Abstract
Although there have been recent advances in surgery, radiotherapy, and chemotherapy, the survival of patient with glioma remains poor. Increased expression of polymeric immunoglobulin receptor (pIgR) in tumor tissue has been detected in various cancer forms. However, the clinical relevance of pIgR in glioma remains unclear. The aim of this study was to assess the prognostic value of pIgR in patients with glioma after surgical resection. pIgR expression was evaluated by immunohistochemistry in paraffin-embedded glioma tissues from 146 patients. The relation between pIgR expression and clinicopathologic factors and long-term prognosis in these 146 patients was retrospectively examined. The prognostic significance of negative or positive pIgR exspression in glioma was assessed using Kaplan-Meier survival analysis and log-rank tests. Positive expression of pIgR was statistically significantly associated with poor prognosis of patients with glioma. Our results indicated that pIgR could be a novel predictor for poor prognosis of patients with glioma after surgical resection.
Keywords: Glioma, polymeric immunoglobulin receptor (pIgR), prognosis
Introduction
Gliomas are the most common primary brain tumors in the central nervous system and are also the most lethal and least successfully treated solid tumors [1]. The World Health Organization (WHO) classification grading system for human gliomas is usually used to evaluate the prognosis of glioma patients. According to the WHO guidelines [2], gliomas are histologically classified into four grades: pilocytic astrocytoma (grade I), diffuse astrocytoma (grade II), anaplastic astrocytoma (grade III) and glioblastoma multiforme (GBM, grade IV). Among these, the relatively slower-growing WHO I-II lesions are referred to as low grade gliomas and the more rapidly growing WHO III-IV lesions are referred to as high-grade gliomas. Gliomas arise from the constituent glial cells of the brain, or their precursors, and diffusely invade surrounding brain, making curative surgical resection almost impossible [3]. Despite progress in tumor diagnosis and treatment, including surgery, radiotherapy and chemotherapy, the median survival time is only one year and few patients survive for two years [4]. However, some glioma patients with similar grades have obvious discrepancies in survival. It is therefore necessary to identify some new certain tumor biomarkers that are more suitable for the prognostic assessment of gliomas than the grading system.
The polymeric immunoglobulin receptor (pIgR) is a transporter of dimeric IgA (dIgA) and pentameric IgM (pIgM), which are the first-line antibodies in response to initial infection. Widely expressed in epithelial cells, pIgR expression is also commonly increased by proinflammatory cytokines in response to viral or bacterial infections, thus linking innate and adaptive immunity [5-8]. Up-regulation of pIgR was detected in colon cancer [9], breast cancer [10,11], endometrial carcinoma [12,13], bladder carcinoma [14], and hepatocellular carcinoma (HCC) [15,16]. High levels of the cleaved extracellular domain of pIgR, designated as the secretory component, were also detected in the sera of patients with lung cancer [17,18], pancreatic cancer [19], and colon cancer with liver metastasis [20]. However, the clinical relevance of pIgR in gliomas remains uncertain.
In the present study, formalin-fixed and paraffin-embedded tissues and the clinicopathological parameters from 146 patients with glioma were collected and the expression level of the pIgR protein was evaluated by immunohistochemistry. Furthermore, the associations of patient prognosis and clinicopathological parameters with the expression of pIgR protein were investigated. To the best of our knowledge, this is the first study to detect pIgR expression in gliomas and to show a correlation between its expression level and patient prognosis.
Materials and methods
Patients and tumor tissue samples
Paraffin-embedded glioma tissue samples were obtained from 146 patients undergoing surgical resection at the Department of Neurosurgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine and Department of Neurosurgery, Taizhou Hospital, Wenzhou Medical University from January 1998 to December 2010. None of 146 patients had received chemotherapy or radiotherapy before resection. This study was approved by the Research Ethics Committee of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine and Taizhou Hospital, Wenzhou Medical University. Written informed consent was obtained from all of the patients. All the slides were reevaluated according to WHO classifications [2,21] by two pathologists. A total of 83 males and 63 females (1.32:1) were enrolled in this study, and the median age was 45 years (range, 15-78). Fortythree of the 146 gliomas were classified as low-grade 25 pilocytic astrocytomas (WHO I) and 18 diffuse astrocytomas (WHO II), and 103 were classified as high-grade gliomas 53 anaplasia astrocytomas (WHO III), and 50 primary glioblastomas (WHO IV). All patients were assessed by the Karnofsky Performance Status (KPS) scale: (1) minor disability (80 to 100 points); (2) moderate disability (60 to 70 points); and (3) severe disability (10 to 50 points) [22]. The clinicopathological features and the treatment strategies of all the patients were indicated in Table 1.
Table 1.
Clinicopathological features of 146 patients with gliomas
| Features | WHO grade | |||
|---|---|---|---|---|
|
| ||||
| WHO I | WHO II | WHO III | WHO IV | |
| Patients (n) | 25 | 18 | 53 | 50 |
| Mean age (year) | 39.7 | 45.9 | 44.1 | 44.2 |
| Gender | ||||
| Male (n) | 15 | 8 | 26 | 34 |
| Female (n) | 10 | 10 | 27 | 16 |
| KPS score | ||||
| ≥ 80 (n) | 21 | 15 | 12 | 12 |
| < 80 (n) | 4 | 3 | 41 | 38 |
| Surgery | ||||
| Gross total resection (n) | 25 | 18 | 30 | 38 |
| Partial resection (n) | 0 | 0 | 23 | 12 |
| Adjuvant treatment | ||||
| Radiotherapy (n) | 0 | 0 | 32 | 15 |
| Chemotherapy (n) | 0 | 0 | 0 | 6 |
| Radiotherapy and chemotherapy combination (n) | 0 | 0 | 7 | 23 |
Immunohistochemistry
Selected tumor specimen were fixed in 10% neutral-buffered formalin and embedded in paraffin. Five micromolar sections were cut, dewaxed, rehydrated, and subjected to antigen retrieval. After blocking endogenous peroxidase activity, the sections were incubated with the primary antibody against pIgR (Epitomics, Burlingame, CA) (1:100) (overnight at 4 at 4°C). Immunohistochemistry was performed using the streptavidin-biotin peroxidase complex method (Lab Vision, Fremont, CA). The slides were examined and pictures were taken using an Olympus BX60 (Olympus, Japan). Sections known to stain positively were incubated in each batch and negative controls were also prepared by replacing the primary antibody with preimmune sera.
Expression analysis of pIgR in tumor tissue was performed by comparing staining intensity and the percentage of immunoreactive cells. Staining intensity was arbitrarily scored on a scale of four grades: 0 (no staining of cancer cells), 1 (weak staining), 2 (moderate staining), and 3 (strong staining), and the percentage of positive cells was scored as follows: 0 (0%), 1 (1% to 25%), 2 (26% to 50%), and 3 (> 50%). pIgR staining positivity was determined using the following formula: overall score = positive percentage score × intensity score. A score of 0 was defined as “0”, > 0 to ≤ 2 as “1”, > 2 to ≤ 6 as “2”, and > 6 to ≤ 9 as “3”. In the end, tumor samples rated as level 0 or 1 were defined as negative for expression, whereas samples rated as level 2 or 3 were defined as positive.
Follow-up
Patient follow-up consisted of assessment of CT and MRI every 3 months for the first 5 years. The patients were followed up until death or until the date of last follow-up. Follow-up was finished on December 31, 2013. The median follow-up was 28.2 months (range, 3-58 months).
Statistical analysis
All statistical analyses were performed by SPSS 16.0 software (SPSS, Chicago, IL). Data are expressed as a mean ± SEM. Clinicopathologic parameters were analyzed using the two-tailed chi-square test, and the two-tailed t test was used to evaluate association between pIgR expression and clinicopathologic parameters. Overall survival (OS) curves for positive- and negative-pIgR patients were estimated with the Kaplan-Meier method, and the survival functions were compared by the log rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. Factors that significantly influenced overall survival were used in the Cox proportional regression model for multivariate analysis. All P-values were considered statistically significant when the associated probability was less than 0.05.
Results
pIgR expression in glioma
We evaluated the expression of pIgR in 146 paraffin-embedded glioma tissue samples using the method of immunohistochemical staining. We found that among these 146 samples, pIgR was positive in 101/146 (69.2%) glioma tissue samples (Figure 1). These results suggested that pIgR might play a key role in glioma. Table 2 showed the distribution of pIgR expression level in 146 glioma tissue samples and the relationship between pIgR expression level and clinicopathologic characteristics, including age, gender, and histological grade.
Figure 1.
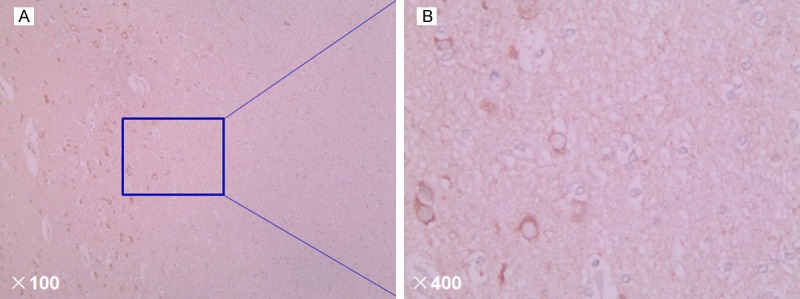
Representative immunohistochemical staining of pIgR in glioma tissues. pIgR protein was mainly expressed in the membrane with brown yellow (original magnification, A: × 100; B: × 400).
Table 2.
Clinicopathological characteristics in relation to pIgR expression in patients with glioma (n = 146)
| Clinicopathological features | Patients (n) | pIgR expression | P value | |
|---|---|---|---|---|
|
| ||||
| Positive (n, %) | Negative (n, %) | |||
| WHO grade | ||||
| I | 25 | 17 | 8 | < 0.001 |
| II | 18 | 13 | 5 | |
| III | 53 | 37 | 16 | |
| IV | 50 | 34 | 16 | |
| Gender | ||||
| Male | 83 | 59 | 24 | NS |
| Female | 63 | 42 | 21 | |
| Age (year) | ||||
| < 55 | 50 | 33 | 17 | NS |
| ≥ 55 | 96 | 68 | 28 | |
| KPS score | ||||
| ≥ 80 | 60 | 41 | 19 | NS |
| < 80 | 86 | 60 | 26 | |
Note: NS, the difference with no statistical significance.
Positive-pIgR is associated with poor survival in patients with glioma
The OS curves for glioma patients subdivided on the basis of pIgR expression are shown in Figure 2. Positive-pIgR expression was associated with poor prognosis in glioma patients (log-rank test, P < 0.001). Univariate analysis showed that pIgR-positive patients had a significantly poorer prognosis than pIgR-negative patients (P < 0.001; Table 3). Multivariate analysis showed that positive-pIgR expression was an independent and significant predictor in OS (Table 4).
Figure 2.
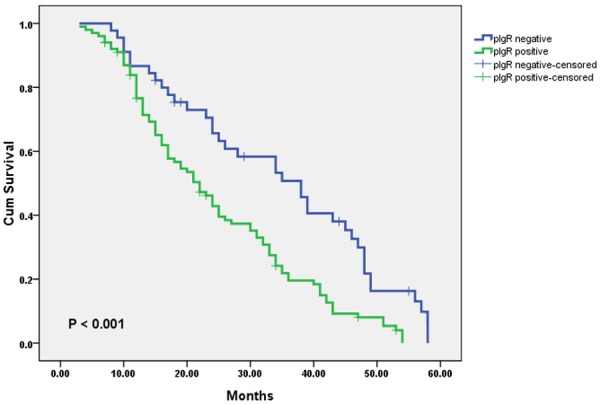
Kaplan-Meier survival curves of patients with glioma undergoing surgical resection, grouped by pIgR expression in tumor tissues. The survival rate for patients with glioma in the pIgR-negative expression group (n = 45) was significantly higher than that for patients in the pIgR-positive expression group (n = 101, log-rank, P < 0.001).
Table 3.
Univariate analysis of overall survival of glioma patients after surgicalresection
| Factor | Overall survival | ||
|---|---|---|---|
|
| |||
| Patients (n) | P value | ||
| pIgR expression in glioma tissue samples | Positive | 101 | < 0.001 |
| Negative | 45 | ||
| Total | 146 | ||
Table 4.
Multivariate analysis of overall survival of glioma patients after surgicalresection
| Factor | HR (95% CI) | P value |
|---|---|---|
| pIgR expression in glioma tissue samples (Positive) | 2.371 (1.523-3.373) | < 0.001 |
HR, hazards ratio. CI, confidence interval.
Discussion
Glioma accounts for nearly one-third of all intrinsic neoplasms in the central nervous system including well-differentiated low-grade astrocytomas, anaplastic astrocytomas, and glioblastoma [23]. This tumor is aggressive and has a tendency to invade the surrounding brain tissue. Although recent advances in surgery, radiotherapy, photodynamic therapy, and chemotherapy, survival of patients with gliomas remains poor. The median overall survival of patients with malignant gliomas is no more than 1 year and local recurrence occurs in more than 90% of patients [4]. It is extremely important to find biomarkers that can offer prognostic insight and eventually guide clinical treatment.
As the most common and deadly brain tumors, human gliomas have prompted many studies which focus on the genetic variation and molecular expression patterns in order to characterize different tumor subgroups, to understand the malignant tumor behavior and to identify valuable, reliable molecular targets for future targeted therapies. In the current study, our data showed that the abnormal expressions of pIgR protein appeared to be correlated with the WHO grade of glioma, clinicopathological features, as well as patient survivals. To the best of our knowledge, this is the first study to demonstrate the prognostic value of the expression of pIgR in gliomas.
The aim of this study was to evaluate the prognostic value of pIgR in patients with glioma after surgical resection. pIgR is a glycoprotein presents on glandular epithelial cells that functions as a receptor for polymeric immunoglobulin. pIgR transports polymeric immunoglobulin A (IgA) into external secretions as secretory IgA (S-IgA), which is critical for the defence of mucosal tissues [24]. As mentioned above, pIgR was overexpressed in tumor tissue of colon cancer [9], breast cancer [10,11], endometrial carcinoma [12,13], bladder carcinoma [14], and HCC [15,16], but its clinical relevance remains uncertain. The prognostic value of pIgR in patients with malignancy was also not ascertained. Ai and colleagues, for the first time, reported the clinical relevance of pIgR in HCC [16]. In their study, pIgR was identified as a prognostic biomarker for HCC and a molecular player in hepatitis B infection, chronic liver inflammation, the induction of the epithelial–mesenchymal transition, HCC recurrence, and metastatic progression [16]. Since the role of pIgR in glioma has not been studied so far, we investigated a set of 146 tumor samples immunohistochemically and correlated our findings with clinico-pathological parameters to also identify potential prognostic implications of pIgR in this brain tumor.
We evaluated the pIgR expression in paraffin-embedded glioma tissue samples from 146 patients with glioma, which had clinical follow-up records. The result of positive expression of pIgR was confirmed in 101 (69.2%) paraffin-embedded glioma tissue samples. Univariate analysis indicated significantly worse OS for patients with a positive pIgR expression in glioma tissues than for patients with a negative pIgR expression. Multivariate analysis showed positive-pIgR in glioma tissues to be an independent prognostic factor for OS after surgical resection (P < 0.001). These data, for the first time, imply that pIgR has distinct roles in glioma and is worthy of further investigation.
In summary, this is the first study showing positive expression of pIgR was statistically significantly associated with poor prognosis of patients with glioma. Our results indicated pIgR can be a novel predictor for poor prognosis of patients with glioma after surgical resection and pIgR might be a promising candidate for targeted therapy of glioma.
Acknowledgements
This work was supported by Zhejiang Provincial Medical and Healthy Science and Technology Projects (Grant No. 201466094).
Disclosure of conflict of interest
None.
References
- 1.Schneider T, Mawrin C, Scherlach C, Skalej M, Firsching R. Gliomas in adults. Dtsch Arztebl Int. 2010;107:799–807. doi: 10.3238/arztebl.2010.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–42. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 4.Parney IF, Hao C, Petruk KC. Glioma immunology and immunotherapy. Neurosurgery. 2000;46:778–91. doi: 10.1097/00006123-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Denning GM. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases. J Immunol. 1996;156:4807–14. [PubMed] [Google Scholar]
- 6.Kvale D, Løvhaug D, Sollid LM, Brandtzaeg P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J Immunol. 1988;140:3086–9. [PubMed] [Google Scholar]
- 7.Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–55. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- 8.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 9.Poger ME, Hirsch BR, Lamm ME. Synthesis of secretory component by colonic neoplasms. Am J Pathol. 1976;82:327–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Harris JP, Caleb MH, South MA. Secretory component in human mammary carcinoma. Cancer Res. 1975;35:1861–4. [PubMed] [Google Scholar]
- 11.Harris JP, South MA. Secretory component: a glandular epithelial cell marker. Am J Pathol. 1981;105:47–53. [PMC free article] [PubMed] [Google Scholar]
- 12.DeSouza LV, Krakovska O, Darfler MM, Krizman DB, Romaschin AD, Colgan TJ, Siu KW. mTRAQ-based quantification of potential endometrial carcinoma biomarkers from archived formalin-fixed paraffin-embedded tissues. Proteomics. 2010;10:3108–16. doi: 10.1002/pmic.201000082. [DOI] [PubMed] [Google Scholar]
- 13.DeSouza LV, Romaschin AD, Colgan TJ, Siu KW. Absolute quantification of potential cancer markers in clinical tissue homogenates using multiple reaction monitoring on a hybrid triple quadrupole/linear ion trap tandem mass spectrometer. Anal Chem. 2009;81:3462–70. doi: 10.1021/ac802726a. [DOI] [PubMed] [Google Scholar]
- 14.Rossel M, Billerey C, Bittard H, Ksiazek P, Alber D, Revillard JP, Vuitton DA. Alterations in polymeric immunoglobulin receptor expression and secretory component levels in bladder carcinoma. Urol Res. 1991;19:361–6. doi: 10.1007/BF00310151. [DOI] [PubMed] [Google Scholar]
- 15.Rossel M, Seilles E, Voigt JJ, Vuitton D, Legait N, Revillard JP. Polymeric Ig receptor expression in hepatocellular carcinoma. Eur J Cancer. 1992;28A:1120–4. doi: 10.1016/0959-8049(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 16.Ai J, Tang Q, Wu Y, Xu Y, Feng T, Zhou R, Chen Y, Gao X, Zhu Q, Yue X, Pan Q, Xu S, Li J, Huang M, Daugherty-Holtrop J, He Y, Xu HE, Fan J, Ding J, Geng M. The role of polymeric immunoglobulin receptor in inflammation-induced tumor metastasis of human hepatocellular carcinoma. J Natl Cancer Inst. 2011;103:1696–712. doi: 10.1093/jnci/djr360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao T, Ying W, Li L, Hu Z, Ma Y, Jiao L, Ma J, Cai Y, Lin D, Guo S, Han N, Di X, Li M, Zhang D, Su K, Yuan J, Zheng H, Gao M, He J, Shi S, Li W, Xu N, Zhang H, Liu Y, Zhang K, Gao Y, Qian X, Cheng S. An approach to studying lung cancer-related proteins in human blood. Mol Cell Proteomics. 2005;4:1480–6. doi: 10.1074/mcp.M500055-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Rossel M, Brambilla E, Billaud M, Vuitton DA, Blanc-Jouvan F, Biichle S, Revillard JP. Nonspecific increased serum levels of secretory component in lung tumors: relationship to the gene expression of the transmembrane receptor form. Am J Respir Cell Mol Biol. 1993;9:341–6. doi: 10.1165/ajrcmb/9.3.341. [DOI] [PubMed] [Google Scholar]
- 19.Makawita S, Smith C, Batruch I, Zheng Y, Rückert F, Grützmann R, Pilarsky C, Gallinger S, Diamandis EP. Integrated proteomic profiling of cell line conditioned media and pancreatic juice for the identification of pancreatic cancer biomarkers. Mol Cell Proteomics. 2011;10:M111.008599. doi: 10.1074/mcp.M111.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kvale D, Norstein J, Meling GI, Børmer OP, Brandtzaeg P, Langmark F, Rognum TO. Circulating secretory component in relation to early diagnosis and treatment of liver metastasis from colorectal carcinomas. J Clin Pathol. 1992;45:568–71. doi: 10.1136/jcp.45.7.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunbar E, Yachnis AT. Glioma diagnosis: immunohistochemistry and beyond. Adv Anat Pathol. 2010;17:187–201. doi: 10.1097/PAP.0b013e3181d98cd9. [DOI] [PubMed] [Google Scholar]
- 22.Wen PY, Brandes AA. Treatment of recurrent high-grade gliomas. Curr Opin Neurol. 2009;22:657–64. doi: 10.1097/WCO.0b013e32833229e3. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau A, Mokhtari K, Duyckaerts C. The 2007 WHO classification of tumors of the central nervous system - what has changed? Curr Opin Neurol. 2008;21:720–7. doi: 10.1097/WCO.0b013e328312c3a7. [DOI] [PubMed] [Google Scholar]
- 24.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]


