Abstract
Elevated D-dimer is frequently found among cancer patients especially for advanced stage patients. Activation of the coagulation system and the fibrinolytic cascade are supposed to be associated with higher risk of invasion, metastases and worse outcome. The purpose of this study is to investigate the dynamic variation of plasma D-dimer and its relationship with other markers of the coagulation system including platelet counts, fibrinogen levels, prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT) in terminal stage cancer patients. We designed a self-controlled study to compare plasma D-dimer dynamic variation from 0-4 weeks to 4-8 weeks before patients’ death. The plasma D-dimer levels pointed an elevated tendency and revealed statistically significant difference as patients gradually near death. Prolonged PT, APTT and TT were found. D-dimer levels were positively correlated with PT, APTT and TT but showed negative correlation with platelet counts and fibrinogen levels. Plasma D-dimer levels gradually increased as terminal stage cancer patients approaching to death. Increasing D-dimer levels may predict worse outcome.
Keywords: D-dimer, dynamic variation, terminal stage, cancer, disease progression
Introduction
Thrombosis is a complication frequently seen in cancer patients. Studies showed that approximately 20% of the total burden of venous thrombosis was caused by cancer [1]. The relationship between cancer and hemostasis has been established. Malignant condition usually causes physiological changes leading to deterioration in the hemostatic status. Tumor growth, angiogenesis, metastasis and coagulation activation are often accompanied by the activation of the fibrinolytic system. D-dimer is a degradation product of fibrinogen and cross-linked fibrin, appear in the blood, signaling the activation of hemostasis and fibrinolysis. Increased D-dimer levels are often observed in patients with suspected venous thromboembolic events (VTE) [2] as well as in patients with other clinical conditions including pregnancy, surgery, sepsis, and critical illness [3]. There is increasing evidence that the coagulation cascade may also be triggered by oncogenic events, leading to a prothrombotic environment [4]. Importantly, rather than being merely a trigger of increased thromboembolic events, cancer induced hemostatic activity has been shown to promote tumor growth and cancer cell dissemination [5]. In cancer patients, the contributing factors for high D-dimer levels include chemotherapy, prolonged hospital stays, and tumor progression itself. It is generally believed that both the tumors, through production of procoagulant factors, and the host, through its inflammatory response, take part in the processes [6].
High D-dimer levels have been found to be associated with decreased survival in cancer patients with malignancies such as lung cancer [7,8], breast cancer [9], colorectal cancer [10] and ovarian cancer [11]. However, most studies focused the role of plasma D-dimer on operable early stage cancer patients, there was relatively few studies explore hypercoagulability in advanced cancer patients. In addition, coagulation status of cancer patients is a long-term dynamic process, so a single measurement of plasma D-dimer is not conducive to make accurate judgments about the disease progression of cancer patients. Our retrospective study was conducted to observe the dynamic variation of D-dimer levels and analysis the impact of plasma D-dimer on terminal stage cancer patients.
Materials and methods
Patients
We designed a retrospective self-controlled study using data obtained from medical records with approval from our institutional ethical review board. Eighty one (81) terminal stage malignant tumor patients were included in the present study. Patients were included if they met the following criteria: (1) histological confirmation of diagnosis (2) patients died of complications of malignancy or cachexy (3) adequate clinical information in the records (4) plasma D-dimer levels were recorded during 0-4 weeks period and 4-8 weeks period before patients’ death. Patients were excluded if the presence of any the following criteria: (1) venous or arterial thromboembolism before plasma D-dimer levels were measured; and continuous anticoagulation with vitamin K antagonists or low-molecular-weight heparins (2) surgery within the preceding 3 weeks and chemotherapy within 1 months prior to s plasma D-dimer levels were measured.
Blood sampling and reagents
Peripheral venous blood was collected in Vacutainer tubes (Becton Dickinson) containing 1/10 volume of 0.129 M sodium citrate. Samples were prepared by centrifugation at 2200 g for10 min. D-dimer, PT, APTT, TT and fibrinogen levels were determined on SYSMEX CA-7000, a fully automated multiparameter hemostasis analyzer equipped with a photo-optical clot detection unit and a cap-piercing system, according to the manufacturer’s instructions. Platelet counts were measured with Sysmex XE-2100™ Automated Hematology System. All coagulation parameters are measured on citrated plasma within 2 h of sample collection. At our institution, plasma D-dimer levels less than or equal to 250 ng/ml, blood platelet counts between 100 and 300 (×109/L), and plasma fibrinogen levels between 200 and 400 mg/dl are considered to be within the normal range.
Statistics
The results are reported either as mean or as median values depending on the type of distribution and interquartile range. Assessment of relationships between D-dimer and other markers of the coagulation system (platelet counts, fibrinogen levels, PT, TT and APTT) were accomplished using Pearson correlation analysis. Paired-Sample T test was performed to evaluate dynamic change of D-dimer, platelet counts and fibrinogen levels. SPSS17.0 was used for calculation of all these significance tests.
Results
Patient characteristics
The study involved 81 terminal stage cancer patients died of malignancy from September 2008 to September 2012. The 81 patients had a mean age of 62 ± 12 with 45 males and 36 females. Twenty-six patients had lung cancer, thirty patients had digestive tract malignancy, six had breast tumors, four had a pancreatic cancer and fifteen patients had other malignant tumors. Seventy-eight patients were stage IV, two patients were stage III b and one patient was III c. The results showed that, among all the patients, 38 had normal plasma D-dimer levels (≤ 0.25 mg/L) and 43 had elevated D-dimer levels (> 0.25 mg/L) 4-8 weeks before their death; however, 25 had normal plasma D-dimer levels (≤0.25 mg/L) and 56 had elevated D-dimer levels (> 0.25 mg/L) 0-4 weeks before their death. The test results and patient characteristics are listed in Table 1.
Table 1.
Characteristics of the patients
| Characteristic | No. (%) |
|---|---|
| Overall/N | 81 (100) |
| Sex | |
| Male | 45 (56) |
| Female | 36 (44) |
| Age/year | |
| ≤ 60 | 38 (47) |
| > 60 | 43 (53) |
| Tumor stage | |
| IV | 78 (96) |
| III b | 2 (2.5) |
| III c | 1 (1.5) |
| 0-4 weeks | |
| ≤ 0.25 mg/L | 25 (31) |
| > 0.25 mg/L | 56 (69) |
| 4-8 weeks | |
| ≤ 0.25 mg/L | 38 (47) |
| > 0.25 mg/L | 43 (53) |
| Site of cancer | |
| Lung | 26 (32) |
| Digestive Tract | 30 (37) |
| Breast | 6 (7.4) |
| Pancreatic | 4 (4.9) |
| Endometrium | 3 (3.7) |
| Ovarian | 2 (2.5) |
| Liver | 2 (2.5) |
| Prostate | 2 (2.5) |
| Others | 6 (7.5) |
Distribution of plasma D-dimer levels
The D-dimer levels of 53.1% of all examined terminal stage patients were above the normal reference cut-off value during 4-8 weeks period, however, during 0-4 weeks period, 69.1% of the patients displayed D-dimer levels above the upper limit of the normal cut-off point. A significant increasing tendency of plasm D-dimer was seen from 4-8 weeks to 0-4 weeks. Using Pearson χ2 Test to assess the distribution of plasma D-dimer values for the two periods, we found significant difference between the two periods that patients had higher D-dimer levels during the 0-4 weeks than during the 4-8 weeks, P = 0.036). The Paired-Samples T Test results showed much more significant elevated tendency (P < 0.001) (see Figure 1).
Figure 1.
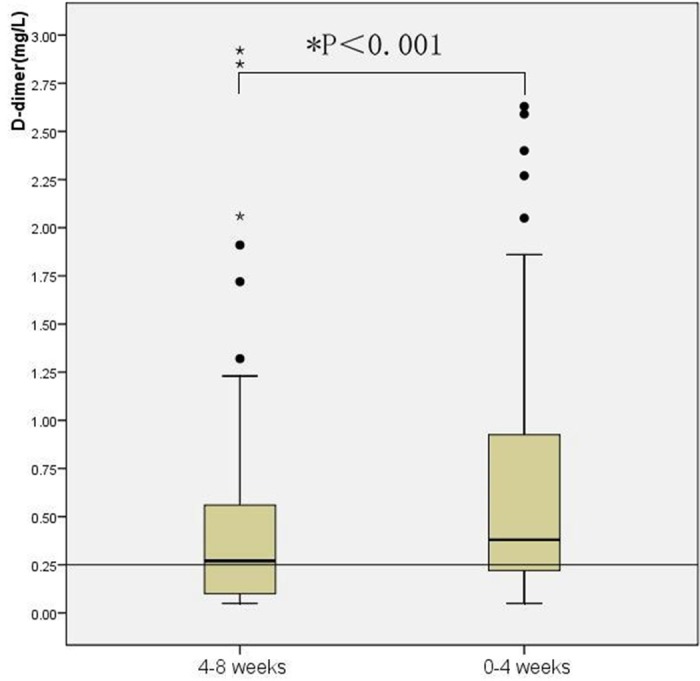
Dynamic variation of plasma D-dimer levels. Box plot of plasma D-dimer results from 4-8 weeks and 0-4 weeks before patients’ death. Box plots represent the range of data from the 25th to the 75th interquartile range, while the horizontal line in the middle of each box plot represents the median value. Circles and asterisks indicate outliers (1.5 x the interquartile range) and extreme values (3.0 x the interquartile range) outside the central box, respectively. The area below the horizontal line represents normal expected values established in our laboratory.
Variation of D-dimer levels according to sites of tumor
The association of D-dimer levels with tumor sites was analyzed separately. With the Paired-Samples T Test, an elevated D-dimer level is associated with increased mortality risk in patients with lung cancer (P = 0.015) and digestive tract tumor (P = 0.004), but not in patients with breast cancer (P = 0.28) (Table 2).
Table 2.
Dynamic variation results of plasma D-dimer in different malignant tumor sites (x̅ ± S, n = 62)
| Groups | N | 4-8 weeks before death | 0-4 weeks before death | P-value |
|---|---|---|---|---|
| lung cancer | 26 | 0.33 ± 0.38 | 0.60 ± 0.76 | 0.015 |
| malignant tumor of digestive tract | 30 | 0.50 ± 0.64 | 0.84 ± 1.0 | 0.004 |
| breast cancer | 6 | 0.33 ± 0.31 | 0.48 ± 0.40 | 0.28 |
Correlation analysis of plasma D-dimer levels with platelet counts, fibrinogen levels and other markers of coagulation time
Platelet counts gradually declined from 4-8 weeks to 0-4 weeks before patients’ death with a statistically significant differences between the two time intervals (P < 0.001). While the mean platelet counts remained above the normal conventional low cut -off point for both time period (see Figure 2). Higher D-dimer levels were associated with lower platelet counts, as evidenced by results of the correlation analysis using all data collected in the study (p = 0.045, r = -0.158). The levels of fibrinogen also declined throughout the terminal stage. There were significant differences between the parameter of the 4-8 weeks period and 0-4 weeks period (P = 0.012) (see Figure 3). The correlation analysis showed that there was a negative linear correlation between D-dimer and fibrinogen levels (p = 0.001, r = -0.27). An analysis of the dynamic variation of APTT, PT and TT found that all these three blood coagulation time parameters were prolonged significantly from the 4-8 weeks period to 0-4 weeks period before patient’ death. Significant positive linear correlations were observed D-dimer levels and APTT (p = 0.023, r = 0.18), TT (p ≤ 0.001, r = 0.29) and PT (p ≤ 0.001, r = 0.47). In case of the hemoglobin levels, statistically significant decreased tendency was observed between the 4-8 weeks period and the 0-4 week period. Although patients’ hemoglobin levels decreased significantly as disease progressed, no linear correlation was found between hemoglobin and D-dimer levels (p = 0.34, r = 0.075) (Table 3).
Figure 2.
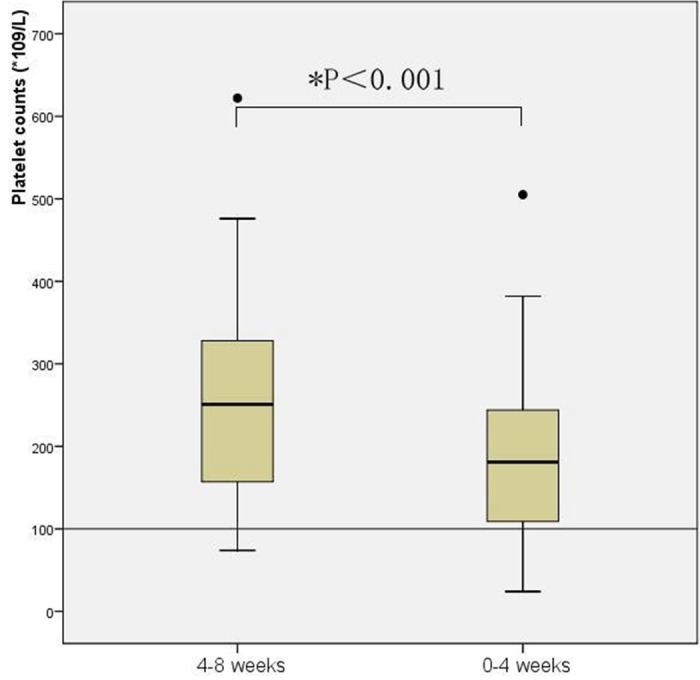
Dynamic variation of platelet counts. Box plot of platelet counts results from 4-8 weeks and 0-4 weeks before patients’ death. Box plots represent the range of data from the 25th to the 75th interquartile range, while the horizontal line in the middle of each box plot represents the median value. Circles and asterisks indicate outliers (1.5 x the interquartile range) and extreme values (3.0 x the interquartile range) outside the central box, respectively. The area below the horizontal line represents normal expected values established in our laboratory.
Figure 3.
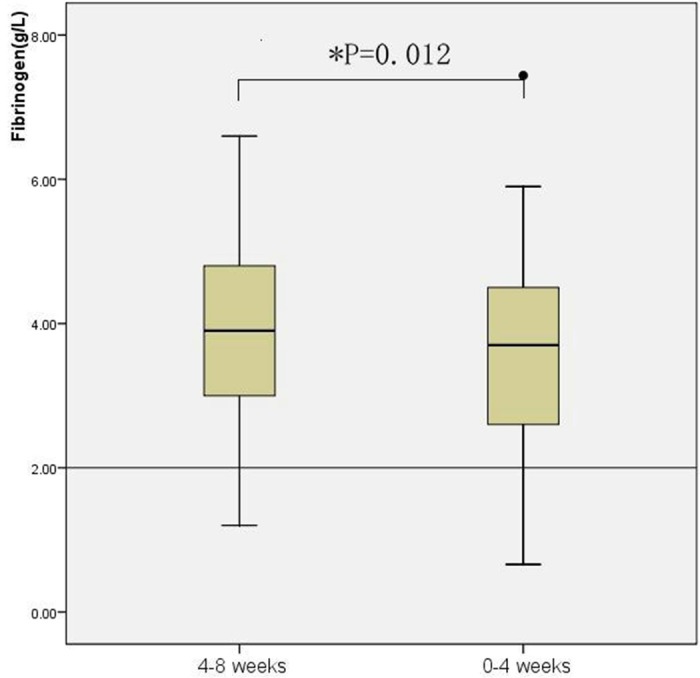
Dynamic variation of fibrinogen levels. Box plot of fibrinogen levels results from 4-8 weeks and 0-4 weeks before patients’ death. Box plots represent the range of data from the 25th to the 75th interquartile range, while the horizontal line in the middle of each box plot represents the median value. Circles and asterisks indicate outliers (1.5 x the interquartile range) and extreme values (3.0 x the interquartile range) outside the central box, respectively. The area below the horizontal line represents normal expected values established in our laboratory.
Table 3.
Pearson correlation analysis of D-dimer levels with markers of coagulation and fibrinolytic system and hemoglobin
| Pearsoncorrelation | Fibrinogen | Platelet counts | Hemoglobin | APTT | PT | TT |
|---|---|---|---|---|---|---|
| D-dimer | ||||||
| Correlation coefficient | -0.27 | -0.158 | 0.075 | 0.18 | 0.47 | 0.29 |
| P-value | 0.001 | 0.045 | 0.34 | 0.023 | < 0.001 | < 0.001 |
Discussion
Activation of clotting system often leads to more aggressive tumor biology, mechanistically, it is mainly the result of increased expression of tissue factor, which is the primary trigger in the initial activation of the clotting cascade leading to fibrin deposition. Tumor cells can express tissue factor which contributes to metastatic spread, tumor growth, and tumor angiogenesis. Tissue factor can also lead to the fibrin coating of the cells that enables the cells to be captured within the microvasculature and facilitates hematogenous metastasis [12]. Duration and severity of coagulation activation strongly were associated with cancer prognosis. Advanced cancer stage with high tumor burden and high proliferation rate are associated with high coagulation activation, and, finally, fibrin formation provides suitable extracellular matrix for cancer cells to migrate and grow [13]. D-dimer levels can be easily measured with standardized methods and are routinely used in the clinical setting. They may be a global surrogate marker of the association between cancer and the activation of hemostasis and fibrinolysis.
Fukumoto K divided patients into three groups according to the D-dimer level: group A (≤ 0.50 μg/ml), group B (0.51-0.86 μg/ml) and group C (> 0.86 μg/ml) and found that D-dimer level is an independent prognostic factor for patients with completely resected NSCLC [14]. Zhang PP et al. reported that the preoperative plasma D-dimer level is an important prognostic biomarker in patients with operable NSCLC (TNM stage I, II and IIIA) that is independent of VTE [15]. However, majority studies reported the significant role of plasma D-dimer for early stage cancer patients. In our study, we retrospectively reviewed the dynamic variation of each terminal stage patients’ plasma D-dimer levels in 0-4 weeks period and 4-8 weeks period separately and found that the mean levels of plasma D-dimer were above the normal conventional cut-off point in both time intervals. D-dimer levels increased gradually when patients close to death. D-dimer levels in 0-4 weeks period was significantly higher than 4-8 weeks. Even in terminal stage cancer patients with normal plasma D-dimer levels in both time period, their plasma D-dimer levels had an increasing tendency as disease progressed (P = 0.013). Dynamic variation of plasma D-dimer might help to predict disease progression of terminal stage cancer patients.
Platelet is an important component of hemostasis processes and was found to increase with disease progression in gastric cancer [16]. Allensworth SK reported preoperative thrombocytosis portends worse DFS in epithelial ovarian cancer and associated with extensive initial disease burden, measurable residual disease, and postoperative sequelae [17]. Plasma components stored in platelets can contribute to tumor growth and invasiveness of the cancer cells by releasing various cytokines, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which has a significant role in regulating angiogenesis [18]. In this study, although the mean levels of platelet counts were above the normal conventional cut-off point in both time intervals, platelet counts showed decreasing tendency in terminal stage patients with disease progression. Platelet counts measured in 0-4 week period were significant lower than those measured in 4-8 weeks period.
Plasma fibrinogen levels, as another important haemostatic variable, have prognostic significance in patients with cancer exhibiting a role in tumor growth, invasion and metastasis by promoting tumor neovascularization and supporting the sustained adhesion of tumor cells [19]. Tanaka N reported preoperative elevated plasma fibrinogen levels were an independent predictor for poor survival after RNU (nephroureterectomy) and for worse pathological features [20]. Son HJ et al. found that preoperative hyperfibrinogenemia was significantly associated with shorter prognostic marker in colon cancer [21]. In this study, we found that fibrinogen levels had a tendency to decrease along with the condition progress in terminal patients and had a negative correlation with D-dimer levels.
Decreased platelet counts and fibrinogen levels may be caused by the following reasons. Firstly, as hypercoagulable state developing, a large number of platelets, clotting factors and fibrinogen were consumed but repressed marrow’s building blood function and liver dysfunction resulted in those factors can’t be supplied properly. Secondly, chronic nutrient depletions, anorexia, and long-term chemotherapy induced side effects such as nausea, vomiting, loss of appetite, oral diseases, which can result in debilitating conditions and malnutrition. In addition, majority of terminal cancer patients suffered from cachexia which is a multiorgan syndrome associated with cancer, characterised by body weight loss. According to Warren, cachexia is responsible for the deaths of 22% of cancer patients [22].
The association of D-dimer levels variation with cancer sites was analyzed separately in the current study. It was concluded that variation of D-dimer level could be qualified as a disease progression indicator in lung and digestive tract cancers but not in breast cancers. Our findings are consistent with those previously reported for patients with lung and colorectal cancers [8,10]. However, for patients with breast cancers, although the mean values of plasma D-dimer levels appeared to rise as disease worsened, the increase could not be statistically significant. This could be explained by the small sample size of the tumor type. In a larger prospective cohort study of patients diagnosed with breast cancer, plasma levels of D-dimers are significantly elevated compared to healthy controls [9].
The deficiency or depletion of coagulation factors are often characterized by a prolonged PT. Ferrigno reported that prolongation of PT was strongly associated with poor prognosis in non-small cell lung cancer patients [23]. Tas also reported a tendency toward decreased survival for patients with prolonged APTT, but it was not statistically significant [8]. Prolonged PT, APTT and TT were found in our study and D-dimer levels were positively correlated with PT, APTT and TT.
There are some limitations concerning the present study. Terminal cancer patients are usually accompanied with inflammatory conditions, characterized by elevated biochemical or hematologic markers, including CRP, neutrophils, and platelets, and a combination of those factors may influence test results of D-dimer levels. In this study, D-dimer levels were measured only in 0-4 weeks and 4-8 weeks periods prior to patients’ death. Future studies are recommended to include measurements of D-dimer levels for more time intervals to investigate the relationship between D-dimer and disease progression for advanced stage cancer patients.
In conclusion, plasma D-dimer levels pointed an elevated tendency and revealed statistically significant difference as patients gradually near death. Dynamic variation of plasma D-dimer might help to predict disease progression.
Acknowledgements
This article is supported by The National Natural Science Foundation of China (NO. 30872591) and Shanghai Science and Technology Commission (NO. 11411950602).
Disclosure of conflict of interest
None.
References
- 1.Sousou T, Khorana AA. New insights into cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2009;29:316–20. doi: 10.1161/ATVBAHA.108.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sood SL. Cancer-associated thrombosis. Curr Opin Hematol. 2009;16:378–385. doi: 10.1097/MOH.0b013e32832ea31b. [DOI] [PubMed] [Google Scholar]
- 3.Sumney M, Whiteman K, editors. D-dimer: Past, present, and future. CE Connection available at: http://www.nursingcenter.com. Accessed Oct. 10, 2010.
- 4.Lee AY. Thrombosis and cancer: the role of screening for occult cancer and recognizing the underlying biological mechanisms. Hematology Am Soc Hematol Educ Program. 2006;6:438–43. doi: 10.1182/asheducation-2006.1.438. [DOI] [PubMed] [Google Scholar]
- 5.Amirkhosravi A, Meyer T, Amaya M, Davila M, Mousa SA, Robson T, Francis JL. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin Thromb Hemost. 2007 Oct;33:643–52. doi: 10.1055/s-2007-991531. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak HF. Thrombosis and cancer. Hum Pathol. 1987;18:275–84. doi: 10.1016/s0046-8177(87)80010-2. [DOI] [PubMed] [Google Scholar]
- 7.Komurcuoglu B, Ulusoy S, Gayaf M, Guler A, Ozden E. Prognostic value of plasma D-dimer levels in lung carcinoma. Tumori. 2011 Nov-Dec;97:743–8. doi: 10.1177/030089161109700611. [DOI] [PubMed] [Google Scholar]
- 8.Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med. 2013 Mar;107:451–7. doi: 10.1016/j.rmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Batschauer AP, Figueiredo CP, Bueno EC, Ribeiro MA, Dusse LM, Fernandes AP, Gomes KB, Carvalho MG. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010 Jun;21:1267–72. doi: 10.1093/annonc/mdp474. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Yoshinaga K, Matsuyama A, Iwasa T, Osoegawa A, Tsujita E, Yamashita Y, Tsutsui S, Ishida T. Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology. 2012;83:10–5. doi: 10.1159/000338329. [DOI] [PubMed] [Google Scholar]
- 11.Tas F, Kilic L, Bilgin E, Keskin S, Sen F, Ciftci R, Yildiz I, Yasasever V. Clinical and Prognostic Significance of Coagulation Assays in Advanced Epithelial Ovarian Cancer. Int J Gynecol Cancer. 2013 Feb;23:276–81. doi: 10.1097/IGC.0b013e31827b8796. [DOI] [PubMed] [Google Scholar]
- 12.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J. Clin. Oncol. 2009;27:4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012 Aug;97:1158–64. doi: 10.3324/haematol.2011.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukumoto K, Taniguchi T, Usami N, Kawaguchi K, Fukui T, Ishiguro F, Nakamura S, Yokoi K. The preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today. 2014 doi: 10.1007/s00595-014-0894-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Zhang PP, Sun JW, Wang XY, Liu XM, Li K. Preoperative plasma D-dimer levels predict survival in patients with operable non-small cell lung cancer independently of venous thromboembolism. Eur J Surg Oncol. 2013 Sep;39:951–6. doi: 10.1016/j.ejso.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Matowicka-Karna J, Kamocki Z, Kemona H. Assessment of platelet activation and phagocytic activity in gastric cancer patients. World J Gastrointest Pathophysiol. 2013 Feb 15;4:12–7. doi: 10.4291/wjgp.v4.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allensworth SK, Langstraat CL, Martin JR, Lemens MA, McGree ME, Weaver AL, Dowdy SC, Podratz KC, Bakkum-Gamez JN. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2013 Sep;130:499–504. doi: 10.1016/j.ygyno.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka N, Kikuchi E, Matsumoto K, Hayakawa N, Ide H, Miyajima A, Nakamura S, Oya M. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carcinoma. BJU Int. 2013 May;111:857–64. doi: 10.1111/j.1464-410X.2012.11353.x. [DOI] [PubMed] [Google Scholar]
- 21.Son HJ, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, Park SC, Choi HS, Oh JH. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013 Sep;20:2908–13. doi: 10.1245/s10434-013-2968-8. [DOI] [PubMed] [Google Scholar]
- 22.Warren S. The immediate cause of death in cancer. Am J Med Sci. 1932;184:610–3. [Google Scholar]
- 23.Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J. 2001;17:667–73. doi: 10.1183/09031936.01.17406670. [DOI] [PubMed] [Google Scholar]


