Abstract
Galanthamine is a naturally occurring acetylcholinesterase (AchE) inhibitor that has been well established as a drug for treatment of mild to moderate Alzheimer disease, but the role of the compound in plant metabolism is not known. The current study was designed to investigate whether galanthamine could redirect morphogenesis of Artemisia tridentata Nutt. cultures by altering concentration of endogenous neurosignaling molecules acetylcholine (Ach), auxin (IAA), melatonin (Mel), and serotonin (5HT). Exposure of axenic A. tridentata cultures to 10 µM galanthamine decreased the concentration of endogenous Ach, IAA, MEL, and AchE, and altered plant growth in a manner reminiscent of 2–4D toxicity. Galanthamine itself demonstrated IAA activity in an oat coleoptile elongation bioassay, 20 µM galanthamine showed no significant difference compared with 5 μM IAA or 5 μM 1-Naphthaleneacetic acid (NAA). Metabolomic analysis detected between 20,921 to 27,891 compounds in A. tridentata plantlets and showed greater commonality between control and 5 µM treatments. Furthermore, metabolomic analysis putatively identified coumarins scopoletin/isoscopoletin, and scopolin in A. tridentata leaf extracts and these metabolites linearly increased in response to galanthamine treatments. Overall, these data indicate that galanthamine is an allelopathic phytochemical and support the hypothesis that neurologically active compounds in plants help ensure plant survival and adaptation to environmental challenges.
Keywords: Artemisia tridentata, Galanthamine, Acetylcholine, Melatonin, Auxin, cholinergic signalling, metabolomics
Introduction
The classic hypothesis of the regulation of plant growth and regeneration attributes morphological development to changes in the relative ratio of auxins (IAA) to cytokinins.1 Interpretations and applications of this approach are the foundation of modern plant tissue culture and biotechnology. However, not all plants respond in the same way as model systems such as tobacco, and other plant growth regulators have been discovered that mediate, accelerate, or enhance fundamental plant regeneration mechanisms, especially in medicinal plants.2 In particular, human neurosignaling compounds have been shown to influence plant metabolism, growth, and development.3-5 In plant tissue culture, the capacity for neurologically active phytochemicals to enhance cell survival and regeneration warrants further study.
Artemisia tridentata Nutt and its subspecies are collectively known as Big Sagebrush and are found in arid regions of North America from steppe to subalpine zones, dry shrub lands, foothills, rocky outcrops, scablands, and valleys.6,7 Traditionally, species of Big Sagebrush have been used as a ceremonial medicine to treat headaches or protect individuals from metaphysical forces.8 A total of 220 phytochemicals have been described in A. tridentata and related species in the Tridentatae.9 Recently, the neurologically active compounds melatonin (MEL), serotonin (5HT), and acetylcholine (Ach) were identified and quantified in axenic cultures of A. tridentata.10 MEL and 5HT have previously been identified and quantified in axenic culture of several plant species.11-14 Tryptophan was identified as a precursor for biosynthesis of MEL and 5HT in Hypericum perforatum11 and key enzymes and genes in this pathway have been described in rice.15 Current research indicates that MEL and 5HT may be important in plant metabolic systems such as photosynthesis16 and physiological responses to environmental stresses such as tolerance to cold stress.17-19 Ach was first described in plants in the early 1970s using bioassays and detected in plants by gas chromatography in 1974.20 Ach occurs in all parts of plants, but higher concentrations have been quantified in roots and dark-adapted tissues than in shoots or light-exposed cells.20-23 The hypothesized roles of Ach in plants include almost all aspects of growth and development,23 including phytochrome-linked signaling,24 phytochrome-modulated calcium signaling,25 induction of primary and secondary rooting,26 regulation of phloem transport,27 and regulation of pollen tube development.28
Previous work has shown that many synthetic and natural drugs that affect human neurotransmitter metabolism can also mediate plant growth and development.4,12,13 In order to investigate the potential role of the cholinergic system in plant growth and development, we hypothesized that the acetylcholinesterase (AchE) inhibiting natural product galanthamine could redirect morphogenesis in A. tridentata cultures by altering the concentration of endogenous neurosignaling molecules such as Ach, MEL, 5HT, and IAA. To investigate this hypothesis, we designed the following specific objectives: 1) quantify neurosignaling molecules in axenic cultures of A. tridentata, 2) determine whether inhibition of plant cholinergic signaling affects MEL, 5HT, and/or IAA in A. tridentata, and 3) develop and analyze a metabolomic data set as a hypothesis generating tool for abiotic stresses.
Results
In vitro regeneration
A. tridentata shoot proliferation was sustained on basal MSO medium, and altered morphologies were observed in response to galanthamine (Fig. 1A and 1B). Treatment with 5 μM galanthamine appeared to increase leaf length and reduce turgor pressure. Cultures treated with 10 µM galanthamine appeared chlorotic, exhibited leaf curling, and were vitrified compared with the control or 5 µM treatments (Fig. 1B).
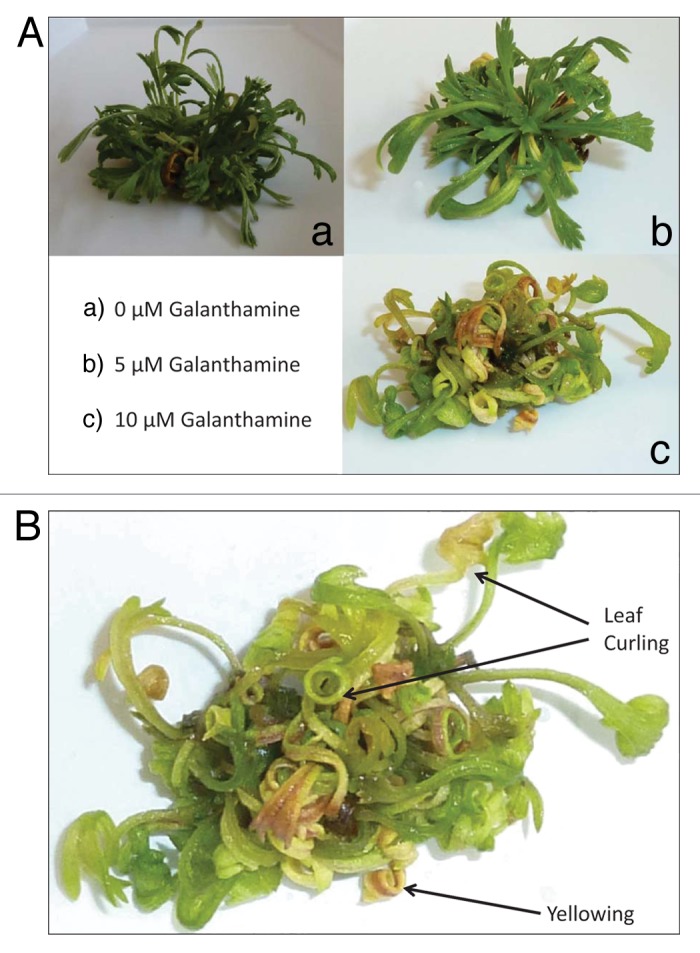
Figure 1. (A) Effect of galanthamine on the growth and development of Artemisia tridentata cultures. (a) 0 µM galanthamine. (b) 5 µM galanthamine. (c) 10 µM galanthamine. (B) Effect of 10 µM galanthamine on growth and development of Artemisia tridentata cultures.
Ach and acetycholinesterase activity
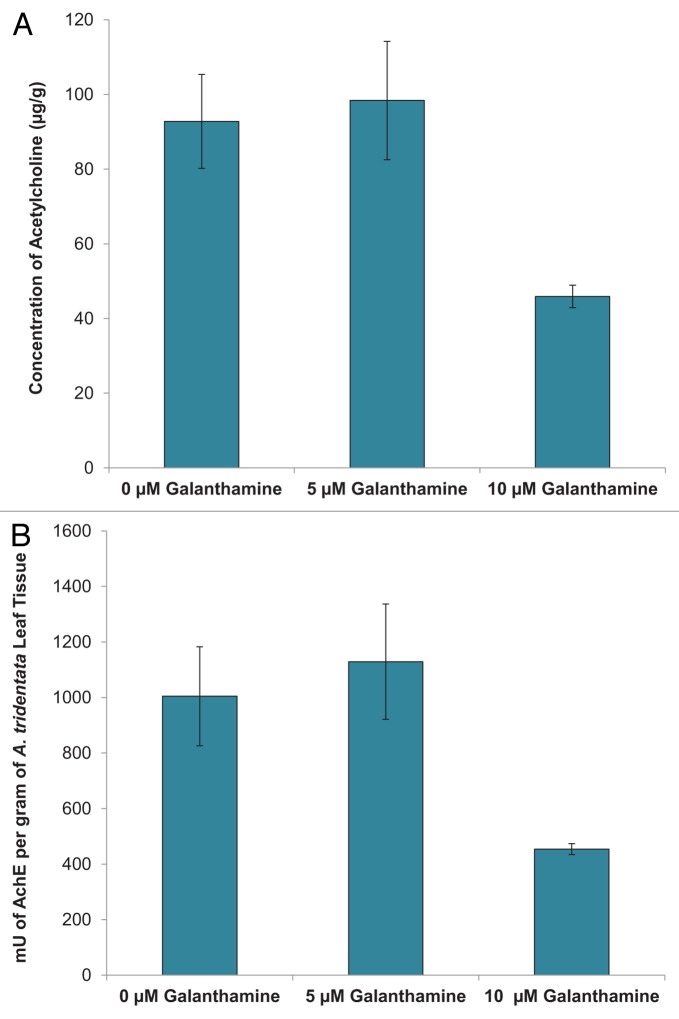
Ach concentration in A. tridentata cultures was significantly lower in plants treated with 10 µM galanthamine (45.9 ± 3.01 µg/g) compared with control plants (92.8 ± 12.6 µg/g) or 5 µM galanthamine treated plants (98.4 ± 3.01 µg/ g) (Fig. 2A). Exposure of plant tissues to 10 µM galanthamine also significantly decreased the activity of AchE in the tissues (454.1 ± 19.8 mU/g) as compared with the control (1004.6 ± 178.4 mU/ g) and 5 µM galanthamine treatments (1129.1 ± 207.3 mU/ g) (Fig. 2B).
Figure 2. Effect of galanthamine on (A) Ach and (B)AchE concentration.
Effects of galanthamine on IAA and indoleamines
Regression analysis demonstrated a significant negative linear relationship (R2 = 0.991) between the concentration of galanthamine and their endogenous levels of MEL. Significant differences were found between the control (9.6 ± 3.1 ng/g) and 10 µM treatments (2.3 ± 0.9 ng/g) (Fig. 3A). Interestingly, 5HT levels were significantly lower in plants exposed to 5 µM galanthamine (10.7 ± 3.6 ng/g) compared with controls (37.1 ± 16.5 ng/g) or the 10 µM treatment (35.4 1 ± 15.3 ng/g) (Fig. 3B). Endogenous IAA levels also demonstrated a negative linear relationship with galanthamine treatment (R2 = 0.9989), but the overall differences between treatments were not significant (Fig. 3C).
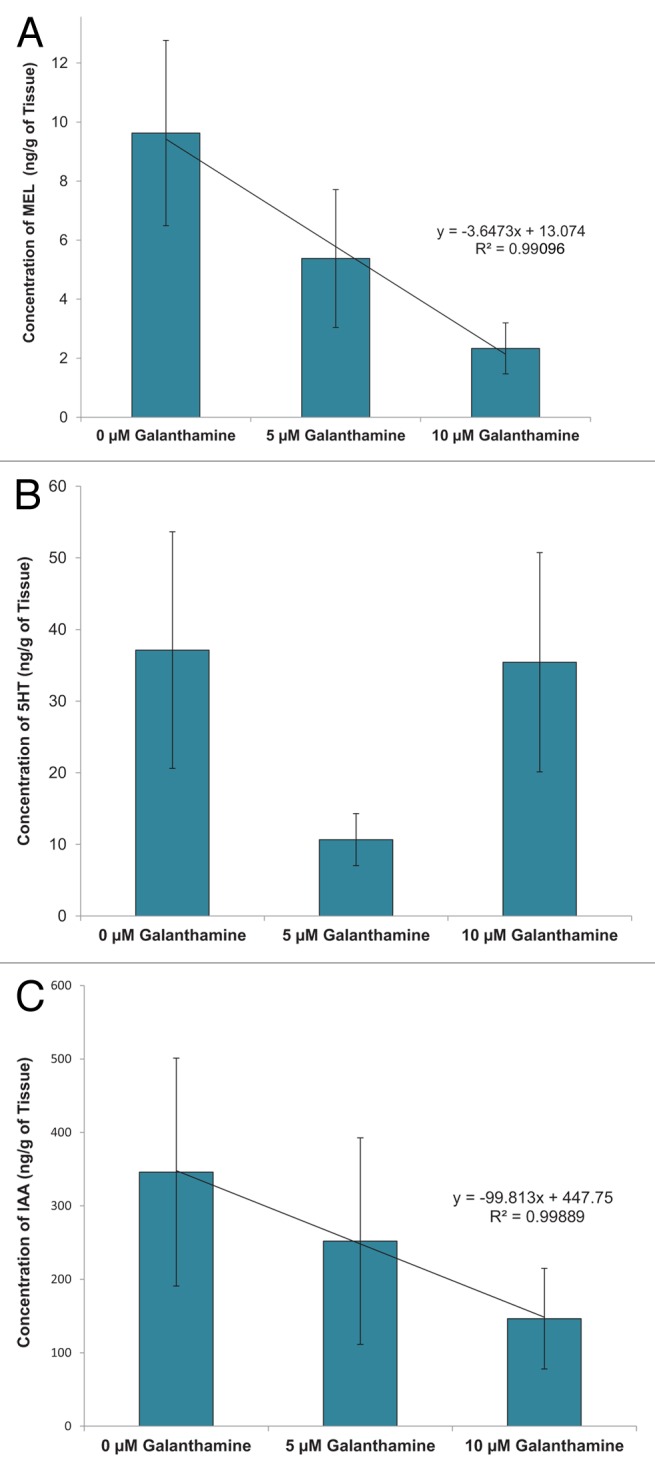
Figure 3. Effect of galanthamine on indoleamine and IAA in Artemisia tridentata cultures. (A) MEL. (B) 5HT. (C) IAA.
Analysis of oxidative stress in galanthamine treated cultures
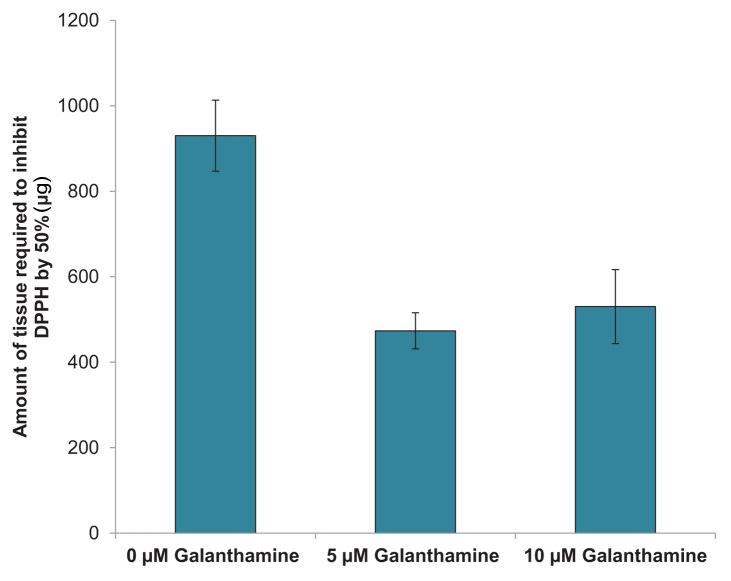
Quantification of the antioxidant potential of control and galanthamine-treated tissues using a DPPH microplate assay indicated that significantly less tissue was required to quench DPPH by 50% in 5 µM (473 ± 42 µg) and 10 µM (529 ± 87 µg) treatments as compared with the control (930 ± 83 µg) (Fig. 4). This demonstrates that plates treated with galanthamine had a higher antioxidant potential than control plants.
Figure 4. Effect of galanthamine on the antioxidant capacity of Artemisia tridentata tissues grown in vitro.
Activity of galanthamine in an IAA bioassay
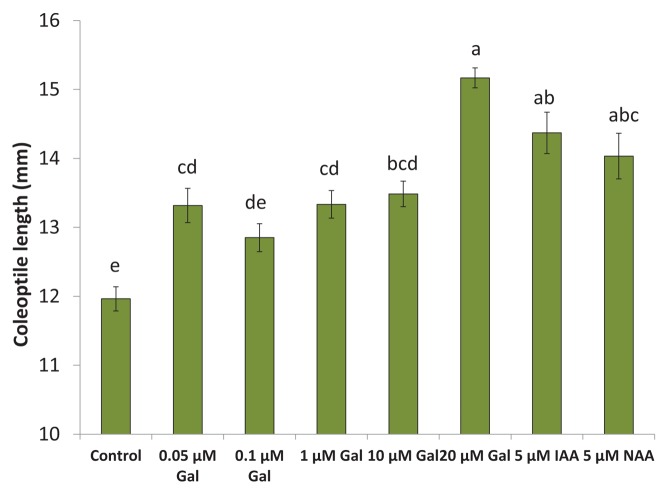
Inclusion of galanthamine in the solution significantly increased the elongation of oat coleoptiles in a manner similar to the classic IAA response (Fig. 5). Exposure of coleoptiles to 20 μM galanthamine significantly increased elongation as compared with controls and was not significantly different from 5 μM IAA or 5 μM 1-Naphthaleneacetic acid (NAA).
Figure 5. Galanthamine showingIAA-like activity in an oat coleoptile bioassay.
Untargeted phytochemical analysis on galanthamine treated cultures
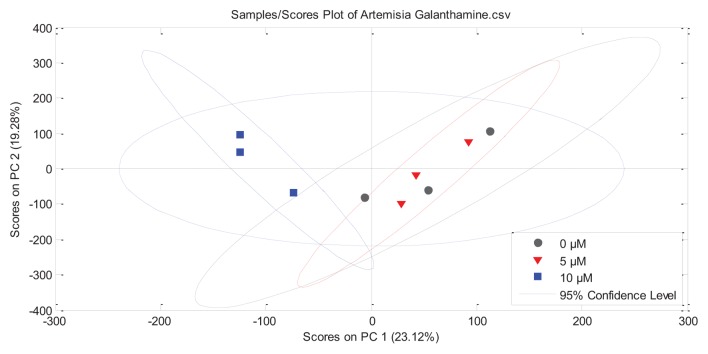
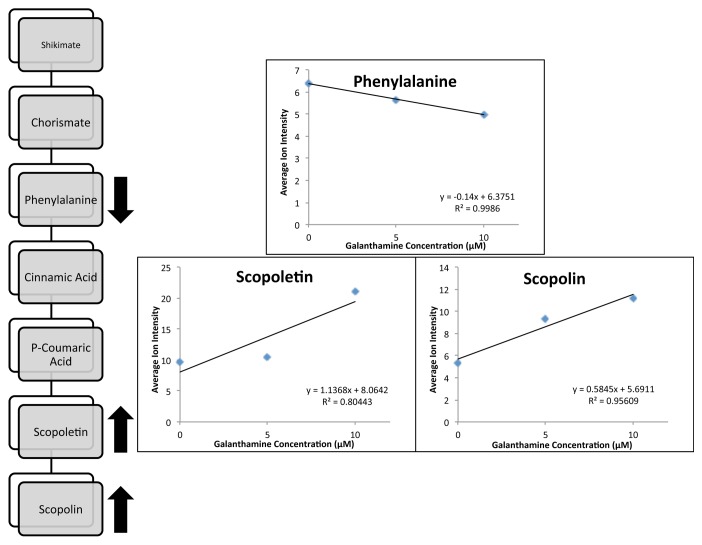
The metabolomes of A. tridentata shoot cultures contained more than 20,000 distinct compounds (Table 1) with about 27,000 detected in controls and 5 µM galanthamine treatments. Fewer compounds were detected in the 10 µM galanthamine treated tissues than the control or 5 μM galanthamine treatments. Scores plots for PC-1(23.12%) and PC-2 (19.28%) showed significant clustering for 10 µM treated cultures in contrast to the significant overlap observed between the control and 5 µM cultures (Fig. 6). Of the 20921–27891 compounds found, 161 had average ion intensities greater than 5.0 (> 0.09% of average total ion count). Linear or exponential responses to galanthamine treatments were observed for 32 (decreasing) and 50 (increasing) compounds (Table 2). Putative identifications were performed for all prominent compounds (data not shown). The most significant findings were the putative identification of coumarins (scopoletin/isoscopoletin and scopolin) and their precursor phenylalanine as compounds significantly impacted by the exposure to galanthamine (Table 2). The average ion intensity of phenylalanine (m/z = 165.0790) significantly decreased (R2 = 0.9986), while the ion intensities of scopoletin/isoscopoletin (m/z = 192.0423, R2 = 0.8044) and its glucocylated derivative scopolin (m/z = 354.0951, R2 = 0.9561) increased in response to galanthamine treatments (Fig. 7).
Table 1. Summary of Metabolomic Data of Galanthamine Treated Cultures.
| Control | 5 µM Galanthamine | 10 µM Galanthamine | |
| Total Number of compounds | 27891 | 27851 | 20921 |
| Average Number of Compounds | 16663 +/− 241 | 17083 +/− 491 | 13786 +/− 192 |
| Compounds Common to All | 6219 | 6799 | 4798 |
| Compounds Unique Between Treatments | |||
| Control | 5 µM Galanthamine | 10 µM Galanthamine | |
| 10 µM Galanthamine | 2291 | 2213 | 0 |
| 5 µM Galanthamine | 9137 | 0 | 2213 |
Figure 6. Principle Component Analysis identifying the characteristic responses of axenic cultures of Artemisia tridentata to galanthamine treatment.
Table 2. Most Prominent Ions (Average Ion Counts > 5.0 (0.09%)) Showing Linear or Exponential Trends.
| Decreasing with Treatment | |||||
| ID | Ret. Time | Mass | Line of Best fit | Equation | R2 |
| 1 | 6.9558 | 340.0927 | Linear | y = -0.2425x + 29.744 | 0.9929 |
| 2 | 6.0175 | 241.0592 | Linear | y = -0.2425x + 29.744 | 0.9929 |
| 3 | 13.5308 | 334.1383 | Linear | y = -2.1247x + 28.83 | 0.8788 |
| 4 | 1.7363 | 213.0418 | Linear | y = -1.0884x + 21.545 | 0.9578 |
| 5 | 8.9819 | 434.1102 | Exponential | y = 22.657e^-0.087x | 0.9545 |
| 6 | 6.7 | 233.113 | Exponential | y = 16.501e^-0.016x | 0.9827 |
| 7 | 1.8035 | 258.0591 | Linear | y = -2.3942x + 26.101 | 0.9964 |
| 8 | 13.5301 | 233.1084 | Linear | y = -1.4829x + 21.035 | 0.8013 |
| 9 | 6.0152 | 276.0961 | Linear | y = -0.4833x + 14.438 | 0.9926 |
| 10 | 6.2874 | 227.0927 | Linear | y = -0.7726x + 15.239 | 0.9506 |
| 11 | 6.0038 | 517.1004 | Linear | y = -1.4169x + 18.422 | 0.9510 |
| 12 | 15.1925 | 505.1853 | Linear | y = -1.4143x + 15.892 | 0.8489 |
| 13 | 1.6532 | 349.1665 | Exponential | y = 16.153e^-0.169x | 0.9935 |
| 14 | 7.4304 | 480.1565 | Linear | y = -0.6014x + 11.605 | 0.9843 |
| 15 | 9.9656 | 434.1394 | Exponential | y = 9.5567e^-0.04x | 0.9144 |
| 16 | 15.2634 | 375.0408 | Linear | y = -0.6859x + 11.272 | 0.9619 |
| 17 | 8.5777 | 399.0983 | Exponential | y = 9.3338e^-0.046x | 0.9243 |
| 18 | 6.6231 | 352.0954 | Linear | y = -0.2273x + 8.1602 | 0.9322 |
| 19 | 9.8383 | 345.0929 | Linear | y = -0.3421x + 8.5447 | 0.9997 |
| 20 | 12.416 | 284.1271 | Exponential | y = 18.081e^-0.391x | 0.9544 |
| 21 | 5.658 | 406.0925 | Linear | y = -0.8878x + 10.342 | 0.9593 |
| 22 | 6.0164 | 205.0516 | Linear | y = -0.2074x + 6.8009 | 0.9392 |
| 23 | 2.0929 | 284.0493 | Linear | y = -0.5524x + 8.4621 | 0.9663 |
| 24 | 14.6013 | 284.1245 | Linear | y = -0.6884x + 9.1244 | 0.9908 |
| 25 A | 3.9644 | 166.0617 | Linear | y = -0.14x + 6.3751 | 0.9986 |
| 26 | 8.6167 | 210.11 | Exponential | y = 7.8604e^-0.079x | 0.9960 |
| 27 | 8.9827 | 399.0978 | Exponential | y = 8.0114e^-0.086x | 0.9980 |
| 28 | 1.7025 | 387.1295 | Linear | y = -0.7391x + 9.0713 | 0.9968 |
| 29 | 8.4876 | 169.1004 | Linear | y = -0.6547x + 8.467 | 0.7922 |
| 30 | 22.367 | 235.1366 | Linear | y = -0.7584x + 8.9401 | 0.9744 |
| 31 | 10.7236 | 582.172 | Linear | y = -0.7747x + 8.91 | 0.9526 |
| 32 | 9.8427 | 327.0888 | Linear | y = -0.2612x + 6.339 | 0.9882 |
| Increasing with Treatment | |||||
| ID | Ret. Time | Mass | Line of Best fit | Equation | R2 |
| 21 | 9.5537 | 199.0965 | Linear | y = 1.1095x + 34.389 | 0.9410 |
| 22 | 1.8738 | 380.991 | Linear | y = 1.3206x + 33.245 | 0.9778 |
| 23 | 13.9895 | 215.116 | Linear | y = 1.1765x + 31.268 | 0.9722 |
| 24 | 6.3407 | 245.0937 | Exponential | y = 28.216e^0.0427x | 0.7988 |
| 25 | 5.61 | 355.004 | Linear | y = 1.8953x + 14.458 | 0.9560 |
| 26 | 5.1247 | 205.0568 | Exponential | y = 5.5215e^0.1735x | 0.8681 |
| 27 | 8.4323 | 354.1065 | Linear | y = 0.2937x + 17.805 | 0.8137 |
| 28 | 8.5697 | 516.9717 | Linear | y = 3.1768x - 0.6695 | 0.9845 |
| 29 B | 5.3907 | 193.0583 | Exponential | y = 8.7431e^0.0777x | 0.8284 |
| 30 | 7.9198 | 399.0968 | Exponential | y = 8.8366e^0.078x | 0.9997 |
| 31 | 5.6303 | 355.0657 | Linear | y = 0.7487x + 7.7082 | 0.9981 |
| 32 | 5.3821 | 390.1043 | Exponential | y = 7.8235e^0.0536x | 0.8414 |
| 33 | 5.1447 | 341.0321 | Exponential | y = 5.4765e^0.1026x | 0.8517 |
| 34 | 9.8245 | 523.021 | Exponential | y = 2.0228e^0.2333x | 1 |
| 35 | 1.742 | 290.7999 | Linear | y = 0.9645x + 4.58 | 0.9966 |
| 36 C | 5.5567 | 355.0838 | Linear | y = 0.5845x + 5.6911 | 0.9561 |
| 37 | 11.4011 | 453.0584 | Exponential | y = -0.6014x + 11.605 | 0.9843 |
| 38 | 7.8306 | 534.1533 | Linear | y = 0.4806x + 5.0447 | 0.7993 |
| 39 | 5.1249 | 188.0433 | Exponential | y = 1.9949e^0.1862x | 0.8455 |
| 40 | 1.8441 | 218.9918 | Exponential | y = 3.5563e^0.1186x | 0.9998 |
| 41 | 1.5434 | 184.9998 | Linear | y = 0.3741x + 5.2801 | 0.9420 |
| 42 | 5.131 | 246.0826 | Exponential | y = 2.4741e^0.1565x | 0.7967 |
| 43 | 8.0024 | 194.0502 | Exponential | y = 4.3066e^0.0751x | 0.7676 |
| 44 | 22.1758 | 593.2024 | Linear | y = 1.3187x - 0.1007 | 0.9993 |
| 45 | 12.5212 | 469.1415 | Exponential | y = 1.108e^0.2394x | 0.9468 |
| 46 | 11.8184 | 418.1451 | Exponential | y = 4.0479e^0.06x | 0.8826 |
| 47 | 12.2741 | 416.1586 | Exponential | y = 0.3947e^0.3309x | 0.9005 |
| 48 | 6.3831 | 422.0663 | Exponential | y = 2.3529e^0.1352x | 0.9879 |
| 49 | 1.5636 | 122.5363 | Linear | y = 0.1846x + 4.1701 | 0.9528 |
| 50 | 9.9414 | 659.1121 | Exponential | y = 1.3175e^0.1987x | 0.9541 |
Putative Identifications: A = Phenylalanine, B = Scopoletin/Isoscopoletin, C = Scopolin*
Figure 7. Putative identification of prominent ions with linear responses to galanthamine treatment.
Discussion
Galanthamine is a natural inhibitor of AchE, first isolated from snowdrop (Galanthus spp) and commonly used in the treatment of Alzheimer disease.29 In humans, galanthamine increases Ach levels in the brain by reversibly inhibiting AchE, while increasing sensitivity of neuronal nicotinic receptors to surrounding Ach.30 The initial observation of Ach in Artemisia tissues10 prompted studies to investigate the role of Ach in plant growth and development. The most significant findings of the current investigations were the degree and diversity of responses of A. tridentata tissues to galanthamine exposure. There was an overall inhibition of the cholinergic system with decreases in both Ach and AchE in response to galanthamine. In addition, there was a decrease in IAA and MEL in the galanthamine treated A. tridentata but the effects on plant 5HT were less clear. Interestingly, galanthamine induced a clear IAA-like growth response in the classic oat coleoptile IAA bioassay and the galanthamine treated A. tridentata plants appeared elongated, chlorotic, and vitrified. The metabolomic analysis identified the increase of polyphenols as the most significant phytochemical response to galanthamine. Together these data indicate the induction of a cascade of responses that include growth regulation, stress, and the induction of defense mechanisms by the natural product.
Cholinergic system effects
It has been proposed that Ach regulates basic cellular functions that are required for survival in vertebrates, invertebrates, plants, fungi, and mosses.31-33 Ach has been reported in many plant species but specific physiological roles remain under debate.23 Our data indicated that an overall decrease in cholinergic metabolism in A. tridentata manifested mainly as stress in the tissues. Bamel et al. (2007) found that the application of exogenous Ach or inhibitors of its breakdown induced rhizogenesis in tomato cultures (Lycopersicon esculentum Miller), which led them to propose that Ach may interact with other plant regulators such as IAA or MEL.26 Our data support this observation by demonstrating an interaction between galanthamine and IAA. The observation that oat coleoptiles elongated in response to galanthamine could indicate that the mechanism of action may involve cross talk between IAA and Ach pathways.
IAAs and indoleamines
Regulation of shoot and root development by 5HT and MEL was previously investigated with a variety of pharmaceuticals used to regulate neuronal 5HT metabolism in humans3,4,12 and served as a model for the current study. Several researchers have proposed roles for MEL and 5HT in mediation of stress including protection of plant organelles and structures from oxidative damage caused by free radicals16,34-36 and the elimination of extraneous metabolic products through detoxification.37 The current data are the first suggestion of synergy between cholinergic metabolism and neuroindoleamine signaling in plants. In human systems, the interaction between cholinergic and serotonergic systems during cognitive processes has been well documented,38 and 5HT receptor subtypes are under investigation as potential drug targets for diseases such as Alzheimer.39
Metabolomics as a hypothesis generating tool
Metabolomics is the qualitative and quantitative analysis of all small metabolites in a biological sample.40,41 In recent years, the application of metabolomics has provided an interesting avenue for understanding how plants respond to specific environmental stressors.42,43 Understanding how the plant metabolome responds to neurologically active phytochemicals has not been investigated, but environmental cues such as light, temperature, drought, or salt stresses have been examined44 with broader applications toward crop selection.45,46 There are 3 distinctly different research approaches that are taken in metabolomics research. Some researchers are using a strictly targeted approach with quantification of known compounds using authenticated standards and spectral libraries sometimes associated with genomic or proteomic data.47 Other researchers are using the genomic or proteomic data as chemical leads in a pseudo-untargeted approach that quantifies known compounds from other species but that have not previously been described in the species of interest. These data may be mapped in association with the genomic data for compound and pathway discovery but are not truly untargeted analyses.48 The third approach is completely untargeted identification and quantification of the entire metabolome using statistics modeling and mapping to eliminate false discoveries, explore relationships between unknowns, and to target specific characteristic unknowns for fingerprinting, future targeted phytochemical studies, pathway elucidation, chemical association with physiological responses, and other applications.49
In the current work, we investigated changes in the whole plant metabolome in response to galanthamine treatment. Untargeted analysis identified phenylpropanoid metabolism as a primary response for further study. Coumarins are a class of secondary metabolites derived from the shikamate pathway via phenylalanine and contribute to plant defense through their anti-fungal and anti-microbial properties.50 Previous research identified coumarins, including scopoletin, isoscopoletin, esculin, esculetin, methylesculin, and umbelliferone, in leaf extracts of A. tridentata.51,52 Our exploratory analysis found increasing amounts of scopoletin and its monoglucoside scopolin in response to galanthamine exposure. Increased levels of scopoletin and scopolin have been shown to occur in response to stresses such as microbial attack, water stress, and wounding.50 Furthermore, manipulation of cultures with growth regulators such as 2–4D, kinetin, salicylic acid, and methyl jasmonate was previously found to affect levels of these compounds,50 most notably in tobacco cultures.53-55 Although more enquiry is needed, our untargeted exploratory analysis has allowed us to formulate the hypothesis that cultures treated with galanthamine increase production of phenolic compounds.
Conclusions and implications
Galanthamine is a very interesting natural drug that is found in a wide range of species including snowdrops, Narcissis, and other common bulbs. The utility of galanthamine for modulation of human neurochemistry is well established but less is known about the role of galanthamine in plant metabolism. Our data indicate a role for galanthamine as an allelopathic phytochemical released by one species into the environment and inducing a wide spectrum of responses in the phytochemistry of other plants. In order to survive abiotic and biotic environmental stresses, plants use many phytochemicals, including neurotransmitters or neurologically active signaling molecules, and our data indicate this potential role for IAA, indoleamines, and polyphenols. Previous researchers have hypothesized that these neuro-mimicking plant responses indicate plant communication, plant neurosignaling, or plant avoidant behaviors necessary for survival.56,57 The systems approach to generating novel phytochemical hypotheses through metabonomic analysis provides new avenues for future studies of the responses of plant tissues to environmental stimuli, allelochemicals, and natural products.
Materials and Methods
Establishment of germplasm lines
A collection of axenic stock plants of A. tridentata plants were established from individual seeds in vitro with previously established protocols10 and maintained on a sterile medium hereafter referred to as “MSO,” containing 4.4g/L Murashige and Skoog salts,58 B5 vitamins,59 and 30 g/l sucrose at pH 5.7. 3g/L phytagel was added to all media prior to autoclaving for 20 min at 121 °C at 1.4 x 104 kg/m/s2. Germplasm lines were established by regeneration of shoot cultures from individual seedlings as described previously,10 maintained at standard temperature (28 °C) with a 16 h photoperiod (35–50 μmol/m2/s) and sub-cultured on MSO medium into GA7 boxes (Magenta Corp) at 2 wk intervals.
Galanthamine treatments
Shoot tissue from the A. tridentata germplasm line DCO-1–09 were sub-cultured onto MSO medium in petri dishes (n = 12) (see ref. 30). For each treatment (n = 4), a solution of galanthamine hydrobromide (1.0 mL of 0, 5, or 10 µM; Chromadex, Vancouver) was filter sterilized (Millipore PVDF, 0.22 μm, 25 mm sterile syringe filters; Fisher Scientific, USA) and layered onto the sterile media twice weekly for a period of 1 month to ensure a continual dose. At the end of the experiment, tissues were excised, flash frozen and stored at –80 ◦C until further testing.
Preparation of extracts
A method was developed for analysis of neurotransmitters, IAA, and antioxidant potential in the same extract to ensure valid data comparison. In brief, an explant of A. tridentata leaf tissue was extracted in MeOH (0.5 g/mL) in complete darkness and an aliquot of 100 µL of each sample was dried under nitrogen gas and re-suspended in 18 mΩ water (Millipore). Remaining MeOH extract was stored at –80 ◦C for further analysis.
Quantification of ach and acetylcholinesterase
The cholinergenic system was quantified with an Amplex© Red Ach / Acetylcholinesterase Assay Kit (Molecular Probes Inc, Eugene, Oregon).60,61 Ach was quantified in tissues using a modification of the spectrophotometric method originally described in 1961.62 In brief, 100µL of Ach standard (n = 3) (0, 1, 5, 10, 20, 40, 60, 80, and 100µM) or AchE standard (n = 3) (0, 1, 2.5, 5, 10, 15, 25, 50, and 100 mu/mL), positive/negative control (n = 4), and A. tridentata tissue sample (0.5 g/mL) were added to a 96 well plate. All samples were prepared in 1X buffer. 100 µL of working solution (200 µM Amplex red reagent, 1 U/mL Horseradish peroxidase, 0.1 U/mL choline oxidase, 0.5 U/mL AchE, or 50 µM Ach, Buffer) was added to each well, lightly shaken and placed into a Synergy multi-detection microplate reader (BioTek Instruments Inc, USA), with excitation and emission wavelengths set to 530–560 nm and 590 nm, respectively. Fluorescence was measured every 5 min until the reaction was complete (1 h). A standard curve for Ach and AchE was generated by subtracting standards by the negative control (0 µM Ach or AchE). Ach and AchE were then quantified for each sample by subtracting the observed fluorescence values for each sample by the negative control and sample blank and comparing to standard curve data.
Analysis of MEL, 5HT, and IAA
IAA and indoleamines were separated from a 1 µL aliquot of the extract on a reverse phase column (150 x 2.1 mm, 1.7 μm C18 BEH, Waters) using a Waters I-Class UPLC. A gradient of 0.1% formic acid (Eluent A) and acetonitrile (Eluent B) [(A%:B%): 0.0–0.5 min, 99:1; 0.5–2.5 min, 70:30; 2.5–2.6 min, 5:95; 2.6–3.0 min, 5:95; 3–3.5 min, 5:95; 3.5–3.6 min, 99:1; 3.6–5.0 min, 99:1] separated MEL, 5HT, and IAA with a flow rate of 0.5 ml/min. Analytes were detected and quantified with a tandem mass spectrometer (Xevo TQ-S triple quadrupole mass spectrometer, Waters, Mississauga) using detection parameters optimized for signal intensity and specificity (Table 3). The capillary voltage was 3500, desolvation gas rate was 800 L/hr, cone gas rate was 150 L/hr, desolvation temperature was 400 °C, and the source temperature was 150 °C for all analyses. Authentic standards were injected over a wide range to establish the limits of detection (LOD) and limits of quantification (LOQ). The linear ranges were 1–100 ng/mL for MEL and 1–500 ng/mL for 5HT and IAA. In preliminary experiments it was determined that a gradient of IAA and indoleamine was present across the plant tissues. Therefore, to determine the response to galanthamine, plantlets were subsampled by sectioning into 3–5 pieces (259 ± 10 mg) prior to analysis and each subsample was analyzed in triplicate.
Table 3. Optimized detection of Indoleamines and IAA by Tandem Mass Spectrometry (Waters Xevo TQ-S).
| Cone voltage (V) | Collision voltage (V) | MRM Transition | |
| IAA | 30 | 25 | 176 > 103 |
| IAA | 30 | 13 | 176 > 130 |
| 5HT | 45 | 27 | 177 > 115 |
| 5HT | 45 | 10 | 177 > 160 |
| MEL | 30 | 23 | 233 > 159 |
| MEL | 30 | 15 | 233 > 174 |
IAA and indoleamine data analysis
Indoleamines and IAA were quantified by comparison to the linear range of standard curves established with authenticated standards (Sigma, USA). Data for each plantlet were determined by a proportioned approach. In brief, the fresh weight of each excised section was divided by the total weight of the plantlet it originated from in order to scale the proportionate MEL, 5HT, and IAA concentrations in the entire tissues.
Antioxidant potential
Antioxidant potential was determined from the same extracts with a DPPH bioassay as described previously.63 In brief, the extracts were diluted (n = 6), replicated (n = 24) and randomly assigned to a 96 well microplate alongside extract blanks (n = 24), assay blanks (n = 3), negative control (n = 3), and a positive control of Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid; Sigma, purity ≥ 97%) dilutions (1, 8, 16, 24, 32, 40, and 50 µM) in triplicate. An aliquot of each extract or Trolox standard (100 µL) reacted with 100 µL of 0.1 mM DPPH for 15 min at 25 °C. Absorbance was recorded every 60s at 520 nm using a Synergy multi-detection microplate reader. Radical scavenging activity was calculated at t = 15 min and expressed as required to reduce free radicals by 50%.
IAA bioassay
IAA activity was determined using an oat (Avena sativa) coleoptile elongation bioassay as previously described.64 In brief, coleoptile tips (about 0.3 mm) were removed and 10 mm sections were incubated in 10 ml of sterile distilled water supplemented with various concentrations of galanthamine (0, 0.05, 0.1, 1, or 10 μM), NAA (5 μM), or IAA (5 μM) in 15 mm petri plates. After 24 h, the coleoptiles were measured again to determine the degree of elongation. Each plate contained 10 sections and each treatment was replicated thrice for a total of 30 sections per treatment. The plates were arranged in a completely randomized design and the experiment was conducted twice. Significance between treatments was determined using the Tukey means separation test (α=0.05).
Metabolomic analysis
A. tridentata shoot cultures treated with 0, 5, and 10 µM of galanthamine were sampled in triplicate and subjected to untargeted phytochemical analysis using previously published protocols.49,66 In brief, shoot tissues were extracted in 70% ethanol (0.05 g/mL), homogenized, and filtered by centrifugation (UltraFree MC, Millipore). Extracts were then separated on a Waters BEH Acquity C18 (2.1 X 150 mm, 1.7 µm) column with the following gradient: 0.1% aqueous formic acid:acetonitrile (0.0–25 min, 95:5–5:95 v/v, 25.01–30.0 min, 95:5 v/v). The flow rate was set to 0.25 mL/min for 30 min at 30 °C (Waters Acquity UPLC). A steady flow of leucine enkephalin (Waters 1525 HPLC binary solvent manager, 2 ng/mL) was used as the internal standard for calibration of the Micromass LCT Premier series ToF-MS (Waters Inc). Time of Flight mass spectrometry used previously published optimized conditions including: electrospray ionization and positive and negative ion detection in W mode, mass range of 100–1000 amu and a scan time of 0.1 s. Data were collected with MassLynx V4.1 and exported via MarkerLynx. Data were processed in Excel (Microsoft) to align retention times and remove multiply charged ions as described previously.49,65
Analysis of metabolomic data
Average ion intensity was calculated for each compound in order to select for metabolites that are most prominent throughout all treatments and replicates. Once ion averages were calculated, they were divided by the average total ion count in order to identify compounds that possess average ion intensities greater than 5.0 (0.09% of the average total ion intensity). Using Excel, average ion intensity for each prominent ion (dependent) was plotted against 0, 5, and 10 µM galanthamine treatments (independent), in order to determine whether a linear relationship exists. Compounds exhibiting exponential or linear behavior (R2 > 0.75) in response to galanthamine treatments were then subjected to putative identification using Metabosearch http://omics.georgetown.edu/MetaboSearch.html, Plant Metabolic Pathway Database http://www.plantcyc.org/, and phytochemical information gathered from a comprehensive review on North American Sagebrush.9 Putative identifications (± 0.02 daltons) were performed using a previously published protocol63,66 (Figs. 8 and 9).
Glossary
Abbreviations:
- Ach
Ach
- AchE
Acetylcholinesterase
- IAA
Auxin
- MEL
Melatonin
- 5HT
Serotonin
Disclosure of Potential Conficts of Interest
No conflicts of interest have been disclosed.
References
- 1.Skoog F, Miller CO. . Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 1957; 11:118 - 30; PMID: 13486467 [PubMed] [Google Scholar]
- 2.Murch SJ, Saxena PK, Journey of a Single Cell to a Plant. Enfield, NH, ed. Science Publishers, Inc: 2005 [Google Scholar]
- 3.Murch SJ, Saxena PK. . Melatonin: A potential regulator of plant growth and development?. In Vitro Cell Dev Pl 2002; 38:531 - 536; http://dx.doi.org/ 10.1079/IVP2002333 [DOI] [Google Scholar]
- 4.Murch SJ, Saxena PK. . Role of indoleamines in regulation of morphogenesis in in vitro cultures of St. John's Wort (Hypericum perforatum L.). Future for Medicinal and Aromatic Plants 2004; 425 - 432 [Google Scholar]
- 5.Cao J, Cole IB, Murch SJ. . Neurotransmitters, neuroregulators and neurotoxins in the life of plants. Can J Plant Sci 2006; 86:1183 - 8; http://dx.doi.org/ 10.4141/P06-034 [DOI] [Google Scholar]
- 6.Stanton DJ, McArthur ED, Freeman DC, Golenberg EM. . No genetic substructuring in Artemisia subgenus Tridentatae despite strong ecotypic subspecies selection. Biochem Syst Ecol 2002; 30:579 - 93; http://dx.doi.org/ 10.1016/S0305-1978(01)00118-1 [DOI] [Google Scholar]
- 7.Douglas, GW., Stanley, GB., Meidinger, D., Pojar, J. Illustrated flora of British Columbia, gymnosperms and dicotyledons, Vol 1. British Columbia; 1998. [Google Scholar]
- 8.Moerman DE. Native American Ethnobotany. Portland, OR: Timber Press, Inc, 2009. [Google Scholar]
- 9.Turi CE, Shipley PR, Murch SJ. . North American Artemisia species from the subgenus Tridentatae (Sagebrush): a phytochemical, botanical and pharmacological review. Phytochemistry 2014; 98:9 - 26; http://dx.doi.org/ 10.1016/j.phytochem.2013.11.016; PMID: 24359634 [DOI] [PubMed] [Google Scholar]
- 10.Turi CE, Axwik KE, Murch SJ. . In vitro conservation, phytochemistry, and medicinal activity of Artemisia tridentata nutt: Metabolomics as a hypothesis-generating tool for plant tissue culture. Plant Growth Regul 2014; Forthcoming http://dx.doi.org/ 10.1007/s10725-014-9915-y [DOI] [Google Scholar]
- 11.Murch SJ. . KrishnaRaj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's Wort (Hypericum perforatum L. cv. anthos) plants. Plant Cell Rep 2000; 19:698 - 704; http://dx.doi.org/ 10.1007/s002990000206 [DOI] [PubMed] [Google Scholar]
- 12.Murch SJ, Campbell S, Saxena PK. . The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John's Wort (Hypericum perforatum L.). In Vitro Cell Dev Pl 2001; 37:786 - 793; http://dx.doi.org/ 10.1007/s11627-001-0130-y [DOI] [Google Scholar]
- 13.Jones MPA, Cao J, O’Brien R, Murch SJ, Saxena PK. . The mode of action of thidiazuron: auxins, indoleamines, and ion channels in the regeneration of Echinacea purpurea L. Plant Cell Rep 2007; 26:1481 - 90; http://dx.doi.org/ 10.1007/s00299-007-0357-0; PMID: 17483954 [DOI] [PubMed] [Google Scholar]
- 14.Cole IB, Cao J, Alan AR, Saxena PK, Murch SJ. . Comparisons of Scutellaria baicalensis, Scutellaria lateriflora and Scutellaria racemosa: genome size, antioxidant potential and phytochemistry. Planta Med 2008; 74:474 - 81; http://dx.doi.org/ 10.1055/s-2008-1034358; PMID: 18484546 [DOI] [PubMed] [Google Scholar]
- 15.Park S, Byeon Y, Back K. . Functional analyses of three ASMT gene family members in rice plants. J Pineal Res 2013; 55:409 - 15; PMID: 24033370 [DOI] [PubMed] [Google Scholar]
- 16.Lazár D, Murch SJ, Beilby MJ, Al Khazaaly S. . Exogenous melatonin affects photosynthesis in characeae Chara australis.. Plant Signal Behav 2013; 8:e23279; http://dx.doi.org/ 10.4161/psb.23279; PMID: 23299331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murch SJ, Alan AR, Cao J, Saxena PK. . Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res 2009; 47:277 - 83; http://dx.doi.org/ 10.1111/j.1600-079X.2009.00711.x; PMID: 19732299 [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Qi LW, Wang WM, Saxena PK, Liu CZ. . Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata.. J Pineal Res 2011; 50:83 - 8; http://dx.doi.org/ 10.1111/j.1600-079X.2010.00817.x; PMID: 21073518 [DOI] [PubMed] [Google Scholar]
- 19.Uchendu EE, Shukla MR, Reed BM, Saxena PK. . Melatonin enhances the recovery of cryopreserved shoot tips of American elm (Ulmus americana L.). J Pineal Res 2013; 55:435 - 42; PMID: 24117864 [DOI] [PubMed] [Google Scholar]
- 20.Hartmann E, Gupta R. Acetylcholine as a signaling system in plants. In: Second Messengers in Plant Growth and Development. Boss W, Marre D, eds. New York: Alan R Liss, 1989:257-288 [Google Scholar]
- 21.Hartmann E, Kilbinger H. . Occurrence of light-dependent acetylcholine concentrations in higher plants. Experientia 1974; 30:1397 - 8; http://dx.doi.org/ 10.1007/BF01919649; PMID: 4442525 [DOI] [PubMed] [Google Scholar]
- 22.Tretyn A, Kendrick R. . Acetylcholine in plants - presence, metabolism and mechanism of action. Bot Rev 1991; 57:33 - 73; http://dx.doi.org/ 10.1007/BF02858764 [DOI] [Google Scholar]
- 23.Roshchina VV. Neurotransmitters in Plant Life. Science Publishers: 2001 [Google Scholar]
- 24.Jaffe MJ. . Evidence for the regulation of phytochrome-mediated processes in bean roots by the neurohumor, acetylcholine. Plant Physiol 1970; 46:768 - 77; http://dx.doi.org/ 10.1104/pp.46.6.768; PMID: 5500205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta M, Malik M, Khurana J, Maheshwari S. . Phytochrome modulation of calcium fluxes in wheat (Triticum aestivum L) protoplasts. Plant Growth Regul 1993; 12:293 - 302; http://dx.doi.org/ 10.1007/BF00027211 [DOI] [Google Scholar]
- 26.Bamel K, Gupta SC, Gupta R. . Acetylcholine causes rooting in leaf explants of in vitro raised tomato (Lycopersicon esculentum Miller) seedlings. Life Sci 2007; 80:2393 - 6; http://dx.doi.org/ 10.1016/j.lfs.2007.01.039; PMID: 17328922 [DOI] [PubMed] [Google Scholar]
- 27.Yang C, Zhai Z, Guo Y, Gao P. . Effects of acetylcholine, cytochalasin B and amiprophosmethyl on phloem transport in radish (Raphanus sativas). J Integr Plant Biol 2007; 49:550 - 5; http://dx.doi.org/ 10.1111/j.1744-7909.2007.00434.x [DOI] [Google Scholar]
- 28.Tezuka T, Akita I, Yoshino N, Suzuki Y. . Regulation of self-incompatibility by acetylcholine and cAMP in Lilium longiflorum.. J Plant Physiol 2007; 164:878 - 85; http://dx.doi.org/ 10.1016/j.jplph.2006.05.013; PMID: 16882455 [DOI] [PubMed] [Google Scholar]
- 29.Heinrich M, Lee Teoh H. . Galanthamine from snowdrop--the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharmacol 2004; 92:147 - 62; http://dx.doi.org/ 10.1016/j.jep.2004.02.012; PMID: 15137996 [DOI] [PubMed] [Google Scholar]
- 30.Ago Y, Koda K, Takuma K, Matsuda T. . Pharmacological aspects of the acetylcholinesterase inhibitor galantamine. J Pharmacol Sci 2011; 116:6 - 17; http://dx.doi.org/ 10.1254/jphs.11R01CR; PMID: 21498956 [DOI] [PubMed] [Google Scholar]
- 31.Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, Kato T, Kawashima K. . Evolutional study on acetylcholine expression. Life Sci 2003; 72:1745 - 56; http://dx.doi.org/ 10.1016/S0024-3205(02)02478-5; PMID: 12559395 [DOI] [PubMed] [Google Scholar]
- 32.Wessler I, Kirkpatrick CJ, Racké K. . The cholinergic ‘pitfall’: acetylcholine, a universal cell molecule in biological systems, including humans. Clin Exp Pharmacol Physiol 1999; 26:198 - 205; http://dx.doi.org/ 10.1046/j.1440-1681.1999.03016.x; PMID: 10081614 [DOI] [PubMed] [Google Scholar]
- 33.Grando SA, Kawashima K, Kirkpatrick CJ, Meurs H, Wessler I. . The non-neuronal cholinergic system: basic science, therapeutic implications and new perspectives. Life Sci 2012; 91:969 - 72; http://dx.doi.org/ 10.1016/j.lfs.2012.10.004; PMID: 23141771 [DOI] [PubMed] [Google Scholar]
- 34.Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. . Phytomelatonin: a review. J Exp Bot 2009; 60:57 - 69; http://dx.doi.org/ 10.1093/jxb/ern284; PMID: 19033551 [DOI] [PubMed] [Google Scholar]
- 35.Galano A, Tan DX, Reiter RJ. . Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 2011; 51:1 - 16; http://dx.doi.org/ 10.1111/j.1600-079X.2011.00916.x; PMID: 21752095 [DOI] [PubMed] [Google Scholar]
- 36.Park WJ. . Melatonin as an endogenous plant regulatory signal: Debates and perspectives. J Plant Biol 2011; 54:143 - 9; http://dx.doi.org/ 10.1007/s12374-011-9159-6 [DOI] [Google Scholar]
- 37.Grobe W. . Function of serotonin in seeds of walnut. Phytochem 1982; 21:819 - 22; http://dx.doi.org/ 10.1016/0031-9422(82)80071-X [DOI] [Google Scholar]
- 38.Matsukawa M, Ogawa M, Nakadate K, Maeshima T, Ichitani Y, Kawai N, Okado N. . Serotonin and acetylcholine are crucial to maintain hippocampal synapses and memory acquisition in rats. Neurosci Lett 1997; 230:13 - 6; http://dx.doi.org/ 10.1016/S0304-3940(97)00460-6; PMID: 9259452 [DOI] [PubMed] [Google Scholar]
- 39.Terry AV Jr., Buccafusco JJ, Wilson C. . Cognitive dysfunction in neuropsychiatric disorders: selected serotonin receptor subtypes as therapeutic targets. Behav Brain Res 2008; 195:30 - 8; http://dx.doi.org/ 10.1016/j.bbr.2007.12.006; PMID: 18241938 [DOI] [PubMed] [Google Scholar]
- 40.Yuliana ND, Khatib A, Choi YH, Verpoorte R. . Metabolomics for bioactivity assessment of natural products. Phytother Res 2011; 25:157 - 69; PMID: 20658470 [DOI] [PubMed] [Google Scholar]
- 41.Verpoorte R, Choi YH, Kim HK. . Metabolomics: will it stay?. Phytochem Anal 2010; 21:2 - 3; http://dx.doi.org/ 10.1002/pca.1191; PMID: 20013869 [DOI] [PubMed] [Google Scholar]
- 42.Sanchez DH, Schwabe F, Erban A, Udvardi MK, Kopka J. . Comparative metabolomics of drought acclimation in model and forage legumes. Plant Cell Environ 2012; 35:136 - 49; http://dx.doi.org/ 10.1111/j.1365-3040.2011.02423.x; PMID: 21902697 [DOI] [PubMed] [Google Scholar]
- 43.Obata T, Fernie AR. . The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 2012; 69:3225 - 43; http://dx.doi.org/ 10.1007/s00018-012-1091-5; PMID: 22885821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kral'ova K, Jampilek J, Ostrovsky I. . Metabolomics - useful tool for study of plant responses to abiotic stresses. Ecol Chem Eng S 2012; 19:133 - 61 [Google Scholar]
- 45.Arbona V, Manzi M, Ollas Cd, Gómez-Cadenas A. . Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci 2013; 14:4885 - 911; http://dx.doi.org/ 10.3390/ijms14034885; PMID: 23455464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen S, Parsons AJ, Jones CS. . Metabolomics of forage plants: a review. Ann Bot 2012; 110:1281 - 90; http://dx.doi.org/ 10.1093/aob/mcs023; PMID: 22351485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis MC, Fiehn O, Durnford DG. . Metabolic acclimation to excess light intensity in Chlamydomonas reinhardtii.. Plant Cell Environ 2013; 36:1391 - 405; http://dx.doi.org/ 10.1111/pce.12071; PMID: 23346954 [DOI] [PubMed] [Google Scholar]
- 48.Brady SM, Provart NJ. . Web-queryable large-scale data sets for hypothesis generation in plant biology. Plant Cell 2009; 21:1034 - 51; http://dx.doi.org/ 10.1105/tpc.109.066050; PMID: 19401381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown PN, Turi CE, Shipley PR, Murch SJ. . Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Med 2012; 78:630 - 40; http://dx.doi.org/ 10.1055/s-0031-1298239; PMID: 22337317 [DOI] [PubMed] [Google Scholar]
- 50.Gnonlonfin GJB, Sanni A, Brimer L. . Review scopoletin - A coumarin phytoalexin with medicinal properties. Crit Rev Plant Sci 2012; 31:47 - 56; http://dx.doi.org/ 10.1080/07352689.2011.616039 [DOI] [Google Scholar]
- 51.Wilt FM, Geddes JD, Tamma RV, Miller GC, Everett RL. . Interspecific variation of phenolic concentrations in persistent leaves among 6 taxa from subgenus Tridentatae of Artemisia (Asteraceae). Biochem Syst Ecol 1992; 20:41 - 52; http://dx.doi.org/ 10.1016/0305-1978(92)90071-K [DOI] [Google Scholar]
- 52.Wilt FM, Miller GC. . Seasonal-variation of coumarin and flavonoid concentrations in persistent leaves of Wyoming Big Sagebrush (Artemisia-tridentata ssp wyomingensis, asteraceae). Biochem Syst Ecol 1992; 20:53 - 67; http://dx.doi.org/ 10.1016/0305-1978(92)90072-L [DOI] [Google Scholar]
- 53.Sharan M, Taguchi G, Gonda K, Jouke T, Shimosaka M, Hayashida N, Okazaki M. . Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Sci 1998; 132:13 - 9; http://dx.doi.org/ 10.1016/S0168-9452(97)00260-4 [DOI] [Google Scholar]
- 54.Petit-Paly G, Franck T, Brisson L, Kevers C, Chenieux JC, Rideau M. . Cytokinin modulates catalase activity and coumarin accumulation in in vitro cultures of tobacco. J Plant Physiol 1999; 155:9 - 15; http://dx.doi.org/ 10.1016/S0176-1617(99)80134-5 [DOI] [Google Scholar]
- 55.Taguchi G, Yoshizawa K, Kodaira R, Hayashida N, Okazaki M. . Plant hormone regulation on scopoletin metabolism from culture medium into tobacco cells. Plant Sci 2001; 160:905 - 11; http://dx.doi.org/ 10.1016/S0168-9452(00)00464-7; PMID: 11297787 [DOI] [PubMed] [Google Scholar]
- 56.Darwin C. The Power of Movement in Plants. New York, USA: NYU Press, 1880 [Google Scholar]
- 57.Baluska F, Mancuso S. . Plant neurobiology: from sensory biology, via plant communication, to social plant behavior. Cogn Process 2009; 10:Suppl 1 S3 - 7; http://dx.doi.org/ 10.1007/s10339-008-0239-6; PMID: 18998182 [DOI] [PubMed] [Google Scholar]
- 58.Murashige T, Skoog F. . A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol Plant 1962; 15:473 - 97; http://dx.doi.org/ 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 59.Gamborg OL, Miller RA, Ojima K. . Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 1968; 50:151 - 8; http://dx.doi.org/ 10.1016/0014-4827(68)90403-5; PMID: 5650857 [DOI] [PubMed] [Google Scholar]
- 60.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. . A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 1997; 253:162 - 8; http://dx.doi.org/ 10.1006/abio.1997.2391; PMID: 9367498 [DOI] [PubMed] [Google Scholar]
- 61.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. . A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods 1997; 202:133 - 41; http://dx.doi.org/ 10.1016/S0022-1759(96)00244-X; PMID: 9107302 [DOI] [PubMed] [Google Scholar]
- 62.Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM. . A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7:88 - 95; http://dx.doi.org/ 10.1016/0006-2952(61)90145-9; PMID: 13726518 [DOI] [PubMed] [Google Scholar]
- 63.Turi CE, Murch SJ. . Targeted and untargeted phytochemistry of Ligusticum canbyi: indoleamines, phthalides, antioxidant potential, and use of metabolomics as a hypothesis-generating technique for compound discovery. Planta Med 2013; 79:1370 - 9; http://dx.doi.org/ 10.1055/s-0033-1350618; PMID: 23877920 [DOI] [PubMed] [Google Scholar]
- 64.Sirois JC. . Studies on growth regulators. I. Improved Avena coleoptile elongation test for auxin. Plant Physiol 1966; 41:1308 - 12; http://dx.doi.org/ 10.1104/pp.41.8.1308; PMID: 16656401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown PN, Murch SJ, Shipley PR. . Phytochemical diversity of cranberry (Vaccinium macrocarpon Aiton) cultivars by anthocyanin determination and metabolomic profiling with chemometric analysis. J Agric Food Chem 2012; 60:261 - 71; http://dx.doi.org/ 10.1021/jf2033335; PMID: 22148867 [DOI] [PubMed] [Google Scholar]
- 66.Murch SJ, Rupasinghe HP, Goodenowe D, Saxena PK. . A metabolomic analysis of medicinal diversity in Huang-qin (Scutellaria baicalensis Georgi) genotypes: discovery of novel compounds. Plant Cell Rep 2004; 23:419 - 25; http://dx.doi.org/ 10.1007/s00299-004-0862-3; PMID: 15449017 [DOI] [PubMed] [Google Scholar]