Abstract
Background
This study tests whether the genetic predictor (CHRNA5 nicotine receptor gene variants) and an environmental risk factor (partner smoking) interact in the prediction of smoking reduction.
Methods
Subjects were from a community-based, longitudinal study of women (N=1,856) who smoked before pregnancy, and a randomized comparative effectiveness smoking cessation trial (N=1,065). Smoking reduction was defined as the trajectory of self-reported smoking quantities over time in the observational study, and as the trajectory of alveolar CO levels in the cessation trial.
Results
In the pregnancy study, rs16969968 genotype and partner smoking status interacted such that the smoking reduction was lowest for expectant mothers with high genetic risk and partner smoking, and highest for those with high genetic risk but not partner smoking (interaction of genotype*partner smoking on smoking quantity trajectory slope β=0.071, 95%CI=0.013, 0.13, p=0.017). In the clinical trial, a similar interaction was found (interaction β=0.20, 95%CI=0.049, 0.36, p=0.010). Furthermore, these associations were moderated by pharmacotherapy such that the interactive relation of genetic and environmental factors occurred in the placebo group, but not in the active pharmacotherapy group (interaction of genotype*partner smoking*pharmacotherapy on CO trajectory slope β=-0.25, 95%CI=-0.42, -.091, p=0.0023).
Conclusions
The CHRNA5 genetic risk synergized the effect of partner smoking, producing an especially low likelihood of successful smoking reduction in two complementary studies. This suggests that the genetic vulnerability may be mitigated by altering environmental factors. In addition, cessation pharmacotherapy neutralizes the increase in cessation failure associated with combined genetic and environmental risks, which has possible relevance to treatment algorithms.
Keywords: Smoking Reduction, CHRNA5, Partner Smoking, ALSPAC, UW-TTURC
1. Introduction
Tobacco smoking is a continuing global public health concern despite effective smoking cessation treatments and public health policies (Jha et al., 2013; Schroeder, 2013; Thun et al., 2013), and rates of smoking cessation failure remain high in both clinical and general populations (Baker et al., 2007; Breslau and Johnson, 2000; West, 2005). Identification of the genetic and environmental predictors of quitting success is critical in understanding the causes of smoking cessation outcomes and developing more effective clinical interventions and health policies.
Growing evidence suggests that genetic variants predict cessation success (Baker et al., 2009; Breitling et al., 2010; Conti et al., 2008; Freathy et al., 2009; King et al., 2012; Munafo et al., 2011; Rose et al., 2010; Sarginson et al., 2011; Uhl, 2009, 2008, 2012). Specifically, rs16969968, a non-synonymous coding variant in the nicotinic receptor gene (CHRNA5), is not only unequivocally associated with heavy smoking in multiple large scale meta-analyses, but also is associated with a functionally significant change in nicotinic receptor binding to agonist (Bierut et al., 2008; Liu et al., 2010; Saccone et al., 2010; TAG, 2010; Thorgeirsson et al., 2010; Ware et al., 2011). This CHRNA5 variant has been shown to predict smoking cessation success and response to cessation pharmacotherapy in multiple studies. Individuals with the rs16969968 risk variant (A) are less likely to be abstinent at the end of treatment and more likely to benefit from cessation pharmacotherapy such as nicotine replacement (Bergen et al., 2013; Chen et al., 2012b; Munafo et al., 2011).
Having a partner who smokes is a well-established risk factor for low motivation to quit smoking and failure to quit smoking successfully (Bolt et al., 2009; Harmer and Memon, 2013; Homish and Leonard, 2005; Okechukwu et al., 2012; Ruge et al., 2008). This may be because partner smoking allows immediate access to cigarettes and greater exposure to smoking cues. It is currently unknown how this major environmental risk affects smoking cessation in the context of the major genetic risk (i.e., CHRNA5 risk alleles). It is possible that the two factors merely produce additive effects, or they interact such that one amplifies the risk posed by the other. For instance, it is possible that partner smoking affects only those low in genetic risk; i.e., those high in genetic risk will likely relapse regardless of cigarette availability and exposure. Conversely, it is possible that environmental risk is most damaging to those high in genetic risk; i.e., partner smoking is especially challenging to those with a strong genetic vulnerability to cessation failure. The current research aims to address a clinically significant question: i.e., Do major genetic and environmental risks synergize to produce individuals with an especially high risk of cessation failure?
Using data from a community-based study, the Avon Longitudinal Study of Parents and Children (ALSPAC(Golding et al., 2001)), and a University of Wisconsin Transdisciplinary Tobacco Use Research Center (UW-TTURC; Piper et al., 2009)) smoking cessation clinical trial, we examine the main and interactive effects of partner smoking and CHRNA5 genetic risk on smoking reduction likelihood. The two studies differ in type of participants, study duration, and design. However, complementary hypotheses are developed for these two research designs.
The ALSPAC study includes pregnant women smokers who are likely to limit their smoking or quit completely during pregnancy with health and social concerns (Cnattingius, 2004; Triche et al., 2008). In the ALSPAC study, the primary outcome is smoking reduction defined by a trajectory of decreasing self-reported smoking quantity during pregnancy. In the Wisconsin smoking cessation trial, a biomarker for smoking heaviness (alveolar CO level) was assessed both before and after the quit date through 8 weeks post-quit. CO level over time constitutes an objective biomarker of smoking reduction. In sum, smoking reduction is assessed by trajectories of self-reported smoking quantity in a community sample and alveolar CO level in a treatment trial. Use of continuous measures of smoking outcomes provides a more sensitive index of outcome than does a binary measure such as point prevalence abstinence (Baker et al., 2011).
Analyses address these questions: 1) Whether the CHRNA5 effect on smoking reduction is moderated by partner smoking in the observational community study; 2) Whether the CHRNA5 effect on smoking reduction is moderated by partner smoking in the cessation trial; and 3) Given evidence that the CHRNA5 risk for smoking cessation failure occurs primarily amongst individuals not using pharmacotherapy, does the gene × environmental risk interaction occur only amongst individuals receiving placebo? Given evidence that CHRNA5 risk for smoking cessation varies with pharmacotherapy (Bergen et al., 2013; Chen et al., 2012b), we will also test whether the gene x environmental risk interaction varies with pharmacotherapy.
2. Methods
2.1 Avon Longitudinal Study of Parents and Children (ALSPAC)
The ALSPAC study (Fraser et al., 2012; Golding et al., 2001) is a prospective study that recruited pregnant women from Avon, UK, with expected delivery dates between April 1991 and December 1992 (known as Phase I enrollment). The study website contains details of all the data that are available through a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/). All women gave informed consent and ethical approval was obtained from the ALSPAC Law and Ethics Committee and the local review committee.
Smoking behavior of women before and during pregnancy was determined from questionnaires. A questionnaire was administered in the 18th gestational week, asking about pre-pregnancy and first-trimester smoking behavior (whether or not the woman smoked and, for smokers, the quantity of cigarettes per day). Women were questioned again about current smoking behavior during the 32nd week of pregnancy. At each time point, the data on smoking quantity were categorized into 0, 1–9, 10–19, and 20+ cigarettes per day. Data on known covariates of smoking cessation in pregnancy (Ebert and Fahy, 2007; Lu et al., 2001) were also collected via questionnaire: age and partner's smoking status. Smoking cessation was defined as the trajectory of smoking quantity over 3 time points: pre-pregnancy, first-trimester, and third trimester. Cessation pharmacotherapy was not provided as part of this observational study and likely very rare given the risk of most medication use during pregnancy. The proxy variant for CHRNA5 rs16969968, rs1051730 (r2=1, 1000 Genome CEU, http://www.1000genomes.org/), was genotyped. Genetic and phenotypic data are available on 1,856 subjects of European ancestry.
2.2 University of Wisconsin Transdisciplinary Tobacco Use Research Center (UW-TTURC)
The UW-TTURC study was a randomized, placebo-controlled smoking cessation trial (Piper et al., 2009). The University of Wisconsin-Madison IRB approved this trial, and all subjects provided written informed consent. Participants were 18 years of age or older, smoked 10 or more cigarettes per day, and were motivated to quit smoking. Prior to randomization, participants completed baseline assessments of demographics, smoking history (including cigarettes smoked per day), and environmental risks (e.g., living with a partner who smoked). Participants provided a breath sample for alveolar carbon monoxide (CO) analysis to verify their smoking status and estimate their smoking heaviness at 6 time points: pre-quit, quit-date, and 1, 2, 4 and 8 weeks post-quit during the trial. Smoking reduction was defined as the linear trajectory of CO level over time.
Participants (N=1,065 of European ancestry with genetic data) were randomly assigned to either placebo (n = 134) or active pharmacotherapy ((n=931): (nicotine patch (n = 187); nicotine lozenge (n = 179); bupropion SR (n = 183); nicotine patch and nicotine lozenge (n = 192); or bupropion and nicotine lozenge (n = 190)) for 2 months. All participants received six brief (10 minute) individual counseling sessions.
Genotyping of the UW-TTURC sample was performed by the Center for Inherited Disease Research at Johns Hopkins University using the Illumina Omni2.5 microarray (www.illumina.com). Data cleaning was led by the GENEVA Coordinating Center at the University of Washington.
2.3 Analysis
We examined the association between the CHRNA5 variant rs16969968, coded additively, and smoking cessation in both studies.
In the ALSPAC study, we used a standard series of mixed models to analyze smoking outcome: the linear trajectory of smoking quantity during pregnancy. Cigarettes smoked per day (CPD) were coded as 4 levels (0, 1–9, 10–19, 20+). Self-reported cigarettes smoked per day for 3 time points (pre-pregnancy, 1st trimester, 3rd trimester, with the repeated measures coded as 0, 1, and 2) was analyzed with mixed models for repeated measures. The β coefficient for ‘time’ was the slope characterizing the trajectory of smoking quantity change over time. For example, the interaction term of ‘time’ and rs16969968 was a test of genetic effect on the slope, i.e., the trajectory of smoking quantity. In secondary analyses, we examined dichotomous outcomes (abstinence and reduction) in the 32nd week of pregnancy with logistic regressions.
In the UW-TTURC study, we used a standard series of mixed models to analyze smoking outcome: the linear trajectory of alveolar CO levels during the trial. Because the distribution of CO levels was skewed to the right, it was square root transformed. Alveolar CO level for 6 time points (pre-quit, quit date, and 1, 2, 4 and 8 weeks post-quit, with repeated measure coded as 0-5) was analyzed with mixed models for repeated measures. Covariates included age, gender, and pharmacotherapy (placebo versus active pharmacotherapy in the UW-TTURC study). In secondary analyses, we examined the dichotomous outcome (abstinence) in the end of treatment at 2 months post-quit with logistic regressions.
3. Results
3.1 Avon Longitudinal Study of Parents and Children (ALSPAC)
Subjects were of European descent, identified as smokers pre-pregnancy (defined as active smoking ≥1 cigarettes per day (CPD)), and had genotype data (N=1,856). Demographic data, pre-pregnancy smoking quantity, genotype frequencies are given in Table S1(A)1 and 62.0% of women reported living with a partner who smoked cigarettes. We found a robust association between CHRNA5 rs16969968 and smoking heaviness defined by CPD adjusted for age (β=0.081, 95%CI=0.044 to 0.118, p=1.47×10-5).
During pregnancy, these women had a trajectory of decreased smoking quantity over time (pre-pregnancy, first trimester, 3rd trimester) (β=-0.53, 95% CI=-0.55 to -0.50, p<0.0001). Both genetic risk (rs16969968 (A)) and partner smoking predicted an overall higher level of smoking quantity during pregnancy (β=0.070, 95% CI=0.033-0.11, p=1.8×10-4 for rs16969968 (A); β=0.23, 95% CI=0.19 to 0.29, p<0.0001 for partner smoking; Table 1, Model 1). Genetic risk of variant rs16969968 interacted with partner smoking in the prediction of smoking quantity. The trajectory of smoking quantity remained especially elevated amongst women who had both a smoking partner and high risk in rs16969968 (interaction of genotype*partner smoking on smoking quantity trajectory slope β=0.071, 95% CI=0.013 to 0.13, p=0.017; Table 1, Model 2).
Table 1. ALSPAC: The effect of CHRNA5 rs16969968a on the trajectory of smoking quantity during pregnancy is moderated by partner smoking (n=1856).
| Predictor | B | 95% Confidence Interval | P value | |
|---|---|---|---|---|
|
| ||||
| Lower Bound | Upper Bound | |||
| Model 1 | ||||
| Age | -0.0029 | -.047 | .041 | 0.90 |
| Time | -.53 | -.55 | -.50 | <0.0001 |
| Partner smoking | 0.23 | 0.19 | 0.29 | <0.0001 |
| rs16969968a | .070 | .033 | 0.11 | 1.8×10-4 |
|
| ||||
| Model 2 | ||||
| Age | -0.0030 | -0.047 | 0.041 | 0.90 |
| Time | -0.51 | -0.56 | -0.46 | <0.0001 |
| Partner smoking | 0.16 | 0.082 | 0.24 | <0.0001 |
| rs16969968a | 0.078 | 0.021 | 0.14 | 7.8×10-3 |
| rs16969968a * Time | 0.017 | -0.032 | 0.066 | 0.50 |
| Partner smoking* Time | 0.032 | -0.10 | 0.04 | 0.38 |
| Partner smoking* rs16969968a * Timea | 0.071 | 0.013 | 0.13 | 0.017 |
rs1051730 was used as the proxy for rs16969968 (r2=1.0 in 1000G CEU).
Figure 1 displays the relations of the genetic and environmental risk factors with trajectory of smoking quantity. Figure 1(A) displays the decreasing level of smoking quantity during pregnancy and the pattern of heavier smoking for individuals with the high-risk rs16969968 genotype (AA) compared with those with the low-risk genotype (GG). Further, the right panel shows that the effects of the risk environment (partner smoking) are not equivalent across levels of genetic risk. The risk of partner smoking is increased markedly in the subjects with AA genotypes.
Figure 1.
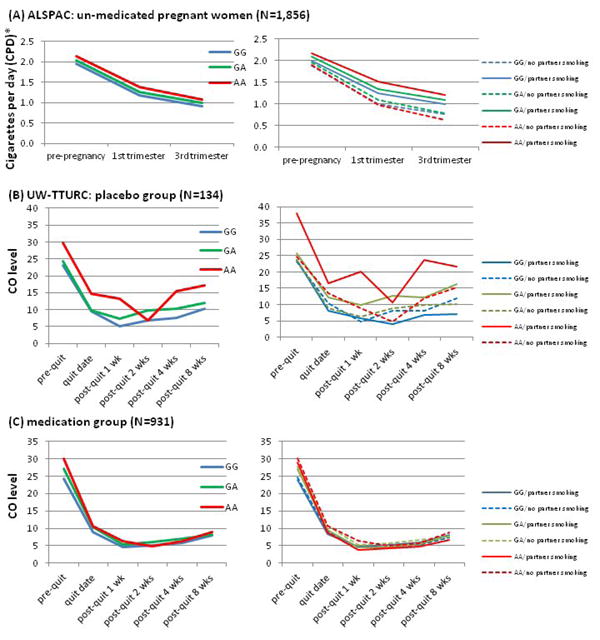
Convergent results in two independent samples: Environmental effect (partner smoking) on quitting is stronger in individuals with CHRNA5 risk allele: Convergent results in two independent samples of un-medicated smokers
(A) and (B)
Interaction of rs16969968 and partner smoking on quitting (decrease of smoking quantity over time) is significant.
(b=0.071, 95% CI 0.013-0.13, p=0.017 in ALSPAC, and b=0.20, 95%CI 0.049-0.36, p=0.010 in TTURC).
(B) and (C)
Medication neutralizes the G effect (b=-0.092, 95% CI=-0.17 to -0.016, 0.018).
Medication neutralizes the G*E effect (b=-0.25, 95% CI=-0.42 to -0.091, p=0.0023)
Reported data points indicate means in each group for that time points.
*CPD coding for 4 levels (0-1, 1=1-9, 2=10-19, 3=20 or more).
In secondary analyses, we found consistent results with dichotomous cessation outcomes at the 32nd week of pregnancy. Many pregnant women either reduced their smoking quantity (26%) or became abstinent (42%) at the 32nd week of pregnancy. These results suggested possible interactions of rs16969968 and partner smoking on whether they were abstinent at the 32nd week during pregnancy (interaction of genotype*partner smoking on abstinence OR=0.49, p=0.078) or reduced their smoking quantity (interaction OR=0.60, p=0.14).
3.2 University of Wisconsin Transdisciplinary Tobacco Use Research Center (UW-TTURC) Study
Subjects of European ancestry with genotype data and alveolar CO data were included in the analysis (N=1,065). The sample characteristics and genotype frequencies are shown in Table S1(B) and 27.7% of the participants lived with someone who smoked. In this treatment-seeking sample, CO was associated with CHRNA5 rs16969968 adjusted for age and gender (β=0.13, 95% CI=0.041 to 0.23, p=0.0050), a modest effect in this sample of heavy smokers.
In this trial, 134 participants were randomized to the placebo group and thus, like the women in the ALSPAC study, did not use smoking cessation pharmacotherapy. During the cessation trial, these smokers showed decreasing alveolar CO level over time (pre-quit, quit date, and 1, 2, 4 and 8 weeks post-quit) (β=-0.29, 95% CI=-0.34 to -0.23, p<0.0001, Table 2, Model 1). The rs16969968 high-risk allele (A) predicted an increased level of alveolar CO level (β=0.36, 95% CI=0.050 to 0.67, p=0.023; Table 2, Model 1). Genetic risk of variant rs16969968 interacted with partner smoking in the prediction of smoking quantity as estimated by CO; the trajectory of CO remained especially elevated amongst subjects who had both partner smoking and high rs16969968 genetic risk (interaction of genotype*partner smoking on CO trajectory slope β=0.20, 95% CI=0.049 to 0.36, p=0.0101; Table 2, Model 2).
Table 2. UW-TTURC Placebo Group: The effect of CHRNA5 rs16969968 on the trajectory of exhaled CO level after quitting is moderated by living with someone who smokes (n=134).
| Parameter | β | 95% Confidence Interval | P value | |
|---|---|---|---|---|
|
| ||||
| Lower Bound | Upper Bound | |||
| Model 1 | ||||
| Age | 0.0076 | -0.0086 | 0.024 | 0.36 |
| Gender | -0.29 | -0.69 | 0.10 | 0.15 |
| Time | -0.29 | -0.34 | -0.23 | <0.0001 |
| rs16969968 | 0.36 | 0.050 | 0.67 | 0.023 |
| Partner smoking | 0.22 | -0.20 | 0.64 | 0.31 |
|
| ||||
| Model 2 | ||||
| Age | 0.0070 | -0.0090 | 0.023 | 0.39 |
| Gender | -0.29 | -0.68 | 0.11 | 0.15 |
| Time | -0.28 | -0.37 | -0.18 | <0.0001 |
| rs16969968 | 0.25 | -0.12 | 0.62 | 0.18 |
| Partner smoking | 0.19 | -0.31 | 0.69 | 0.45 |
| rs16969968 * Time | -0.021 | -0.12 | 0.081 | 0.69 |
| Partner smoking * Time | -0.12 | -0.28 | 0.033 | 0.12 |
| Partner smoking * rs16969968 * Time | 0.20 | 0.049 | 0.36 | 0.010 |
Figure 1(B) displays the decreasing alveolar CO level during the trial among placebo participants, and the pattern of heavier smoking for individuals with the high-risk rs16969968 genotype (AA) compared with those with the low-risk genotype (GG). Further, the right panel shows that the risk of partner smoking is increased in the subjects with AA genotypes. In secondary analyses, we found similar interaction results of partner smoking and rs16969968 on the dichotomous outcome, cross-sectional abstinence at 8 weeks post-quit (interaction of genotype*partner smoking on abstinence OR=4.53, df=1, p=0.049). Thus, the effect of partner smoking is most prominent in subjects with AA genotypes: those with a partner who smoked were less likely to be abstinent (0% vs. 25%) and had higher CO levels (21.7 vs. 15.3) at this time point (8 weeks) than those without a partner who smoked.
3.3 Pharmacotherapy neutralizes the increase in cessation failure associated with combined genetic and environmental risks
Using the same subjects in the TTURC study, we previously showed that pharmacotherapy benefit only those at high CHRNA5 genetic risk (Chen et al., 2012b). That research, however, used a cross-sectional abstinence outcome. In the present study, we attempted to replicate that finding using the CO smoking trajectory outcome. Thus, we compared the genetic effect on smoking trajectory in the placebo and active pharmacotherapy groups. In subjects receiving placebo, there was a clear CHRNA5 rs16969968 effect on smoking cessation trajectory, while in subjects receiving active pharmacotherapy, there was no such effect. There was an interaction between genetic risk and pharmacotherapy (interaction of genotype*pharmacotherapy on CO trajectory slope β=-0.092, 95% CI=-0.17 to -0.016, p=0.018; Table S2 Model 22; Figure 1(B) and 1(C): left panel).
Given that pharmacotherapy appears to mitigate the genetic effects of CHRNA5 on smoking cessation, we deemed it important to determine whether pharmacotherapy also mitigates the gene X environment interaction observed in the placebo group. Therefore, we tested and found a significant 3-way interaction involving genetic risk, environmental risk, and pharmacotherapy (interaction of genotype*partner smoking*pharmacotherapy on CO trajectory slope β=-0.25, 95% CI=-0.42 to -0.091, p=0.0023; Table 3 Model 2; Figure 1(A) and 1(B): right panel). In other words, the interaction of genetic and environmental risks was observed only in the placebo group (β=0.20, 95% CI=0.049 to 0.36, p=0.0101), but not in the active pharmacotherapy group (β=-0.14, 95% CI=-0.36 to 0.075, p=0.20). This gene * environment * pharmacotherapy interaction did not differ across the different active treatment arms (nicotine patch, nicotine lozenge, bupropion, nicotine patch and lozenge, bupropion and nicotine lozenge, F=1.2, df=4, p=0.31).
Table 3.
UW-TTURC: The interactive effects of genetic effect (rs16969968) and environment (partner smoking) on the trajectory of exhaled CO level after quitting is moderated by cessation pharmacotherapy.
| Parameter | β | 95% CI | Sig. | |
|---|---|---|---|---|
|
| ||||
| LB | UB | |||
| Model 1 | ||||
| Age | 0.0024 | -0.0030 | 0.0079 | 0.38 |
| Gender | -0.27 | -0.40 | -0.15 | <.0001 |
| Time | -0.47 | -0.49 | -0.45 | <.0001 |
| rs16969968 | 0.17 | 0.043 | 0.23 | 0.0041 |
| Medication | -0.39 | -0.58 | -0.20 | <0.0001 |
| Partner smoking | 0.0037 | -0.13 | 0.14 | 0.96 |
|
| ||||
| Model 2 | ||||
| Age | 0.0026 | -0.0029 | 0.0080 | 0.35 |
| Gender | -0.28 | -0.40 | -0.15 | <0.0001 |
| Time | -0.47 | -0.51 | -0.44 | <0.0001 |
| rs16969968_A | 0.22 | 0.10 | 0.34 | 2.3×10-4 |
| Medication | 0.074 | -0.16 | 0.31 | 0.54 |
| Partner smoking | -0.075 | -0.25 | 0.098 | 0.39 |
| rs16969968_A * Time | -0.033 | -0.068 | 0.0021 | 0.065 |
| Medication * Time | -0.18 | -0.28 | -0.089 | 1.5×10-4 |
| Partner smoking * Time | 0.060 | -0.0015 | 0.12 | 0.056 |
| Medication * rs16969968 * Time | -0.012 | -0.11 | 0.081 | 0.81 |
| Partner smoking * rs16969968 * Time | -0.043 | -0.10 | 0.014 | 0.14 |
| Medication * Partner smoking * Time | 0.15 | -0.0027 | 0.30 | 0.054 |
| Medication * Partner smoking * rs16969968 * Time | -0.25 | -0.42 | -0.091 | 2.3×10-3 |
rs16969968_A is coded additively as 0,1,2 copy of the minor risk allele.
To further illustrate the effect of pharmacotherapy on CO trajectory, we show in Figure 2 how the pharmacotherapy effect differs as a function of the combined genetic and environmental risks. The strongest pharmacotherapy effect was seen in participants with both high-risk genotype (AA) and high-risk environment (living with someone who smokes), compared with the other three groups who have 0 or 1 risk factor. In secondary analyses, we found similar interaction results of partner smoking, rs16969968, and active pharmacotherapy when modeling a dichotomous outcome, cross-sectional abstinence at end of treatment at 2 months (interaction of genotype*partner smoking*pharmacotherapy on abstinence OR=0.20, df=1, p=0.039).
Figure 2.
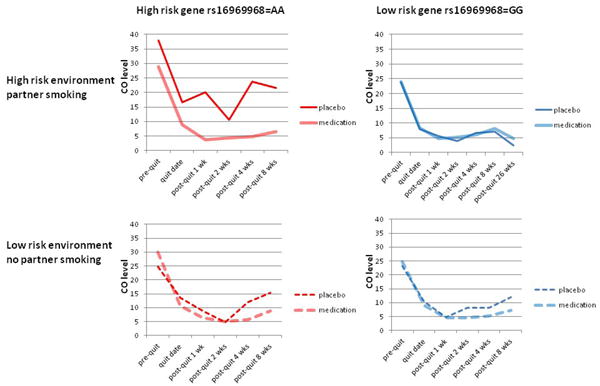
Medication effect on post-quit smoking quantity is moderated by both CHRNA5 rs16969968 genotypes and partner smoking status
| Sample size | AA | GG |
| partner smoking | 33 | 127 |
| no partner smoking | 87 | 304 |
4. Discussion
Our study represents an initial evaluation of the complex interplay of gene, environment, and pharmacotherapy in smoking behaviors. We found, as in prior studies, that the risk variant rs16969968 in CHRNA5 decreases the likelihood of smoking cessation success, as does living with a partner who smokes (Bergen et al., 2013; Bolt et al., 2009; Chen et al., 2012b; Harmer and Memon, 2013; Homish and Leonard, 2005; Munafo et al., 2011; Okechukwu et al., 2012; Ruge et al., 2008). However, across two complementary studies, we found a quantitative interaction between partner smoking and rs16969968 such that relative to other individuals, those with both risk factors were especially unlikely to quit or reduce their smoking successfully. In the ALSPAC community-based observational study of smoking cessation during pregnancy, expectant mothers decreased their smoking quantity over time while receiving no cessation pharmacotherapy. Both the genetic risk (rs16969968 (A)) and environmental risk (partner smoking) independently predicted less smoking reduction during pregnancy. In addition, an interaction was found between the variant rs16969968 and partner smoking: women who possessed the risk allele and lived with a partner who smoked were especially unlikely to reduce their smoking. Conversely, women with the risk allele who lived with a non-smoking partner had the greatest smoking reduction. This same pattern of interaction was found in the UW-TTURC smoking cessation trial; i.e., smokers possessing both risk factors who did not receive active pharmacotherapy were especially unlikely to quit or reduce their smoking, as evidenced by elevated CO levels. Despite differences in study population, motivation and support for smoking cessation, follow up duration, and type of outcome measure, both studies revealed the same interaction between the genetic and environmental risks. The risk associated with CHRNA5 is moderated by partner smoking, a known risk for smoking cessation difficulty.
This gene-environment interaction suggests that the environmental risk effect is not constant, but variable depending on the individual';s genetic makeup. Partner smoking predicts cessation failure, and this effect is more prominent for individuals with the high-risk genotype compared to those with the low-risk genotype. One possible relapse mechanism might be that individuals with the high-risk genotype experience more craving and withdrawal symptoms while trying to quit (Chen et al., 2012a), and they are more likely to relapse if cigarettes are more accessible and triggers are more common when they live with someone who smokes.
This research extends existing evidence that CHRNA5 increases the risk of cessation failure, and this increased risk is ameliorated by cessation pharmacotherapy (Bergen et al., 2013; Chen et al., 2012b). Our prior study evaluated these effects using a cross-sectional abstinence outcome. This study used the longitudinal assessment of a tobacco exposure biomarker (alveolar CO), a quantitative measure over time that should reflect smoking outcomes more sensitively than would a binary measure such as point-prevalence abstinence (Baker et al., 2011). Using repeated assessments of this tobacco exposure biomarker, we find that CHRNA5 predicts higher CO levels, and there is also an interaction between CHRNA5 and pharmacotherapy, with the genetic risk being ameliorated by cessation pharmacotherapy.
The results of this research have potential clinical relevance. First, they show that heightened risk for poor smoking outcomes, whether due to CHRNA5 genotype or the environmental risk of partner smoking, can be mitigated through the use of smoking cessation pharmacotherapy (e.g., see Figure 1(B) and 1(C)). This observation agrees with recent suggestions that an optimal smoking cessation treatment algorithm should comprise both environmental and genetic risk factors (Bough et al., 2013). Second, the results suggest that in the absence of cessation pharmacotherapy, reducing exposure to smoking cues and opportunities may be especially important for individuals with high CHRNA5 genetic risk.
The results of this study should be interpreted in the context of several limitations. First, the placebo group in the cessation trial is fairly small. However, the results obtained in a different study, the ALSPAC observational study of pregnant women, support the validity of the clinical trial results. These results should be treated with caution until further replication by independent studies or meta-analyses. Second, the smoking reports in the ALSPAC sample were not confirmed by biochemical confirmation. Although research shows that self-report is a valid indicant of current smoking when there are no strong incentives to deceive (SRNT, 2002), pregnant women may or may not misreport their smoking status (Dietz et al., 2011; Kvalvik et al., 2012). However, an objective biomarker of tobacco exposure (alveolar CO level) was obtained in the UW-TTURC trial to define smoking cessation. Third, we specifically examined a longitudinal smoking reduction outcome instead of abstinence, a commonly used dichotomous cross-sectional cessation outcome. Another limitation is the choice of a linear model in analyzing these repeated smoking quantity measures instead of others (e.g., quadratic model) which may capture the dynamic fluctuation of smoking quantity over time. Furthermore, this work only studied one genetic locus, and it is clear that multiple genes contribute to smoking cessation success. Fourth, this study took a targeted approach by examining the hypothesized interaction between CHRNA5 and partner smoking in two complementary samples without exploring other possible interactions (Keller, 2014). Multiple differences exist across the two studies including the motivation to reduce of quit smoking, the level of cessation treatment, the demographic distribution, and the prevalence of partner smoking (62.0% in the pregnancy study, and 27.7 % in the clinical trial). These differences are important in the interpretation of these results. Environmental risk levels were not randomly assigned in this research, and so there might have been other factors correlated with environmental risk that were causally active in affecting smoking cessation and reduction. In addition, it is possible that the genetic and environmental effects are not independent; smokers with heightened genetic risk may be more likely to marry another smoker. Finally, this study only included subjects of European descent; therefore, findings may not generalize to other populations. We need more independent treatment studies and investigation of other environmental risks which may be partially correlated with partner smoking (Davey Smith, 2011).
While acknowledging the limitations of our study, we note that this work complements and builds upon existing research on the genetic, environmental, and treatment determinants of smoking cessation. Using diverse samples, this work underscores the importance of incorporating both genetic and environmental factors in order to understand smoking cessation failure and to design and apply smoking cessation treatments in an optimal manner. In addition to theoretical relevance, these results suggest that there is a population of smokers for whom medication, and perhaps environmental change, is especially important in order for them to achieve successful smoking cessation.
Supplementary Material
Acknowledgments
The Wisconsin State Laboratory of Hygiene provided considerable technical assistance in this research effort. Glaxo Wellcome provided bupropion at no cost in the UW-TTURC clinical trial. The authors thank John Budde and Nick McKenna for their technical assistance with Open Array platform genotyping, Joseph Mullaney for his assistance in preparing the data, and Sherri Fisher for her assistance in project coordination and editing/preparing the manuscript.
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Role Of Funding Source: This research was supported by NIH grants P01 CA089392 (LJB), P50 CA84724 (TBB), and K05 CA139871 (TBB) from the National Cancer Institute, P50 DA19706 (TBB), K02 DA021237 (LJB), and K08 DA030398 (LSC) from the National Institute on Drug Abuse, U01 HG004422 (LJB) from the National Human Genome Research Institute, and sub-award KL2 RR024994 (LSC) from the National Center for Research Resources. Genotyping services for the UWTTURC sample were provided by the Center for Inherited Disease Research (CIDR). Funding support for CIDR was provided by NIH grant U01 HG004438 and NIH contract HHSN268200782096C to The Johns Hopkins University. Assistance with genotype cleaning was provided by the Gene Environment Association Studies (GENEVA) Coordinating Center (U01 HG004446). The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors who will serve as guarantors for the contents of this paper.
Footnotes
Contributors: Authors Li-Shiun Chen, Timothy Baker, George Davey Smith, Marcus Munafo, and Laura Bierut designed the study. Authors Li-Shiun Chen, Timothy Baker, Marcus Munafo, and Laura Bierut wrote summaries of previous related work. Authors Charles Gu, Megan Piper, Steven Smith, and Rick Grucza advised on the analysis designs and plans. Authors Li-Shiun Chen undertook the statistical analysis, and author Li-Shiun Chen, Timothy Baker, Marcus Munafo, and Laura Bierut wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: Laura J. Bierut is listed as an inventor on issued U.S. Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. All other authors declare no potential conflict of interest.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, Christiansen BA, Schlam TR, Cook JW, Fiore MC. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, Piper ME, Matsunami N, Smith SS, Coon H, McMahon WM, Scholand MB, Singh N, Hoidal JR, Kim SY, Leppert MF, Cannon DS. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, Liu J, Lee W, Edlund CK, Hall S, Kwok PY, Benowitz NL, Baker TB, Tyndale RF, Lerman C, Swan GE. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23:94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Morgan SD, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PAF, Mayo K, Nurnberger JJ, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan GT, J A, Edenberg HJ, Rice JP, Goate AM. Nicotine dependence and the a5-a3-b4 nicotinic receptor gene cluster: variants in the nicotinic receptors alter the risk for nicotine dependence. Am J Psychiatry. 2008;9:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, Baker TB. The Wisconsin Predicting Patients' Relapse questionnaire. Nicotine Tob Res. 2009;11:481–492. doi: 10.1093/ntr/ntp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Lerman C, Rose JE, McClernon FJ, Kenny PJ, Tyndale RF, David SP, Stein EA, Uhl GR, Conti DV, Green C, Amur S. Biomarkers for Smoking Cessation. Clin Pharmacol Ther. 2013;93:526–538. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling LP, Twardella D, Hoffmann MM, Witt SH, Treutlein J, Brenner H. Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics. 2010;11:527–536. doi: 10.2217/pgs.10.1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Grucza RA, Johnson EO, Breslau N, Hatsukami D, Smith SS, Saccone NL, Saccone SF, Rice JP, Goate A, Bierut LJ. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine Tob Res. 2012a;14:425–433. doi: 10.1093/ntr/ntr231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, Gogarten SM, Johnson EO, Saccone NL, Wang JC, Weiss RB, Goate AM, Bierut LJ. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012b;169:735–742. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, Bergen AW, Swan GE, Tyndale RF, Benowitz NL, Lerman C. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating, population-level causal influences of diet on health. Genes Nutr. 2011;6:27–43. doi: 10.1007/s12263-010-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, Bernert JT. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173:355–359. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- Ebert LM, Fahy K. Why do women continue to smoke in pregnancy? Women Birth. 2007;20:161–168. doi: 10.1016/j.wombi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2012;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, Smith GD, Frayling TM, Hattersley AT. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R. ALSPAC--the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Harmer C, Memon A. Factors associated with smoking relapse in the postpartum period: an analysis of the child health surveillance system data in Southeast England. Nicotine Tob Res. 2013;15:904–909. doi: 10.1093/ntr/nts221. [DOI] [PubMed] [Google Scholar]
- Homish GG, Leonard KE. Spousal influence on smoking behaviors in a US community sample of newly married couples. Soc Sci Med. 2005;61:2557–2567. doi: 10.1016/j.socscimed.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene x environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, Kaprio J, Lerman C, Park PW. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37:641–650. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvalvik LG, Nilsen RM, Skjaerven R, Vollset SE, Midttun O, Ueland PM, Haug K. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72:101–107. doi: 10.1038/pr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tong S, Oldenburg B. Determinants of smoking and cessation during and after pregnancy. Health Promot Int. 2001;16:355–365. doi: 10.1093/heapro/16.4.355. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res. 2011;13:982–988. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okechukwu CA, Dutra LM, Bacic J, El Ayadi A, Emmons KM. Home matters: work and household predictors of smoking and cessation among blue-collar workers. Prev Med. 2012;56:130–134. doi: 10.1016/j.ypmed.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med. 2010;16:247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge J, Ulbricht S, Schumann A, Rumpf HJ, John U, Meyer C. Intention to quit smoking: is the partner's smoking status associated with the smoker's intention to quit? Int J Behav Med. 2008;15:328–335. doi: 10.1080/10705500802365607. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6:1–16. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, Murphy GM., Jr Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- Schroeder SA. New evidence that cigarette smoking remains the most important health hazard. N Engl J Med. 2013;368:389–390. doi: 10.1056/NEJMe1213751. [DOI] [PubMed] [Google Scholar]
- SRNT, Subcommittee on Biochemical Verficqtion. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- TAG. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triche EW, Hossain N, Paidas MJ. Genetic influences on smoking cessation and relapse in pregnant women. J Obstet Gynaecol. 2008;28:155–160. doi: 10.1080/01443610801912725. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Promise of pharmacogenomics in smoking cessation. Pharmacogenomics. 2009;10:1123–1125. doi: 10.2217/pgs.09.87. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, David SP, Niaura R, Lerman C. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Musci R, Fisher C, Anthony JC, Storr CL, Behm FM, Eaton WW, Ialongo N, Rose JE. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Mol Psychiatry. 2012;19:50–54. doi: 10.1038/mp.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JJ, van den Bree MB, Munafo MR. Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis. Nicotine Tob Res. 2011;13:1167–1175. doi: 10.1093/ntr/ntr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. Defining and assessming nicotine dependence in humans. In: Bock G, Goode J, editors. Understanding Nicotine and Tobacco Addiction Novartis Foundation Symposium. No 275. Wiley; Chichester, UK: 2005. pp. 36–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


