Abstract
Objectives
Exogenous administration of cholecystokinin (CCK) induces hypertrophy and hyperplasia of the pancreas with an increase in DNA content. We hypothesized that endogenous CCK is involved with the malignant progression of pancreatic intraepithelial neoplasia (PanIN) lesions and the fibrosis associated with pancreatic cancer.
Methods
The presence of CCK receptors in early PanIN lesions was examined by immunohistochemistry in mouse and human pancreas. Pdx1-Cre/LSL-KrasG12D transgenic mice were randomized to receive either untreated drinking water or water supplemented with a CCK-receptor antagonist (proglumide, 0.1mg/ml). Pancreas from mice were removed and examined histologically for number and grade of PanINs after 1, 2 or 4 months of antagonist therapy.
Results
Both CCK-A and CCK-B receptors were identified in early stage PanINs from mouse and human pancreas. The grade of PanIN lesions was reversed and progression to advanced lesions arrested in mice treated with proglumide compared to controls (p=0.004). Furthermore, pancreatic fibrosis was significantly reduced in antagonist-treated animals compared to vehicle (pitalic>0.001).
Conclusions
These findings demonstrate that endogenous CCK is in part responsible for the development and progression of pancreatic cancer. Use of CCK-receptor antagonists may have a role in cancer prophylaxis in high risk subjects, and may reduce fibrosis in the microenvironment.
Keywords: Transgenic, proglumide, Kras, PanIN lesions, fibrosis
INTRODUCTION
Treatment of advanced pancreatic cancer has been disappointing and the survival from the time of diagnosis remains dismal at about 6 months. 1 The poor prognosis for pancreatic cancer is to some extent due to the inability to diagnose this disease in early stages 2,3 when intervention could potentially improve the outcome. Although pancreatic cancer cells often respond to drugs in vitro, many agents are found to be ineffective in the clinic and fail to improve survival in human subjects. 4 One explanation for the in vivo chemoresistance 5 has been attributed to the dense fibrotic or desmoplastic reaction associated with pancreatic cancer 6 impairing penetration of chemotherapeutic agents. Activated pancreatic stellate cells have been identified as the source of the extracellular matrix and collagen in this malignancy. 6,7 Strategies to improve drug delivery or to decrease the fibrosis are active areas of investigation.
Early stages leading to the development of pancreatic carcinoma have been difficult to study in human subjects. However, a genetically engineered murine animal model, the Pdx1-Cre/LSL-KrasG12D transgenic mouse, has been established 8 that facilitates studying pancreatic carcinogenesis. This mutant Kras murine model progresses through advancing grades of premalignant lesions called pancreatic intraepithelial neoplasia (PanIN) and therefore, provides a means for examining pancreatic carcinogenesis over time. Although Kras is mutated in the majority of human pancreatic cancers, this oncogene may not be sufficient by itself to transform a normal cell 9 and additional stimuli such as inflammation may be required.
The gastrointestinal peptide CCK may represent a key instigator of inflammation and collaborator with Kras. Exogenously administered CCK or its related decapeptide analogue cerulein, in super-physiologic doses have been used for decades to induce acute pancreatitis in animal models.10,11 Using this experimental approach, CCK-induced pancreatitis has been shown to accelerate both carcinogen-induced pancreatic cancer 12 and the development of cancer from PanINs in the mutant Kras transgenic mouse model.13 It has been proposed that this inflammatory state induced by CCK in the pancreas may be the second insult that activates Kras. 9
In contrast to super-physiological doses of CCK utilized to induce experimental pancreatitis, when CCK is administered at lower (physiologic) doses, it induces both hypertrophy 14 (increase in weight and protein content) and hyperplasia 15 (increased cell number and DNA content) of the pancreas. This trophic or proliferative effect of CCK is independent of its effects on inflammation. Both CCK-A and CCK-B receptors have been identified on human pancreatic cancer cells 16,17 and tumors, 18 and exogenous administration of CCK at physiologic doses (i.e., 10−9 to 10−12M) stimulates growth of human pancreatic cancer cells in vitro 19 and transplanted tumors in vivo 20 in nude mice. RNAi techniques that down-regulate CCK receptors significantly impair pancreatic cancer growth and downstream signaling through proliferative pathways, (i.e., pAkt). 21 CCK receptors have also been identified on pancreatic stellate cells and when stimulated with CCK, these cells produce collagen. 22,23 Therefore, it has been proposed that CCK plays an important role in pancreatic carcinogenesis and the fibrosis associated with pancreatic cancer. 24
We hypothesized that that due to its trophic properties at physiologic levels, endogenous CCK may contribute to pancreatic carcinogenesis. Moreover, since CCK receptors on pancreatic stellate cells produce collagen when activated, we postulate that CCK activity is partially responsible for the fibrosis associated with pancreatic cancer. Using the Kras mutant transgenic mouse model, we now demonstrated that endogenous CCK promotes pancreatic carcinogenesis and that blockade of CCK’s interaction at its receptor halts progression of PanINs and reduces fibrosis. We also show herein for the first time that CCK receptors are present on early PanIN lesions in mouse and human tissues. These studies support the important role of CCK in the development of pancreatic cancer and offer insight for new strategies for therapy.
MATERIALS AND METHODS
Materials
The nonselective CCK receptor antagonist, proglumide, was purchased from Sigma and was resuspended in water. Immunohistochemistry reagents were purchased from Vector Laboratories. The rabbit polyclonal CCK-A receptor antibody was obtained from Pierce (PA-3116) and the goat polyclonal CCK-B receptor antibody (ab77077) from Abcam (Toronto, Canada). These CCK receptor antibodies react to both murine and human CCK-B receptors.
Animals
Pdx1-Cre (strain 01XL5) and LSL-KrasG12D (strain 01XJ6) transgenic mice were obtained from the MMHCC repository of the National Cancer Institute, Frederick, Maryland. All genotyping was done by PCR following suggested MMHCC protocols. Institutional guidelines for care and use of laboratory animals were followed throughout the study in accordance with protocols approved by the Penn State Hershey Institutional Animal Use and Care Committee.
Human Tissues
Human pancreatic tissues were obtained from surgical specimens in patients undergoing resection for cancer and donated by the Tumor bank. These protocols were approved by the Human Subjects Protection Committee of the Pennsylvania State University College of Medicine and the Scientific Review Committee of the Penn State Cancer Institute.
Expression of CCK Receptors in Precancerous Pancreatic Tissues by Immunohistochemistry
Identification and localization of CCK-A and CCK-B receptors in early pancreatic lesions (PanINs) from mouse or human pancreas tissues was performed by immunohistochemistry using formalin-fixed tissues that were paraffin embedded and cut into 6-μm sections. Slides were deparaffinized in xylene followed by successive ethanol washes and rehydration. High temperature antigen unmasking was done using a Vector Laboratories citrate-based solution (Cat. #H-3300) followed by quenching of endogenous peroxidases with 3% H2O2 for 30 minutes. After blocking in 1% normal rabbit serum in TBS at room temperature for 1 hour, tissues were incubated with an Avidin D solution (Vector Laboratories #SP-2001) for 15 minutes, rinsed briefly with buffer, then incubated for 15 minutes with a biotin solution. After a 1 hour incubation at room temperature in primary CCK-A receptor antibody (Pierce, PA-3116, 1:700) or CCK-B receptor antibody (Abcam, ab77077, 1:200 dilution), sections were washed 3 times in TBST. HRP conjugated secondary anti-rabbit antibody (CCK-AR; 1:10,000) or anti-goat antibody (CCK-BR; 1:10,000) was incubated with the samples for 30 minutes followed by 3 additional TBST washes. Slides were incubated in Vectastain Elite ABC reagent for 30 minutes at room temperature, washed in TBST, and incubated in ImmPACT DAB chromagen solution until color developed. Slides were counter stained with hematoxylin. Control slides of both mouse and human tissues were stained with the same titers of secondary antibodies only.
Study Design and Treatment
To assess the role of CCK receptors in early, pre-cancerous pancreatic lesions (PanINs), the Pdx1-Cre/LSL-KrasG12D transgenic mouse model was utilized. In this animal model, an oncogenic Kras allele is specifically activated in pancreatic cells early in pancreatic development, 25 and nearly 100% of Pdx1-Cre/LSL-KrasG12D mice develop PanIN lesions by 3 months. 26 Because PanIN-1a lesions are evident as early as 3 months of age in Pdx1-Cre/LSL-KrasG12D mice, animals were prospectively randomized at this age into treatment groups to ensure time for the establishment of PanINs. Controls received untreated drinking water and the treatment groups received drinking water supplemented with the CCK receptor antagonist proglumide (0.1 mg/ml, approximately 30 mg/kg/day). This dose was calculated based upon doses uses in prior studies in rodents20,27 and in human subjects28. Since we observed the mice for 3 months before initiating therapy, the amount of water consumed daily was recorded so an accurate proglumide dose could be added to the water. Proglumide is an orally bioavailable nonselective CCK receptor antagonist that blocks both the CCK-A and CCK-B receptor subtypes29. Three treatment groups were established: Group 1 consisted of three month old mice treated for one month with proglumide or normal water; Group 2 included four month old mice treated for two months with proglumide or normal water; and Group 3 also included four month old mice but treated for four months with proglumide or normal water (Figure 1). Hence, groups of mice were 4, 6 or 8 months of age at the time of sacrifice and pancreas necropsy.
FIGURE 1.
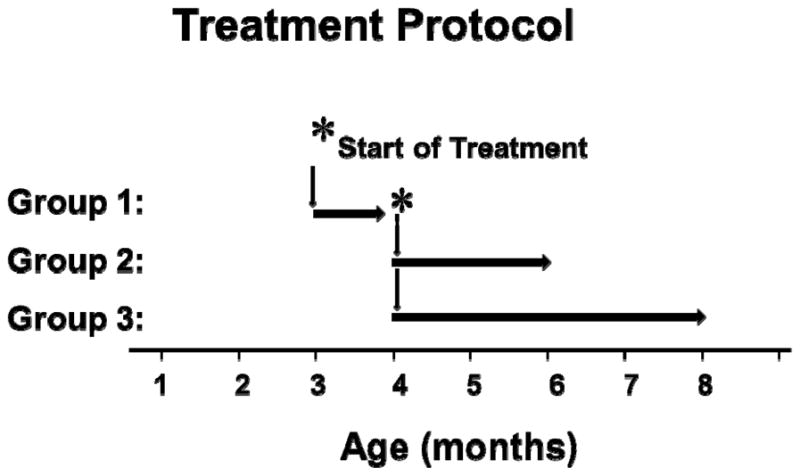
Study design for the CCK-receptor antagonist treatment groups in Kras mice. After 3–4 months when PanIN lesions are known to be present in this transgenic mouse model, the animals were randomized to receive regular, untreated drinking water (controls) or drinking water supplemented with the CCK receptor antagonist proglumide. The mice were also further divided into 3 groups with 6–8 mice per group for duration of therapy. Group 1 received proglumide therapy for 1 month; group 2 received therapy for 2 months; and group 3 received therapy with proglumide for 4 months. The initiation of therapy was delayed to assure PanIN formation, the groups 1–3 were treated and euthanized at ages 4, 6 or 8 months, respectively.
Animal weights were measured and recorded at the time of necropsy. Six to eight mice each from the proglumide or the water treatment groups were sacrificed at specific time points (4, 6 or 8 months of age). Pancreata were dissected, fixed and paraffin-embedded, sectioned and stained with hematoxylin and eosin (H&E). Fibrosis was evaluated using the Masson’s trichrome stain as described.30 Histologic sections were examined and scored by a veterinary pathologist that was blinded to treatment groups and animal age. Pre-cancerous ductal lesions were graded according to standard criteria13,31 and the scoring system is summarized in Table 1. Tissues were independently scored for the most abundant pancreatic pre-cancerous lesion and the highest grade of lesion as adapted from the transgenic adenocarcinoma of the mouse prostate (TRAMP) intra-epithelial neoplasm (mPIN) grading system of Berman-Booty et.al.32 Grading of mouse PanIN lesions, acinar to ductal metaplasia (ADM), cysts, and pancreatic ductal adenocarcinoma (PDAC) was performed according to established criteria.13,31
TABLE 1.
Scoring criteria for grade of PanIN lesions in transgenic Pdx1-Cre/LSL-KrasG12D mice. Each pancreas received two scores, one for the most extensive lesion (geographically) and one for the most advanced lesion (highest grade). Scoring is based on the following: Focal: <3 lesions, Multifocal: >3 lesions to 50% of pancreas, Diffuse: >50% of pancreas 32. PDAC = pancreatic ductal adenocarcinoma.
| Lesion Grade | Distribution | Score |
|---|---|---|
| No Lesions | Normal | 0 |
| PanIN-1a | Focal | 1 |
| Multifocal | 2 | |
| Diffuse | 3 | |
| PanIN-1b | Focal | 4 |
| Multifocal | 5 | |
| Diffuse | 6 | |
| PanIN-2 | Focal | 7 |
| Multifocal | 8 | |
| Diffuse | 9 | |
| PanIN-3 | Focal | 10 |
| Multifocal | 11 | |
| Diffuse | 12 | |
| Microinvasive PDAC | Focal | 13 |
| Multifocal | 14 | |
| Diffuse | 15 | |
| Macroinvasive PDAC | Focal | 16 |
| Multifocal | 17 | |
| Diffuse | 18 |
Fibrosis was assessed according to criteria on grading by Nathan et al.,33 on a scale of 0 to 3 with: 0 = normal and no fibrosis; 1= occasional mild increased collagenous stroma; 2= moderate increase in collagen; and 3= diffuse markedly increased collagenous stroma. Fibrosis was further evaluated within distinct anatomic compartments: intralobular = fibrosis within lobules that separates, surrounds, and dissects exocrine acini; perilobular = circumferential fibrosis around lobules, interlobular = non-circumferential between lobules; and periductal = circumferential around ducts. The degree of inflammatory response was graded was according to previously published criteria,34 and the scoring criteria for inflammation are shown in Table 2.
TABLE 2.
Scoring criteria for pancreatic inflammation in transgenic Pdx1-Cre/LSL-KrasG12D mice. Inflammation was scored based upon a scale of 0 (no inflammation) to 4 (marked inflammation).
| Inflammation Score | Histologic Criteria |
|---|---|
| 0 | No pathological changes |
| 1 | Minimal infiltration of periductal tissue with leukocytes but no parenchymal destruction |
| 2 | Moderate periductal infiltration with leukocytes associated with beginning parenchymal destruction |
| 3 | Severe periductal inflammation and/or more extended parenchymal destruction |
| 4 | Diffuse leukocytes infiltrates, destruction of acini and (partial) replacement by adipose tissue |
Statistical Analysis
The results are expressed as means ± standard error (SEM), and comparisons were made by ANOVA or two sample Student’s t test with a Bonferroni correction for multiple comparisons. Logistic regression analysis was done assuming that there were no treatment effects at Time=0.
RESULTS
CCK Receptors are Expressed in Pre-cancerous Pancreatic Lesions (PanINs) in Murine and Human Tissues
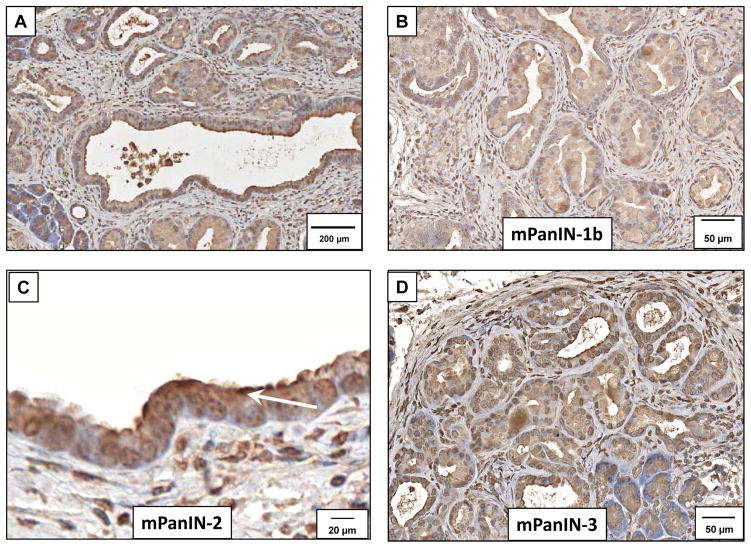
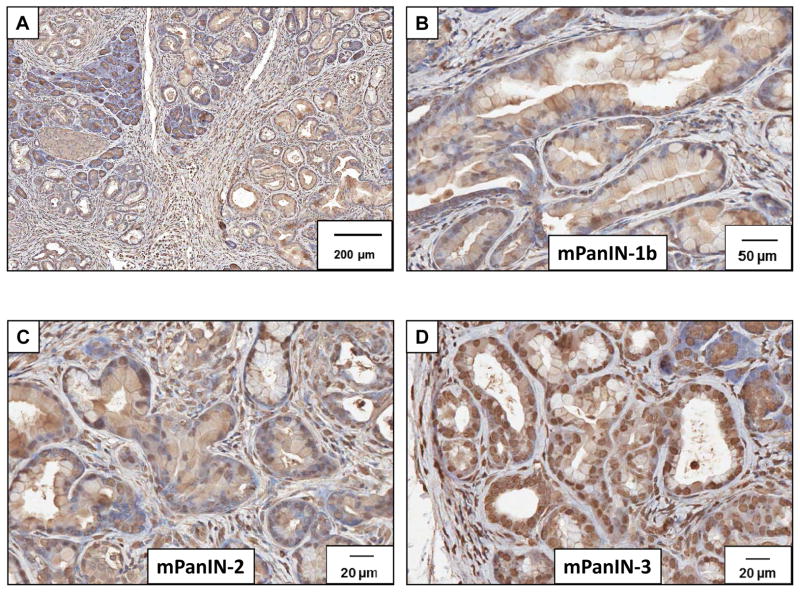
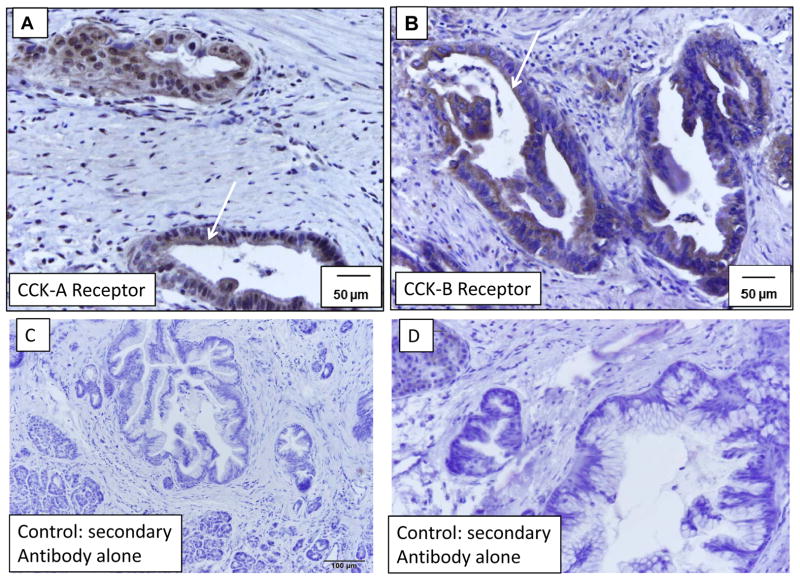
Immunoreactivity for the CCK-A receptor was strong and abundant in murine PanIN (mPanIN) lesions at all stages. Low power magnification demonstrates immunoreactivity at various stages (Figure 2A). A representative higher magnification histologic section demonstrating immunoreactivity for CCK-A receptors is shown for the various grades including PanIN-1b (Figure 2B), PanIN-2 (Figure 2C) and PanIN-3 (Figure 2D). The high magnification in Figure 2C shows CCK-A receptor immunoreactivity at the plasma membrane of PanINs (arrow). CCK-B receptors were also detected in the mouse precancerous pancreas lesions by immunohistochemistry at various stages. Low power magnification of CCK-B immunoreactivity is shown in Figure 3A and staining was also found in PanIN-1b lesions (Figure 3B), PanIN-2 lesions (Figure 3C), and PanIN-3 lesions (Figure 3D). Human pre-cancerous PanIN lesions also stained positive for expression of both the CCK-A (Figure 4A) and CCK-B receptors (Figure 4B). Human tissues reacted with the secondary antibody alone (controls) are shown in Figures 4C and 4D and fail to show significant immunoreactivity. Mouse controls with secondary antibody showed similar lack of staining (not shown).
FIGURE 2.
Detection of CCK-A receptor expression in early mouse PanIN lesions from Pdx1-Cre/LSL-KrasG12D mice by immunohistochemistry. (A) The CCK-A receptor is expressed in all stages of mouse PanIN (mPanIN) lesions. Extensive fibrosis and infiltration of immune cells is evident around the mPanIN lesions. Scale bar=200 μm. (B) Immunoreactivity for the CCK-A receptor is identified in early grade mPanIN-1b lesions with a CCK-A receptor antibody. (Scale bar = 50 μm). (C) Mouse PanIN-2 lesions shows CCK-A receptor immunoreactivity localized to the plasma membrane of the epithelial cells (arrow) (Scale bar = 20 μm) and (D) shows continued expression of the CCK-A receptor in the more advanced mPanIN-3 (Scale bar = 50 μm).
FIGURE 3.
Detection of CCK-B receptor expression in early mouse PanIN lesions from Pdx1-Cre/LSL-KrasG12D mice by immunohistochemistry. (A) CCK-B receptor immunoreactivity is expressed in all stages of mouse PanIN (mPanIN) lesions. Scale bar=200 μm. (B) Immunoreactivity is identified in early mPanIN-1b lesions with a CCK-B receptor antibody. (Scale bar = 50 μm). (C) Mouse PanIN-2 lesions also express CCK-B receptors (Scale bar = 20 μm) and (D) CCK-B receptors are also expressed in the more advanced mPanIN-3 (Scale bar = 20 μm).
FIGURE 4.
CCK-A and CCK-B receptor expression in human PanIN (hPanIN) lesions. Immunohistochemistry with a (A) CCK-A receptor antibody or (B) CCK-B receptor antibody demonstrates expression of both of these receptor types on pre-cancerous pancreatic lesions (hPanINs) in human pancreatic samples. Arrows indicate receptor positive hPanINs with cell surface staining. Scale bars=50 μm. Control human pancreas tissues (C) and (D) that are reacted only with the secondary antibody fail to demonstrate any significant immunoreactivity. This representative pancreas tissue was from a patient that had pancreatic resection for cancer and numerous PanINs were in the specimen.
CCK Receptor Blockade with Proglumide Slows the Progression of PanINs in Mice
Although proglumide is a relatively weak CCK receptor antagonist, the advantage of its use in animal studies includes its ability to block both the CCK-A and CCK-B receptor types; and due to its water solubility and oral bioavailability, the drug could be administered in the animals’ drinking water. This method of administration provides a more steady state of receptor blockade compared to parenteral administration. Proglumide is a broad spectrum CCK receptor antagonist that blocks both the CCK-A and CCK-B forms of the receptor, including heterodimeric receptors.35 Thus, proglumide inhibits the binding of both CCK and gastrin to the receptor. Since proglumide has been reported to increase food intake,36 weight was monitored in the animals. Rather than increased weight, proglumide-treated mice weighed slightly less at months 4, 6 and 8 compared to placebo-treated mice (Table 3), and these differences were not statistically significant.
TABLE 3.
Mean ± SEM of weights of control mice versus proglumide-treated mice at ages 4, 6 and 8 months.
| Age of Mouse, months | Weight, g Controls | Weight, g Proglumide | p- value |
|---|---|---|---|
| 4 | 28.4 ± 2.1 | 24.6 ± 1.9 | 0.21 |
| 6 | 30.6 ± 2.4 | 27.5 ± 1.5 | 0.26 |
| 8 | 37.5 ± 1.8 | 32.5 ± 2.1 | 0.08 |
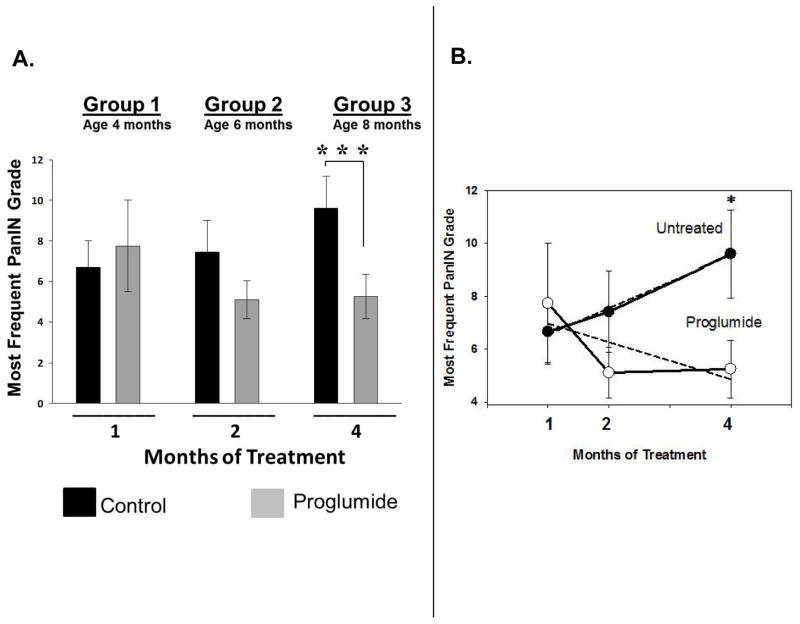
Scores were given to the pancreas histologic sections from each mouse by the pathologist according to Table 1 and the mean score (± SEM) for each group was recorded in the columns for each group in Figure 5A. The grade of the most frequent PanIN lesions in 4-month old mice treated with proglumide for one month was not statistically different from the PanIN grade of untreated mice. In control mice given untreated drinking water, the mean grade of the most abundant PanIN lesion found in the pancreas increased as expected over the course of the experiment, from low grade focal lesions (PanIN 1b-2) at 4 months of age to advanced grade mPanIN-3 lesions by 8 months of age (Figure 5A). In contrast, proglumide-treated mice failed to demonstrate any progression in PanIN grade and these mice even showed a decrease in the mean lesion score over time (Figure 5A). At 8 months of age, when the mice had been treated with proglumide for 4 months, the most frequent pancreatic lesion observed was still the early stage PanIN-1b, suggesting that proglumide therapy arrested PanIN progression and even appeared to reverse lesions to an earlier stage. This difference in mean lesion grade at 8 months between proglumide-treated and untreated mice was significant (p= 0.004). Mice treated with proglumide showed a decrease in the most frequent PanIN lesion grade after 2 months of treatment (p=0.09), which became more significant in 8 month old mice that had received 4 months of treatment. In addition, the overall trend of lesion progression was assessed by poisson regression analysis (Figure 5B). While the untreated mice followed a positive slope with an increase in the linear rate of lesion progression from 4 to 8 months of age, proglumide-treated mice had a negative regression coefficient of -0.404 (Figure 5B). Proglumide therapy arrested pre-cancerous mPanIN lesion progression in treated mice while mPanIN lesions progressed as expected to a significantly more advanced stages in control mice. Perhaps if the intervention were initiated earlier, more significant changes would have been found or even the potential for the prevention of PanIN development.
FIGURE 5.
Blockade of the CCK receptors with proglumide in Pdx1-Cre/LSL-KrasG12D transgenic mice halts the progression of pre-cancerous pancreatic lesions. The pancreas tissue from animals was scored histologically for the most frequently observed mPanIN lesion grade after one, two or four months of proglumide administration. (A) Proglumide treatment (grey bars) delayed the progression of murine pancreatic intraepithelial neoplasia (mPanIN) lesions, particularly with longer treatment duration. Bars represent the mean ± standard error of 4–9 mice per treatment group at each time point; ***p= 0.004 in treated mice compared to water-only controls (black bars). (B) Rate of change in the mean mPanIN lesion grade in proglumide treated mice (open circles) was significantly different (*** p=0.004) compared to untreated control mice (closed circles). Dashed lines represent the linear regression analysis of all data points within each treatment group. Bars are standard errors of the mean at each time point.
CCK Receptor Blockade with Proglumide Reduces Fibrosis and Inflammation
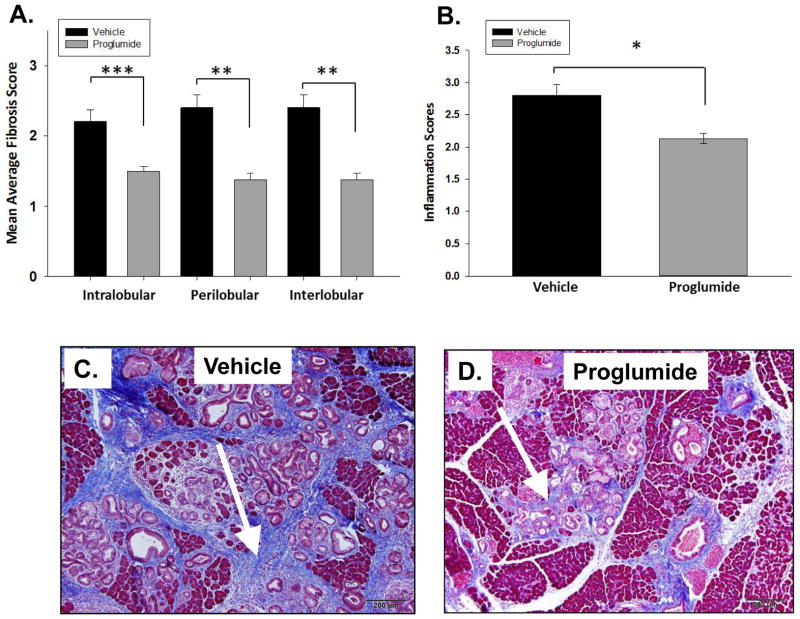
In addition to scoring mouse PanIN lesion grade, pancreatic tissues were also scored at month 8 for fibrosis and inflammation. Pancreas from proglumide-treated mice demonstrated significant decreased fibrosis scores in all anatomic histologic compartments of the pancreas compared to untreated controls. Intralobular fibrosis was reduced by 32% in proglumide-treated mice compared to controls (Figure 6A, pbold>0.001), and both interlobular and perilobular fibrosis were significantly reduced by 43% (Figure 6A, p< 0.005). This reduction in fibrosis is most likely due to the CCK receptor blockade on pancreatic stellate cells. Masson’s trichrome staining highlights the extensive stromal collagen network and fibrosis that surrounded the PanIN lesions in control mice (Figure 6C). In contrast, fibrosis was significantly reduced in proglumide-treated animals (Figure 6D). Pancreatic inflammation is associated with the Kras murine mouse model and also associated with exogenous CCK administration. Histologic scoring of pancreatic inflammation was significantly reduced in proglumide-treated mice compared to water controls (Figure 6B, p< 0.02).
FIGURE 6.
CCK receptor blockade with proglumide reduces pancreatic fibrosis and inflammation. (A) Fibrosis in all 3 anatomical areas of the mouse pancreas (Intralobular, perilobular and interlobular) was significantly reduced in proglumide-treated mice (grey bars) for four months compared to control animals (vehicle, black bars). (B) Proglumide treated mice had less overall inflammation and immune cell infiltration compared to vehicle treated mice. (C) Fibrosis is markedly increased in the pancreas by Trichrome staining (arrow) surrounding the mPanIN lesions in control animals. (D) In contrast, proglumide-treated animals exhibited markedly reduced fibrosis. Scale bar = 200 μm. Significantly different from controls at (*p<0.02, ** p<0.005, *** p<0.001).
DISCUSSION
The present study provides new insights to the mechanisms involved in the carcinogenesis of pancreatic cancer. The major findings of this study include the following: i.) both CCK A and B receptors are present in early PanIN lesions in a mouse pancreatic cancer model as well as in human pancreas tissues; ii.) intervention with CCK receptor blockade prevents progression of the premalignant lesions even after development of PanINs; iii.) CCK and its receptors play a role in the occurrence of fibrosis in the premalignant pancreas; and iv.) endogenous CCK at physiologic levels is involved in pancreatic carcinogenesis.
In human pancreatic cancers, CCK receptors are ubiquitous and over-expressed 16,17 and activation of these receptors with CCK 19,20 or its related peptide gastrin,37 stimulate tumor growth. Although CCK receptors have previously been reported on pancreatic cancer cells, we have identified herein that CCK receptors are also present in very early PanIN-1 lesions in both mouse and human pancreas tissues, suggesting that these receptors play a role in the early stages of pancreatic carcinogenesis. Although the staining with the receptor antibodies appears to be more intense in the PanINs compared to the surrounding cells, immunohistochemistry is not quantitative, and therefore receptor number cannot be discerned between cell types. Since receptor density has previously been reported to be greater in pancreatic cancer tissues compared to normal human pancreas, 24,38 it would not be surprising to find that receptor density increases as PanINs advance in stage. If future receptor binding studies demonstrate that these CCK receptors are increased in density on PanINs compared to normal pancreas cells, one could then speculate that detection of the CCK receptor may represent a biomarker for diagnosis of early stage cancer. Furthermore, since receptor blockade prevented progression of PanIN grade over time, our study then would suggest that endogenous and physiologic levels of CCK play a role in pancreatic carcinogenesis. This discovery is new and different from the adjuvant role exogenously administered CCK and its analogue cerulein have played in animal models of carcinogenesis in the past.
The mechanism by which exogenous administration of CCK or cerulein at high doses in animal models promotes carcinogenesis has been attributed to inflammation and the induction of pancreatitis in animals.12,13 However, this study has demonstrated that CCK plays another important role in carcinogenesis apart from inflammation because pancreatitis was not induced in our model. Alternatively, we suggest that CCK promotes pancreatic carcinogenesis through its trophic actions mediated by the CCK receptor through DNA stimulation, i.e., hyperplasia. Although we waited until PanIN lesions were fully established in our study to test the effects of CCK receptor antagonism on progression of these cancer precursor lesions, future studies are warranted to determine if earlier administration may serve as a preventative therapy to block development of advanced grade PanINs.
While much research has focused on in investigating genetic mutations associated with each stage of PanIN development,39 our report is the first to demonstrate the role of CCK receptors in this progression scenario. Kras is the most frequent mutation associated with pancreatic cancer and its association with PanINs is an early reported event.40 It has been proposed that oncogenic Kras alone is inadequate for cancer development, but that Kras requires stimulation from additional sources, such as CCK, in order to become fully active.41 We suggest that low or physiologic levels of endogenous CCK may be enough to partner with Kras and trigger carcinogenesis in a susceptible host.
Although receptors are present on pancreatic islet cells 42 and are involved in insulin regulation, proglumide does not alter glucose or plasma insulin levels in animals at the doses used in this current experimentation. 43 Therefore, the effects from proglumide observed in this study were most likely due to blockade of CCK at its receptor and not from the actions of insulin or glucose on PanINs.
Proglumide is a nonselective CCK receptor antagonist that was used in this investigation. Several other non-peptide, small molecule CCK receptor antagonists have been developed and characterized.44 Devazepide, also known as L-364,718 or MK-329, is selective for the CCK-A receptor 45 which is often used in animal models of pancreatic carcinogenesis. Other antagonists, such as L-365,260 46 and YF476, 47 are selective for the CCK-B receptor, and since most human pancreatic cancers predominantly harbor the CCK-B receptor type,17,48 these antagonists may be more useful in chemoprevention of human PanIN lesions. Clinical trials have been conducted in patients with advanced pancreatic cancer using a highly selective CCK-B receptor antagonist, Gastrazole 49 where survival was extended compared to placebo controls. However, no survival advantage was identified when comparing this compound to 5-fluoruracil in pancreatic cancer subjects.49 In a Phase 1b/2a clinical trial with another CCK-B receptor antagonist, Z-360 in combination with gemcitabine in naïve subjects with unresectable pancreatic cancer, trends toward improved survival were found but overall Z-360 and gemcitabine therapy did not significantly improve response compared gemcitabine and placebo.50 Using another approach, Gilliam and colleagues 51 immunized subjects against the gastrin peptide and showed a survival advantage in subjects raising an antibody response. These studies suggest a role for CCK receptor blockade in the treatment of pancreatic cancer. Perhaps if therapy were initiated at earlier stages, i.e., during PanIN development or precancerous stage, the antagonist may have be more effective as a preventative agent.
Fibrosis is a common component of pancreatic cancers 7 and many believe it contributes to an adverse microenvironment that impedes the penetration of chemotherapeutic agents. Since pancreatic stellate cells have CCK receptors, 22 the mechanism involved in the reversal of fibrosis in our Kras mouse model is most likely through blockade of CCK receptors on the pancreatic stellate cells. The ability to decrease fibrosis in CCK antagonist treated mice suggests that CCK receptor antagonists may be useful adjuvant therapeutic agents to facilitate delivery and efficiency of chemotherapy by decreasing the barrier desmoplastic reaction associated with cancer.52–54 Patients with chronic pancreatitis also have increased fibrosis histologically suggesting over activity of the stellate cells. Of interest, patients with chronic pancreatitis also have been found to have elevated endogenous CCK plasma levels; 55 therefore, CCK antagonist therapy may have a role in preventing fibrosis or decreasing cancer risk in patients with chronic pancreatitis. A prior study showed that CCK-A receptor antagonist therapy was safely administered and was effective in decreasing pain in patients with chronic pancreatitis, 56 but fibrosis was not evaluated in this study. Since we showed in the current study that nonselective CCK receptor antagonism decreased fibrosis and because both receptor types are associated with human stellate cells, 22 blockade of both the CCK-A and CCK-B receptors with a nonselective receptor antagonist may be useful (and necessary) in subjects with chronic pancreatitis for prevention of fibrosis.
Human pancreatic cancer cell lines and pancreatic tumor samples also produce the ligands for these receptors, CCK57 and gastrin.58 Gastrin exhibits a higher binding affinity for the CCK-B receptor than for the CCK-A receptor, whereas CCK is a high affinity agonist for both the CCK-A and CCK-B receptors.59 Human pancreatic cancer cell lines secrete gastrin, and the expression of gastrin directly correlates with their growth rate in vivo.60 Since gastrin is expressed in the fetal pancreas but not adult pancreas61 and is re-expressed in early stage human PanINs,62 our discovery of expression of the CCK-B receptor to which gastrin binds, may provide new insights to the etiology of pancreatic cancer development.
Overall this pre-clinical study demonstrates the important role of endogenous CCK and its receptor in early PanIN lesion progression. It also shows the significance of CCK receptors in fibrotic conditions of the pancreas. Because potent CCK receptor antagonists have been tested in human subjects for other conditions and are deemed safe, well tolerated, and have few side-effects 44,45,63 it may be possible to use CCK receptor antagonists as a chemopreventive treatment for those patients who are at high risk for pancreatic cancer, including those with multiple risk factors such as a family history of pancreatic cancer, or chronic pancreatitis. Since there are no therapies currently available that impact the survival of patient’s with established pancreatic cancer, perhaps a different strategy with prophylactic treatment of high risk subjects could improve the outcome of this malignancy. The CCK/CCK receptor axis may be a novel and important therapeutic target for both prevention and treatment of preneoplastic pancreatic precursor lesions leading to the decrease incidence of pancreatic cancer.
Acknowledgments
Financial Support: This work was funded by the NIH R01 CA117926 grant and Robert Sullivan Foundation award to J.P.S. We recognize additional support from The V-Foundation for Cancer Research.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Templeton AW, Brentnall TA. Screening and surgical outcomes of familial pancreatic cancer. Surg Clin North Am. 2013;93:629–645. doi: 10.1016/j.suc.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, LED, Yang LX. Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Res. 2013;33:1785–1791. [PubMed] [Google Scholar]
- 5.Warsame R, Grothey A. Treatment options for advanced pancreatic cancer: a review. Expert Rev Anticancer Ther. 2012;12:1327–1336. doi: 10.1586/era.12.115. [DOI] [PubMed] [Google Scholar]
- 6.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuveson DA, Shaw AT, Willis NA, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 9.di Magliano MP, Logsdon CD. Roles for KRAS in Pancreatic Tumor Development and Progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- 11.Saluja AK, Dudeja V. Relevance of animal models of pancreatic cancer and pancreatitis to human disease. Gastroenterology. 2013;144:1194–1198. doi: 10.1053/j.gastro.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howatson AG, Carter DC. Pancreatic carcinogenesis-enhancement by cholecystokinin in the hamster-nitrosamine model. Br J Cancer. 1985;51:107–114. doi: 10.1038/bjc.1985.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriere C, Young AL, Gunn JR, et al. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisset JA, Webster PD. Effects of fasting and feeding on protein synthesis by the rat pancreas. J Clin Invest. 1972;51:1–8. doi: 10.1172/JCI106779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon TE, Vanier M, Morisset J. Cell site and time course of DNA synthesis in pancreas after caerulein and secretin. Am J Physiol. 1983;245:G99–105. doi: 10.1152/ajpgi.1983.245.1.G99. [DOI] [PubMed] [Google Scholar]
- 16.Smith JP, Rickabaugh CA, McLaughlin PJ, et al. Cholecystokinin receptors and PANC-1 human pancreatic cancer cells. Am J Physiol. 1993;265:G149–G155. doi: 10.1152/ajpgi.1993.265.1.G149. [DOI] [PubMed] [Google Scholar]
- 17.Smith JP, Liu G, Soundararajan V, et al. Identification and characterization of CCK-B/gastrin receptors in human pancreatic cancer cell lines. Am J Physiol. 1994;266:R277–R283. doi: 10.1152/ajpregu.1994.266.1.R277. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg DS, Ruggeri B, Barber MT, et al. Cholecystokinin A and B receptors are differentially expressed in normal pancreas and pancreatic adenocarcinoma. J Clin Invest. 1997;100:597–603. doi: 10.1172/JCI119570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JP, Kramer ST, Solomon TE. CCK stimulates growth of six human pancreatic cancer cell lines in serum-free medium. Regul Pept. 1991;32:341–349. doi: 10.1016/0167-0115(91)90027-e. [DOI] [PubMed] [Google Scholar]
- 20.Smith JP, Solomon TE, Bagheri S, et al. Cholecystokinin stimulates growth of human pancreatic adenocarcinoma SW-1990. Dig Dis Sci. 1990;35:1377–1384. doi: 10.1007/BF01536744. [DOI] [PubMed] [Google Scholar]
- 21.Fino KK, Matters GL, McGovern CO, et al. Downregulation of the CCK-B receptor in pancreatic cancer cells blocks proliferation and promotes apoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1244–G1252. doi: 10.1152/ajpgi.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berna MJ, Seiz O, Nast JF, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285:38905–38914. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips PA, Yang L, Shulkes A, et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol. 2014;306:G91–G101. doi: 10.1152/ajpgi.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- 26.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 27.Saia RS, Bertozi G, Mestriner FL, et al. Cardiovascular and inflammatory response to cholecystokinin during endotoxemic shock. Shock. 2013;39:104–113. doi: 10.1097/SHK.0b013e3182793e2e. [DOI] [PubMed] [Google Scholar]
- 28.Chen YP, Yang JS, Liu DT, et al. Long-term effects of proglumide on resection of cardiac adenocarcinoma. World J Gastroenterol. 2005;11:2549–2551. doi: 10.3748/wjg.v11.i17.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herranz R. Cholecystokinin antagonists: pharmacological and therapeutic potential. Med Res Rev. 2003;23:559–605. doi: 10.1002/med.10042. [DOI] [PubMed] [Google Scholar]
- 30.Cooper TK, Zhong Q, Krawczyk M, et al. The haploinsufficient Col3a1 mouse as a model for vascular Ehlers-Danlos syndrome. Vet Pathol. 2010;47:1028–1039. doi: 10.1177/0300985810374842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 32.Berman-Booty LD, Sargeant AM, Rosol TJ, et al. A review of the existing grading schemes and a proposal for a modified grading scheme for prostatic lesions in TRAMP mice. Toxicol Pathol. 2012;40:5–17. doi: 10.1177/0192623311425062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan JD, Romac J, Peng RY, et al. Protection against chronic pancreatitis and pancreatic fibrosis in mice overexpressing pancreatic secretory trypsin inhibitor. Pancreas. 2010;39:e24–e30. doi: 10.1097/MPA.0b013e3181bc45e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asghari F, Fitzner B, Holzhuter SA, et al. Identification of quantitative trait loci for murine autoimmune pancreatitis. J Med Genet. 2011;48:557–562. doi: 10.1136/jmg.2011.089730. [DOI] [PubMed] [Google Scholar]
- 35.Cheng ZJ, Harikumar KG, Holicky EL, et al. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem. 2003;278:52972–52979. doi: 10.1074/jbc.M310090200. [DOI] [PubMed] [Google Scholar]
- 36.Menani JV, Johnson AK. Cholecystokinin actions in the parabrachial nucleus: effects on thirst and salt appetite. Am J Physiol. 1998;275:R1431–R1437. doi: 10.1152/ajpregu.1998.275.5.r1431. [DOI] [PubMed] [Google Scholar]
- 37.Smith JP, Fantaskey AP, Liu G, et al. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995;268:R135–R141. doi: 10.1152/ajpregu.1995.268.1.R135. [DOI] [PubMed] [Google Scholar]
- 38.Smith JP, Hamory MW, Verderame MF, et al. Quantitative analysis of gastrin mRNA and peptide in normal and cancerous human pancreas. Int J Mol Med. 1998;2:309–315. doi: 10.3892/ijmm.2.3.309. [DOI] [PubMed] [Google Scholar]
- 39.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 41.Huang H, Daniluk J, Liu Y, et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2013 doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavine JA, Raess PW, Stapleton DS, et al. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology. 2010;151:3577–3588. doi: 10.1210/en.2010-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niederau C, Schwarzendrube J, Luthen R, et al. Effects of cholecystokinin receptor blockade on circulating concentrations of glucose, insulin, C-peptide, and pancreatic polypeptide after various meals in healthy human volunteers. Pancreas. 1992;7:1–10. doi: 10.1097/00006676-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Berna MJ, Tapia JA, Sancho V, et al. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol. 2007;7:583–592. doi: 10.1016/j.coph.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herranz R. Cholecystokinin antagonists: pharmacological and therapeutic potential. Med Res Rev. 2003;23:559–605. doi: 10.1002/med.10042. [DOI] [PubMed] [Google Scholar]
- 46.Chang RS, Chen TB, Bock MG, et al. Characterization of the binding of [3H]L-365,260: a new potent and selective brain cholecystokinin (CCK-B) and gastrin receptor antagonist radioligand. Mol Pharmacol. 1989;35:803–808. [PubMed] [Google Scholar]
- 47.Semple G, Ryder H, Rooker DP, et al. (3R)-N-(1-(tert-butylcarbonylmethyl)-2,3-dihydro-2-oxo-5-(2-pyridyl)-1H-1,4-benzo diazepin-3-yl)-N′-(3-(methylamino)phenyl)urea (YF476): a potent and orally active gastrin/CCK-B antagonist. J Med Chem. 1997;40:331–341. doi: 10.1021/jm960669+. [DOI] [PubMed] [Google Scholar]
- 48.Galindo J, Jones N, Powell GL, et al. Advanced qRT-PCR technology allows detection of the cholecystokinin 1 receptor (CCK1R) expression in human pancreas. Pancreas. 2005;31:325–331. doi: 10.1097/01.mpa.0000181487.50269.dc. [DOI] [PubMed] [Google Scholar]
- 49.Chau I, Cunningham D, Russell C, et al. Gastrazole (JB95008), a novel CCK2/gastrin receptor antagonist, in the treatment of advanced pancreatic cancer: results from two randomised controlled trials. Br J Cancer. 2006;94:1107–1115. doi: 10.1038/sj.bjc.6603058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer T, Caplin ME, Palmer DH, et al. A phase Ib/IIa trial to evaluate the CCK2 receptor antagonist Z-360 in combination with gemcitabine in patients with advanced pancreatic cancer. Eur J Cancer. 2010;46:526–533. doi: 10.1016/j.ejca.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Gilliam AD, Watson SA. G17DT: an antigastrin immunogen for the treatment of gastrointestinal malignancy. Expert Opin Biol Ther. 2007;7:397–404. doi: 10.1517/14712598.7.3.397. [DOI] [PubMed] [Google Scholar]
- 52.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18:4249–4256. doi: 10.1158/1078-0432.CCR-12-1327. [DOI] [PubMed] [Google Scholar]
- 54.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garces MC, Gomez-Cerezo J, Alba D, et al. Relationship of basal and postprandial intraduodenal bile acid concentrations and plasma cholecystokinin levels with abdominal pain in patients with chronic pancreatitis. Pancreas. 1998;17:397–401. doi: 10.1097/00006676-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Shiratori K, Takeuchi T, Satake K, et al. Clinical evaluation of oral administration of a cholecystokinin-A receptor antagonist (loxiglumide) to patients with acute, painful attacks of chronic pancreatitis: a multicenter dose-response study in Japan. Pancreas. 2002;25:e1–e5. doi: 10.1097/00006676-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Matters GL, McGovern C, Harms JF, et al. Role of endogenous cholecystokinin on growth of human pancreatic cancer. Int J Oncol. 2011;38:593–601. doi: 10.3892/ijo.2010.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JP, Shih A, Wu Y, et al. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am J Physiol. 1996;270:R1078–R1084. doi: 10.1152/ajpregu.1996.270.5.R1078. [DOI] [PubMed] [Google Scholar]
- 59.Jensen SL, Holst JJ, Nielsen OV, et al. Effect of sulfation of CCK-8 on its stimulation of the endocrine and exocrine secretion from the isolated perfused porcine pancreas. Digestion. 1981;22:305–309. doi: 10.1159/000198675. [DOI] [PubMed] [Google Scholar]
- 60.Matters GL, Harms JF, McGovern CO, et al. Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas. 2009;38:e151–e161. doi: 10.1097/MPA.0b013e3181a66fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem. 1988;263:5341–5347. [PubMed] [Google Scholar]
- 62.Prasad NB, Biankin AV, Fukushima N, et al. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 63.Berna MJ, Jensen RT. Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr Top Med Chem. 2007;7:1211–1231. doi: 10.2174/156802607780960519. [DOI] [PMC free article] [PubMed] [Google Scholar]