Abstract
To evaluate exclusion of prostate cancer (PCa) by using empiric antibiotic treatment for patients with total prostate specific antigen (PSA) between 4-10 ng/ml. A hundred asymptomatic men with a PSA between 4-10 ng/ml and normal digital rectal examination (DRE) were enrolled in this randomized prospective study. The treatment group (n=50) was given 400 mg of ofloxacin daily for 4 weeks, whereas the control group (n=50) was followed without any treatment. At the end of the four weeks, repeat PSA were measured and all patients underwent transrectal ultrasound (TRUS) guided biopsy, regardless of the repeat PSA levels. Totally 22 patients (22%) had prostate cancer (9 in treatment group and 13 in control group). A significant PSA decrease was observed in the treatment group at repeat PSA measurements (p=0.001). The PSA drop was also significantly more in patients without PCa than with PCa (p=0.028). In patients whose repeat PSA after antibiotic treatment decreased below 4 ng/ml, 2 times as many patients (16.6%) had PCa in the control group when compared with the treatment group (8.3%). On the other hand, in patients whose repeat PSA remained above 4 ng/ml, PCa was detected in 27.3% of the patients in the control group and 21% in the treatment group. Empirical antibiotic treatment in asymptomatic patients with a PSA level 4-10 ng/ml and a normal DRE may be used to select prostate biopsy candidates. Studies with higher number of patients may result in more powerful associations with narrower confidence intervals for increased confidence.
Keywords: Antibiotic, biopsy, prostate cancer, prostate-specific antigen
Introduction
The total prostate specific antigen (PSA), which was introduced in the late 1980s, is now the most widely used test to detect and follow prostate cancer (PCa) patients [1]. PSA is not cancer-specific. Its levels may be found elevated in some benign conditions such as benign prostate hyperplasia (BPH) and prostatitis [1]. Physiological fluctuation of PSA has also been reported [2].
The PSA range 4 10 ngml is commonly referred to as the diagnostic gray zone [3]. In clinical practice, many specialists use empiric antibiotic treatment followed by a repeat PSA in this group of patients; however, scientific evidence is not clear to support this approach [4,5]. In this study we aimed to examine whether 4 weeks of ofloxacin administration results in a PSA decrease which translates into a decreased risk of prostate cancer detection, whereby biopsy can be safely omitted in asymptomatic patients with an initial PSA level between 4-10 ng/ml.
Materials and methods
The study was conducted at a urology clinic in a tertiary care university hospital and was approved by the local ethics committee. Informed consent was obtained from all participants. From March 2008 to March 2009, 100 consecutive men that met the study criteria with a PSA level between 4-10 ng/ml were enrolled in this randomized prospective study. The following patients were not included in the study: any prostatitis symptoms or history, prior prostate surgery, biopsy or radiotherapy, any abnormal finding in digital rectal examination (DRE), an acute infection of the urinary system, or pyuria and/or bacterial growth in an asymptomatic patient, catheterization in the urinary system during the last 4 weeks, previous use of a 5-α reductase inhibitor, a hypersensitivity to any medical ingredient in the quinolone group.
The patients were primarily assessed with DRE, urinalysis and initial measurement (m1) of serum PSA and free PSA (fPSA) level. A total of 6 six patients refused to sign informed consent for randomization. Eligible 100 patients were randomly divided into two equal groups (n=50 each) using a random number table. None of the patients dropped out after randomization. The blood samples were obtained prior to DRE. Total and free PSA levels were assessed using Roche Elecsys® kit E lecsys System Roche Diagnostics, Mannheim, Germany). The treatment group was given 400 mg of ofloxacin daily for 4 weeks, whereas the control group was followed without any treatment. At the end of four weeks, repeat measurements (m2) were done for PSA and fPSA levels, and all patients underwent transrectal ultrasound (TRUS) guided prostate biopsy, regardless of the repeat PSA levels. In all of the patients TRUS was performed by the same urologist, with the 6 Mhz 150° endorectal probe (PVT-651 VT) of the Toshiba SSA-550A (Toshiba, Tokyo, Japan) device. Twelve-cores systematic transrectal ultrasonographic guided biopsies were obtained with a tru-cut biopsy needle of 18 G 20 cm attached to an automatic-spring driven biopsy gun. The samples were fixed in formaline and sent for pathologic investigation. Results including size of the prostate and pathological findings were reported. The same pathologist evaluated all pathological samples. Prostatic intraepithelial neoplasia (PIN) was not considered as a case of cancer.
Statistical analysis
Statistical Package for Social Sciences (SPSS) for Windows 16.0 was used in analyzing the statistical findings. T-test was used for continuous variables, which showed normal distribution. For nonnormal distributed variables, Mann-Whitney U and Wilcoxon signed rank tests were used. Categorical variables were compared with chi-square (x2) and Fisher’s exact test. Power calculations to confirm the adequacy of the sample size showed that a total sample size of 100 achieved 97.24% power to detect a difference in PSA between two groups with a significance level of 0.05. On the other hand power analysis for biopsy results in patients with PSA levels <4 ng/ml (1 patient in each group) 27.3%. All data are given as mean ± SD, mean ± SE or median ± IQR; a value of p<0.05 was considered statistically significant.
Results
The average age of the patients was 64.16±7.71 in the treatment group, and 64.52±8.61 in the control group. The groups were similar in terms of age, prostate volume, levels of PSA, fPSA, PSA density (PSAD) and percentage of fPSA (fPSA%) (Data not shown). No major biopsy-related complications were noted.
Table 1 shows the comparison of mean change (m2-m1) in PSA and fPSA measurements between the treatment and control groups using paired samples t-test. A significant difference was found between the treatment and control groups in terms of PSA and fPSA change (p=0.001 and p<0.001, respectively).
Table 1.
Comparison of mean decrease in repeat PSA and repeat fPSA between the treatment and control groups
| Parameters, mean ± SE | Treatment Group (n=50) | Control Group (n=50) | p value* |
|---|---|---|---|
| PSA (ng/ml) | 1.71±2.31 | 0.24±1.36 | 0.001 |
| fPSA (ng/ml) | 0.60±0.89 | 0.03±0.32 | <0.001 |
t-test.
Twelve patients (24%) in the treatment group had PSA levels below 4 ng/ml in repeat measurement and 6 patients (12%) in the control group (Fisher’s exact p=0.193). Overall, 22 patients had histologically proven PCa. Nine of these patients (18%) were in the treatment group and 13 (26%) were in the control group (chi-square, p=0.469). In patients whose repeat PSA after antibiotic treatment decreased below 4 ng/ml, 2 times as many patients (16.6%) had PCa in the control group when compared with the treatment group (8.3%). On the other hand, in patients whose repeat PSA remained above 4 ng/ml, PCa was detected in 27.3% of the patients in the control group and 21% in the treatment group (Table 2). One patient in each group with a repeat PSA level <4 ng/ml had cancer, and these patients’ Gleason scores were 3+3=6.
Table 2.
Distribution of PCa detection according to repeat PSA levels in patients with (+PCa) and without (-PCa) cancer
| PSA (ng/ml) | Treatment Group n+PCa/n-PCa (%) | Control Group n+PCa/n-PCa (%) | Total n+PCa/n-PCa (%) | p value* |
|---|---|---|---|---|
| <4 | 1/12 (8.3) | 1/6 (16.6) | 2/18 (11) | 0.569 |
| ≥4 | 8/38 (21) | 12/44 (27.3) | 20/82 (24.3) | 0.513 |
chi-square.
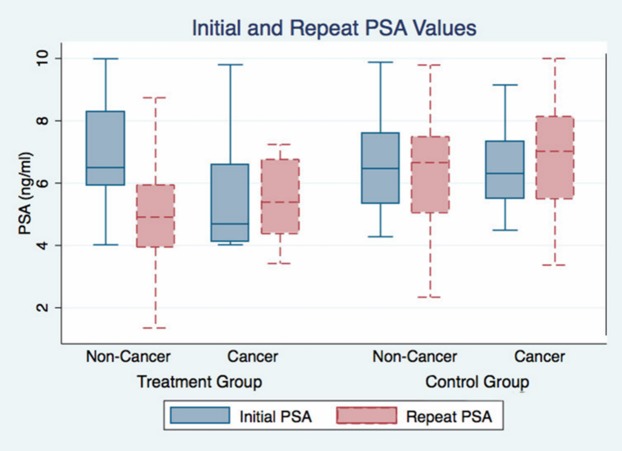
When 13 patients with PCa were compared with the remaining 37 in the control group, there was no statistically significant difference in PSA, fPSA, fPSA% and PSAD levels. In the treatment group, however, the PSA and PSAD drop at repeat measurement was significantly more in 41 patients without cancer compared with 9 patients with cancer (p=0.028, Table 3, Figure 1).
Table 3.
Comparison of mean change in PSA, fPSA, fPSA% and PSAD in patients with (+PCa) and without cancer (-PCa)
| Parameters, mean ± SE | Treatment Group (n=50) | Control Group (n=50) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| +PCa (n=9) | -PCa (n=41) | p value* | +PCa (n=13) | -PCa (n=37) | p value* | |
| PSA (ng/ml) | -0.44±1.64 | -1.99±2.35 | 0.028 | 0.13±0.99 | -0.37±1.46 | 0.293 |
| fPSA (ng/ml) | -0.24±0.31 | -0.67±0.96 | 0.160 | -0.01±0.15 | -0.03±0.37 | 0.707 |
| fPSA% | -0.03±0.04 | 0.05 ±0.57 | 0.357 | -0.00±0.03 | -0.02±0.07 | 0.911 |
| PSAD | -0.01±0.03 | -0.04±0.05 | 0.025 | 0.01±0.03 | -0.01±0.05 | 0.314 |
Mann-Whitney U test.
Figure 1.
Initial and repeat PSA levels in patients with and without prostate cancer in both groups.
Discussion
Serum PSA level, the most commonly used marker for prostate cancer, can rise in conditions other than cancer [6-9]. The use of other parameters such as PSAV, PSAD, and fPSA% has been studied to avoid unnecessary biopsies [4,10-12]. Prostate biopsy should be performed in patients with abnormal DRE findings, regardless of other parameters [13]. When the PSA level is between 4-10 ng/ml, the risk of cancer in the biopsy is approximately 20-30% [3]. There is a high percentage of patients with normal DRE in the PSA gray zone, subjected to unnecessary biopsy. This is still a problem to overcome. The purpose of our study was to determine the effect of antibiotic use in lowering PSA levels below threshold and its role in preventing unnecessary prostate biopsies. The strength of the present study is its randomized, controlled, prospective design. On the other hand, the weakness of the study is that no placebo was used in the control group.
There are numerous studies that show that inflammation in the prostate can lead to an increase in the PSA levels [14-20]. Carver et al. have reported 32% chronic prostatitis NIH-type-IV cases in a randomly chosen group of 300 men [14]. Anim et al. have evaluated 331 patients and observed subclinical prostatitis in 40% [15]. Also some studies showed that prostatic inflammation is not correlated with high PSA levels [7,21,22]. Various studies have reported that PSA levels decreased following antibiotic treatment [5,23,24]. However, the exact mechanism behind this decrease is not elucidated. Kaygisiz et al. administered 400 mg of ofloxacin for 3 weeks to asymptomatic patients whose PSA levels were between 4-10 ng/ml. The mean PSA level dropped from 6.53 ng/ml to 4.76 ng/ml following antibiotic treatment [23]. Schaeffer et al. compared the PSA decreasing effects of 4-weeks levofloxacin and ciprofloxacin treatment in 377 patients [25]. They showed a significant overall decrease in PSA after antibiotic treatment. In our study, we similarly showed in the treatment group that the decrease of mean PSA and fPSA levels after antibiotic treatment was statistically significantly different than that in the control group (Table 1).
The decrease in PSA level may be due to subclinical inflammation or physiological fluctuation as reported in 20% of the cases in a clinical trial with a large number of subjects [2]. In our control group, the change in mean PSA level was not significantly different (Table 3), but we observed a negative or positive fluctuation up to 54%, where 12% (n=6) of the patients had repeat PSA levels below 4 ng/ml.
The most noteworthy finding of our study is the difference in PSA decrease between patients with and without PCa in the treatment group. In our treatment group, mean PSA level at repeat measurement did not decrease significantly in patients with PCa while there was a significant drop in patients without PCa (Table 3). Higher number of patients certainly provides increased confidence with narrower confidence intervals. So, when supported with other studies, this information may be very useful to select prostate biopsy candidates after antibiotic use. Similar to our study, Erol et al. also used a control group and experienced a significant decrease after treatment in PSA levels in patients with pathological diagnosis of inflammation or BPH, but not in cancer patients. However, their inclusion criteria did not include an upper limit for PSA, and they did not exclude patients with abnormal DRE or prostatitis symptoms [26]. In the study by Serretta et al., a 3-week ciprofloxacin treatment was administered to 99 patients with PSA levels >4 ng/ml and normal DRE findings. Fifty-nine patients (59.6%) showed a drop in PSA levels. Cancer was found in 40% and 20.3% of the patients with unchanged or decreased PSA, respectively. No cancer was detected if PSA decreased below 4 ng/ml or more than 70% [27].
The risk of PCa detection in patients with PSA level between 3.1-4 ng/ml was reported to be 26.9% [28]. The study by Baltaci et al. showed that 5 of the 17 patients (30%) whose PSA level dropped below 4 ng/ml after antibiotic treatment had PCa [5]. When patients whose PSA level decreased below 4 ng/ml were taken into consideration, we detected cancer in 1 of the 12 patients (8.3%) in the treatment group, and in 1 of the 6 patients (16.6%) in the control group. The patient with PCa in the treatment group had a repeat PSA of 3.42 ng/ml and had a PSA reduction of 16%. On the other hand the only patient with PCa in the control group had a repeat PSA of 3.37 ng/ml and had a PSA reduction of 24%. Our findings support that the common practice of using the PSA value of 4 ng/ml as cutpoint may be important in the detection of PCa. In our study, 2 times as many patients in the treatment group had repeat PSA levels below 4 ng/ml compared to the control group (24% vs. 12%), and in the treatment group 2 times less PCa was detected than the control group (8.3% vs 16.6%) when PSA drops below 4 ng/ml after antibiotic treatment (Table 2). Although these differences were not statistically significant, our findings suggest that antibiotic treatment may help select patients who have decreased probability of harboring PCa.
Increasing drug resistance to commonly used antibiotics is an important point to address if one aims to evaluate the benefits of empirical antibiotic treatment. Several papers were published in last years which suggest that fluoroquinolones are not recommended for the first line or empirical therapy because of concerns regarding bacterial drug resistance [29,30]. Data shows that patients with recent exposure to fluoroquinolones are more likely to develop sepsis caused by fluoroquinolone-resistant E. Coli after TRUS guided prostate biopsy [4,29]. We did not observe any major biopsy-related complication including sepsis in our patients and we think that the search for the most appropriate antibiotic to be used in prostatitis and prostate biopsy patients should be continued.
In conclusion, 4 weeks ofloxacin treatment decreases PSA and fPSA levels in asymptomatic patients whose PSA levels are between 4-10 ng/ml. After antibiotic treatment, PSA decreases significantly more in patients without PCa. Antibiotic treatment may help select patients who have decreased probability of harboring PCa by effectively lowering PSA level below 4 ng/ml. Studies with higher number of patients may result in more powerful associations with narrower confidence intervals for increased confidence.
Disclosure of conflict of interest
None.
References
- 1.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 2.Komatsu K, Wehner N, Prestigiacomo AF, Chen Z, Stamey TA. Physiologic (intraindividual) variation of serum prostate-specific antigen in 814 men from a screening population. Urology. 1996;47:343–346. doi: 10.1016/s0090-4295(99)80450-6. [DOI] [PubMed] [Google Scholar]
- 3.Raaijmakers R, Wildhagen MF, Ito K, Paez A, de Vries SH, Roobol MJ, Schroder FH. Prostate-specific antigen change in the European Randomized Study of Screening for Prostate Cancer, section Rotterdam. Urology. 2004;63:316–320. doi: 10.1016/j.urology.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Scardino PT. The responsible use of antibiotics for an elevated PSA level. Nat Clin Pract Urol. 2007;4:1. doi: 10.1038/ncpuro0702. [DOI] [PubMed] [Google Scholar]
- 5.Baltaci S, Suer E, Haliloglu AH, Gokce MI, Elhan AH, Beduk Y. Effectiveness of antibiotics given to asymptomatic men for an increased prostate specific antigen. J Urol. 2009;181:128–132. doi: 10.1016/j.juro.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Carter HB, Morrell CH, Pearson JD, Brant LJ, Plato CC, Metter EJ, Chan DW, Fozard JL, Walsh PC. Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res. 1992;52:3323–3328. [PubMed] [Google Scholar]
- 7.Morote J, Lopez M, Encabo G, de Torres IM. Effect of inflammation and benign prostatic enlargement on total and percent free serum prostatic specific antigen. Eur Urol. 2000;37:537–540. doi: 10.1159/000020190. [DOI] [PubMed] [Google Scholar]
- 8.Kirkali Z, Kirkali G, Esen A. Effect of ejaculation on prostate-specific antigen levels in normal men. Eur Urol. 1995;27:292–294. doi: 10.1159/000475183. [DOI] [PubMed] [Google Scholar]
- 9.Oesterling JE, Rice DC, Glenski WJ, Bergstralh EJ. Effect of cystoscopy, prostate biopsy, and transurethral resection of prostate on serum prostate-specific antigen concentration. Urology. 1993;42:276–282. doi: 10.1016/0090-4295(93)90616-i. [DOI] [PubMed] [Google Scholar]
- 10.Seaman E, Whang M, Olsson CA, Katz A, Cooner WH, Benson MC. PSA density (PSAD). Role in patient evaluation and management. Urol Clin North Am. 1993;20:653–663. [PubMed] [Google Scholar]
- 11.Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, Richie JP, deKernion JB, Walsh PC, Scardino PT, Lange PH, Subong EN, Parson RE, Gasior GH, Loveland KG, Southwick PC. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–1547. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 12.Carter HB, Pearson JD. Prostate-specific antigen velocity and repeated measures of prostate-specific antigen. Urol Clin North Am. 1997;24:333–338. doi: 10.1016/s0094-0143(05)70380-3. [DOI] [PubMed] [Google Scholar]
- 13.Carvalhal GF, Smith DS, Mager DE, Ramos C, Catalona WJ. Digital rectal examination for detecting prostate cancer at prostate specific antigen levels of 4 ng/ml or less. J Urol. 1999;161:835–839. [PubMed] [Google Scholar]
- 14.Carver BS, Bozeman CB, Williams BJ, Venable DD. The prevalence of men with National Institutes of Health category IV prostatitis and association with serum prostate specific antigen. J Urol. 2003;169:589–591. doi: 10.1097/01.ju.0000042720.98483.08. [DOI] [PubMed] [Google Scholar]
- 15.Anim JT, Kehinde EO, Sheikh MA, Prasad A, Mojiminiyi OA, Ali Y, Al-Awadi KA. Serum prostate-specific antigen levels in Middle Eastern men with subclinical prostatitis. Med Princ Pract. 2007;16:53–58. doi: 10.1159/000096141. [DOI] [PubMed] [Google Scholar]
- 16.Ozden C, Ozdal OL, Guzel O, Han O, Seckin S, Memis A. The correlation between serum prostate specific antigen levels and asymptomatic inflammatory prostatitis. Int Urol Nephrol. 2007;39:859–863. doi: 10.1007/s11255-006-9125-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoekx L, Jeuris W, Van Marck E, Wyndaele JJ. Elevated serum prostate specific antigen (PSA) related to asymptomatic prostatic inflammation. Acta Urol Belg. 1998;66:1–2. [PubMed] [Google Scholar]
- 18.Kobayashi M, Nukui A, Morita T. Serum PSA and percent free PSA value changes after antibiotic treatment. A diagnostic method in prostate cancer suspects with asymptomatic prostatitis. Urol Int. 2008;80:186–192. doi: 10.1159/000112612. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Hu WL, Yang H, Qiu XF, Zhang CZ. [Effects of antibiotic and anti-inflammatory treatment on serum PSA and free PSA levels in patients with chronic prostatitis IIIA] . Zhonghua Nan Ke Xue. 2006;12:787–790. [PubMed] [Google Scholar]
- 20.Guercio S, Terrone C, Tarabuzzi R, Poggio M, Cracco C, Bollito E, Scarpa RM. PSA decrease after levofloxacin therapy in patients with histological prostatitis. Arch Ital Urol Androl. 2004;76:154–158. [PubMed] [Google Scholar]
- 21.Kwak C, Ku JH, Kim T, Park DW, Choi KY, Lee E, Lee SE, Lee C. Effect of subclinical prostatic inflammation on serum PSA levels in men with clinically undetectable prostate cancer. Urology. 2003;62:854–859. doi: 10.1016/s0090-4295(03)00688-5. [DOI] [PubMed] [Google Scholar]
- 22.Tchetgen MB, Oesterling JE. The effect of prostatitis, urinary retention, ejaculation, and ambulation on the serum prostate-specific antigen concentration. Urol Clin North Am. 1997;24:283–291. doi: 10.1016/s0094-0143(05)70374-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaygisiz O, Ugurlu O, Kosan M, Inal G, Ozturk B, Cetinkaya M. Effects of antibacterial therapy on PSA change in the presence and absence of prostatic inflammation in patients with PSA levels between 4 and 10 ng/ml. Prostate Cancer Prostatic Dis. 2006;9:235–238. doi: 10.1038/sj.pcan.4500885. [DOI] [PubMed] [Google Scholar]
- 24.Karazanashvili G, Managadze L. Prostate-specific antigen (PSA) value change after antibacterial therapy of prostate inflammation, as a diagnostic method for prostate cancer screening in cases of PSA value within 4-10 ng/ml and nonsuspicious results of digital rectal examination. Eur Urol. 2001;39:538–543. doi: 10.1159/000052500. [DOI] [PubMed] [Google Scholar]
- 25.Schaeffer AJ, Wu SC, Tennenberg AM, Kahn JB. Treatment of chronic bacterial prostatitis with levofloxacin and ciprofloxacin lowers serum prostate specific antigen. J Urol. 2005;174:161–164. doi: 10.1097/01.ju.0000162017.24965.2b. [DOI] [PubMed] [Google Scholar]
- 26.Erol H, Beder N, Caliskan T, Dundar M, Unsal A, Culhaci N. Can the effect of antibiotherapy and anti-inflammatory therapy on serum PSA levels discriminate between benign and malign prostatic pathologies? Urologia internationalis. 2006;76:20–26. doi: 10.1159/000089730. [DOI] [PubMed] [Google Scholar]
- 27.Serretta V, Catanese A, Daricello G, Liotta R, Allegro R, Martorana A, Aragona F, Melloni D. PSA reduction (after antibiotics) permits to avoid or postpone prostate biopsy in selected patients. Prostate Cancer Prostatic Dis. 2008;11:148–152. doi: 10.1038/sj.pcan.4500996. [DOI] [PubMed] [Google Scholar]
- 28.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 29.Young JL, Liss MA, Szabo RJ. Sepsis due to fluoroquinolone-resistant Escherichia coli after transrectal ultrasound-guided prostate needle biopsy. Urology. 2009;74:332–338. doi: 10.1016/j.urology.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 30.Arslan H, Azap OK, Ergonul O, Timurkaynak F. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56:914–918. doi: 10.1093/jac/dki344. [DOI] [PubMed] [Google Scholar]