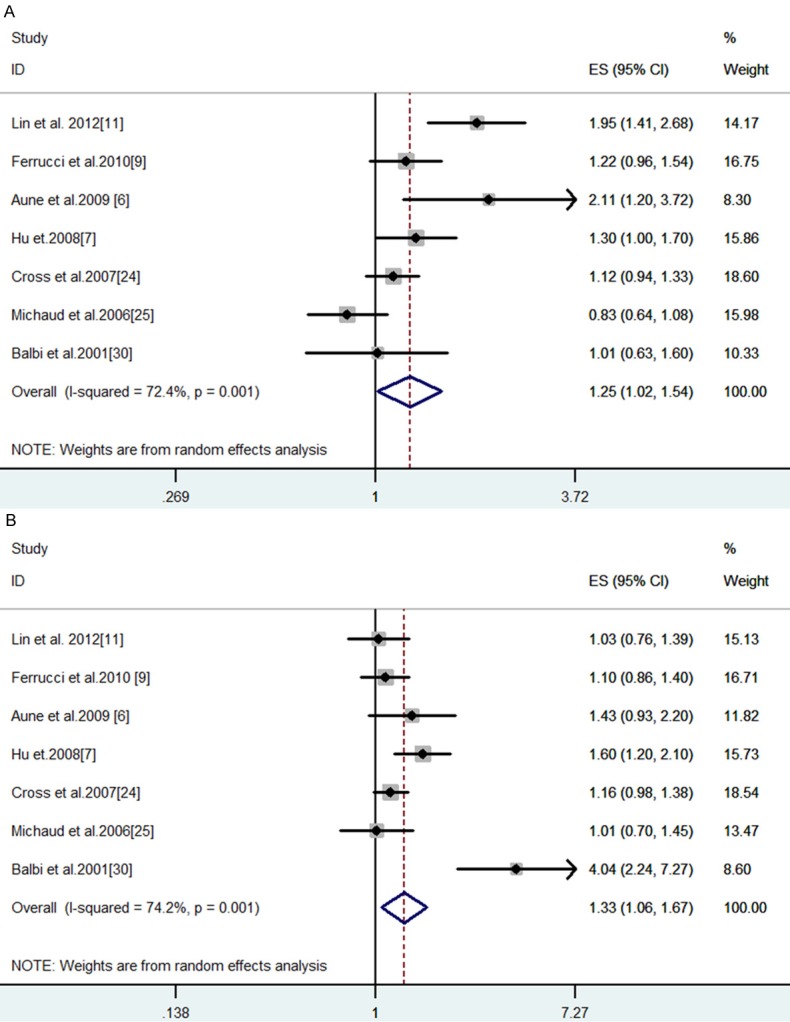
Meta-analysis of red meat (A) and processed meat (B) intake and bladder cancer (high vs. low intake) in the America continent.
The combined effect between processed meat and bladder cancer was elevated (SRRE = 1.22, 95% CI: 1.04-1.43; P-value for heterogeneity = 0.002) (Table 2; Figure 2). The summary association for the processed meat category only was similar to the total model (SRRE = 1.25, 95% CI: 1.09-1.43, P-value for heterogeneity = 0.031). Only two studies reported single processed meat items, the SRRE was 1.71 (95% CI: 0.32-9.16, P-value for heterogeneity < 0.0001). After stratification according to study design, a significant positive association was noted in the summary association from case-control studies only (SRRE = 1.46, 95% CI: 1.10-1.95; P-value for heterogeneity = 0.002) [4,5,9,22,25,27], while no association was observed in the subgroups of cohort studies (SRRE = 1.08, 95% CI: 0.96-1.20; P-value for heterogeneity = 0.553) (Table 2) [7,18-21]. After stratification by geographical region, differences were found among the studies in the American continent and Europe, with SRRE of 1.33 (95% CI: 1.06-1.67; P-value for heterogeneity = 0.001) (Figure 3B) respectively and 1.10 (95% CI: 0.93-1.30; P-value for heterogeneity = 0.608). The Begg (P = 0.44) and Egger (P = 0.35) tests, as well as visual inspection of the funnel plot (not shown), did not suggest a publication bias.
Figure 2.
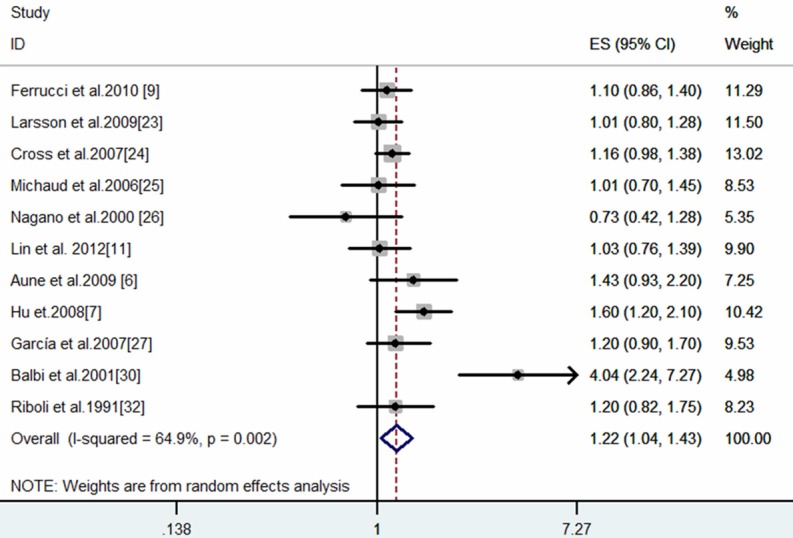
Meta-analysis of processed meat intake and bladder cancer (high vs. low intake).
In addition, we also performed further sensitivity analyses to examine the influence of a single study on the overall estimates. The results showed that none of the study considerably affected the summary of risk estimates in our meta-analysis (data not shown).
Discussion
The association between red or processed meat intake and bladder cancer risk has rapidly received much attention, and the results were inconsistent. Here, we pooled six large cohort studies and nine case-control studies involving 9,198 cases and 1,558,848 participants to get a more stable and creditable result. The results suggested no significant overall association between red meat intake and bladder cancer incidence, but indicating a positive association between processed meat intake and bladder cancer. Among the subgroup analyses based on red/processed meat category, study design, gender, America or European continent, interestingly, increased by 25% and 33% risk of bladder cancer were observed for red meat and processed meat intake respectively in populations from the American continent.
Our study was focused on that the primary outcome defined in the included original article was only bladder cancer, while Wang et al [8]. contained several other types of urinary cancer as well. We could not exclude the possibility that this unmeasured factor may contribute to the results not entirely accurate. Consequently, with recently published evidence [6,9], the number of studies in our analysis on red or processed meat intake and bladder cancer included was more than the previous meta-analysis, and potentially providing a stable and creditable result. Still, the findings of the two meta-analyses on processed meat intake and bladder cancer are consistent. However, the completely novel finding of our analysis compared with the meta-analysis by Wang et al. [8] was an absence of an association between high intake of red meat and bladder cancer incidence, whereas Wang et al. reported an elevated risk of bladder cancer (SRRE=1.07, 95% CI: 1.02-1.34, P-value for heterogeneity = 0.027) [8].
Substantial heterogeneity was observed in our analysis and Wang et al.’s [8] meta-analysis. Not surprisingly, analytical comparisons of red or processed meat intake vary much across included studies and different units were used (g/d, times/d, frequency/wk, times/mon and servings/wk). Those variabilities may, to some extent, contribute to the observed heterogeneity. In this present study, little heterogeneity was observed among cohort studies and studies conducted in Europe regarding processed meat intake. The differences in study design and geographical region might explain proportions of the observed heterogeneity between the individual studies. The pooled results from other subgroups were heterogeneous. However, we did not detect evident publication bias in our meta-analysis based on Egger’s and Begg’s tests.
Overall, most summary associations for red meat were not statistically significant; moreover, significant association was observed between processed meat intake and bladder cancer and many of the pooled results were positive. The highest intake of processed meat probably increased by 22% risk of bladder cancer compared with the lowest intake. Processed meat is a mixed category of meats that are preserved by a variety of mechanical, chemical or enzymatic procedures. Nitrate and nitrite are used in meat products [11]. Concerning significant association between processed meat intake and risk of bladder cancer, one key hypothesis for bladder carcinogenesis involves nitrate and nitrite compounds added to processed meat for preservation and enhancement of color and fl avor. Nitrate and nitrite are precursors to N-nitroso compounds (NOCs), which induce tumors in many organs, including the bladder, in multiple animal species [29]. Additional NOC formation can also occur directly in the bladder when bacterial infection occurs. The N-nitrosamides and N-nitrosoureas have been shown to be direct mutagens [29,30].
In the subgroup analysis by geographical region, we found a significant increase in breast cancer risk with intake of red and processed meat in the American continent. Of note, these positive associations are mainly driven by findings in Uruguay. The stronger summary effects in Uruguay may be attributed to the Uruguayan diet characterized by a low intake of fruits, vegetables and whole grains and by the highest per capita meat consumption in the world [2]. However, the definite underlying mechanism involved in the association between red and processed meat intake and rising risk of bladder cancer in the American population is uncertain.
Our study has several limitations that need to be considered when interpreting our findings. First, the definitions of red or processed meat, categories of exposure, and analytical comparisons vary differently in individual inlcuded studies. Second, because diet in the majority of studies was assessed by food frequency questionnaires (FFQs) to ascertain dietary information pertaining to meat consumption, though the number of food items in the FFQs varied across studies, some measurement error of meat intake assessment is inevitable. Third, though evident publication bias was not detected by Egger’s and Begg’s tests, we could not rule it out because these tests were underpowered. An additional limitation is that genetic factors were not considered in risk assessment in most studies. We could not preclude the possibility that it may affect the association.
In conclusion, our findings based on 1,558,848 participants showed that red meat intake is not associated with bladder cancer, but suggested that high consumption of processed meat probably correlated with rising risk of bladder cancer. In addition, positive relationships were observed regarding people intake of red and processed meat in the American continent. These findings need to be confirmed in future research.
Acknowledgements
This work was supported by Dean’s research fund of Nanfang Hospital, the Southern Medical University (2013C022).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.La Rochelle J, Kamat A, Grossman HB, Pantuck A. Chemoprevention of bladder cancer. BJU Int. 2008;102:1274–8. doi: 10.1111/j.1464-410X.2008.07970.x. [DOI] [PubMed] [Google Scholar]
- 3.Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, Malats N. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007;25:285–95. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 4.Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:429–36. [PubMed] [Google Scholar]
- 5.Hu J, La Vecchia C, DesMeules M, Negri E, Mery L. Meat and fi sh consumption and cancer in Canada. Nutr Cancer. 2008;60:313–24. doi: 10.1080/01635580701759724. [DOI] [PubMed] [Google Scholar]
- 6.Jakszyn P, González CA, Luján-Barroso L, Ros MM, Bueno-de-Mesquita HB, Roswall N, Tjønneland AM, Büchner FL, Egevad L, Overvad K, Raaschou-Nielsen O, Clavel-Chapelon F, Boutron-Ruault MC, Touillaud MS, Chang-Claude J, Allen NE, Kiemeney LA, Key TJ, Kaaks R, Boeing H, Weikert S, Trichopoulou A, Oikonomou E, Zylis D, Palli D, Berrino F, Vineis P, Tumino R, Mattiello A, Peeters PH, Parr CL, Gram IT, Skeie G, Sánchez MJ, Larrañaga N, Ardanaz E, Navarro C, Rodríguez L, Ulmert D, Ehrnström R, Hallmans G, Ljungberg B, Roddam AW, Bingham SA, Khaw KT, Slimani N, Boffetta PA, Jenab M, Mouw T, Michaud DS, Riboli E. Red Meat, Dietary Nitrosamines, and Heme Iron and Risk of Bladder Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2011;20:555–59. doi: 10.1158/1055-9965.EPI-10-0971. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci LM, Sinha R, Ward MH, Graubard BI, Hollenbeck AR, Kilfoy BA, Schatzkin A, Michaud DS, Cross AJ. Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer. 2010;116:4345–53. doi: 10.1002/cncr.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Jiang H. Meat intake and risk of bladder cancer: a meta-analysis. Med Oncol. 2012;29:848–55. doi: 10.1007/s12032-011-9985-x. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Forman MR, Wang J, Grossman HB, Chen M, Dinney CP, Hawk ET, Wu X. Intake of red meat and heterocyclic amines, metabolic pathway genes, and bladder cancer risk. Int J Cancer. 2012;131:1892–903. doi: 10.1002/ijc.27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Alexander DD, Cushing CA. Quantitative assessment of red meat or processed meat consumption and kidney cancer. Cancer Detect Prev. 2009;32:340–51. doi: 10.1016/j.cdp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Li F, An SL, Zhou Y, Liang ZK, Jiao ZJ, Jing YM, Wan P, Shi XJ, Tan WL. Milk and dairy consumption and risk of bladder cancer: a meta-analysis. Urology. 2011;78:1298–1305. doi: 10.1016/j.urology.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70:532S–38S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 14.Radosavljević V, Janković S, Marinković J, Dokić M. Non-occupational risk factors for bladder cancer: a case-control study. Tumori. 2004;90:175–80. doi: 10.1177/030089160409000203. [DOI] [PubMed] [Google Scholar]
- 15.Steineck G, Norell SE, Feychting M. Diet, tobacco and urothelial cancer. A 14-year follow-up of 16,477 subjects. Acta Oncol. 1988;27:323–27. doi: 10.3109/02841868809093549. [DOI] [PubMed] [Google Scholar]
- 16.Chyou PH, Nomura AM, Stemmermann GN. A prospective study of diet, smoking, and lower urinary tract cancer. Ann Epidemiol. 1993;3:211–16. doi: 10.1016/1047-2797(93)90021-u. [DOI] [PubMed] [Google Scholar]
- 17.Wakai K, Hirose K, Takezaki T, Hamajima N, Ogura Y, Nakamura S, Hayashi N, Tajima K. Foods and beverages in relation to urothelial cancer: case-control study in Japan. Int J Urol. 2004;11:11–9. doi: 10.1111/j.1442-2042.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsson SC, Johansson JE, Andersson SO, Wolk A. Meat intake and bladder cancer risk in a Swedish prospective cohort. Cancer Causes Control. 2009;20:35–40. doi: 10.1007/s10552-008-9214-x. [DOI] [PubMed] [Google Scholar]
- 19.Cross AJ, Leitzmann MF, Gail MH. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:1973–85. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud DS, Holick CN, Giovannucci E, Stampfer MJ. Meat intake and bladder cancer risk in 2 prospective cohort studies. Am J Clin Nutr. 2006;84:1177–83. doi: 10.1093/ajcn/84.5.1177. [DOI] [PubMed] [Google Scholar]
- 21.Nagano J, Kono S, Preston DL, Moriwaki H, Sharp GB, Koyama K, Mabuchi K. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. Int J Cancer. 2000;86:132–38. doi: 10.1002/(sici)1097-0215(20000401)86:1<132::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.García-Closas R, García-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, Tardón A, Carrato A, Castaño-Vinyals G, Dosemeci M, Moore L, Rothman N, Sinha R. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer. 2007;43:1731–40. doi: 10.1016/j.ejca.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Baena AV, Allam MF, Del Castillo AS, Díaz-Molina C, Requena Tapia MJ, Abdel-Rahman AG, Navajas RF. Urinary bladder cancer risk factors in men: a Spanish case-control study. Eur J Cancer Prev. 2006;15:498–503. doi: 10.1097/01.cej.0000215618.05757.04. [DOI] [PubMed] [Google Scholar]
- 24.Radosavljević V, Janković S, Marinković J, Dokić M. Diet and bladder cancer: a case-control study. Int Urol Nephrol. 2005;37:283–89. doi: 10.1007/s11255-004-4710-8. [DOI] [PubMed] [Google Scholar]
- 25.Balbi JC, Larrinaga MT, De Stefani E, Mendilaharsu M, Ronco AL, Boffetta P, Brennan P. Foods and risk of bladder cancer: a case-control study in Uruguay. Eur J Cancer Prev. 2001;10:453–58. doi: 10.1097/00008469-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Tavani A, La Vecchia C, Gallus S, Lagiou P, Trichopoulos D, Levi F, Negri E. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86:425–28. doi: 10.1002/(sici)1097-0215(20000501)86:3<425::aid-ijc19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Riboli E, González CA, López-Abente G, Errezola M, Izarzugaza I, Escolar A, Nebot M, Hémon B, Agudo A. Diet and bladder cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49:214–19. doi: 10.1002/ijc.2910490212. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, La Vecchia C, Morrison H, Negri E, Mery L. Salt, processed meat and the risk of cancer. Eur J Cancer Prev. 2011;20:132–39. doi: 10.1097/CEJ.0b013e3283429e32. [DOI] [PubMed] [Google Scholar]
- 29.Joosen AM, Kuhnle GG, Aspinall SM, Barrow TM, Lecommandeur E, Azqueta A, Collins AR, Bingham SA. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009;30:1402–07. doi: 10.1093/carcin/bgp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagengast FM, Grubben MJ, van Munster I. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A:1067–70. doi: 10.1016/0959-8049(95)00216-6. [DOI] [PubMed] [Google Scholar]


