Diffuse intrinsic pontine glioma (DIPG) is the second most common malignant pediatric brain tumor and the leading cause of brain tumor death in childhood [1]. 80 % of DIPG tumors exhibit a specific mutation (H3K27M) in the genes encoding histone 3.1 or 3.3 [2, 3]. standard therapy consisting of local radiotherapy to a dosage of 54–60 Gy extends median survival from 5 months to ∼9 months; 5-year survival remains less than 1 % [1]. The practice of focal radio-therapy to the brainstem is based in part on a 1982 autopsy study reporting DIPG to be relatively localized to the pons and adjacent structures [4]. In contrast, other neuroimaging and autopsy studies have identified widespread disease including supratentorial extension and leptomeningeal spread [5, 6].
Here, we report an autopsy series of 16 patients evaluated from 2009–2014 at stanford (n = 10) and VU (n = 6) University Medical Centers [7]. patient characteristics are listed in Table S1. Consistent with previous reports [5, 6], we found widespread dissemination of DIPG with extension to midbrain and medulla in 63 %, cerebellum in 56 %, thalamus in 56 %, frontal cortex in 25 % and supratentorial leptomeninges in 25 % (Fig. 1). The spinal cord was not consistently examined, but metastases were found in two of three cases examined; both had clinical evidence of spinal cord spread.
Fig. 1.
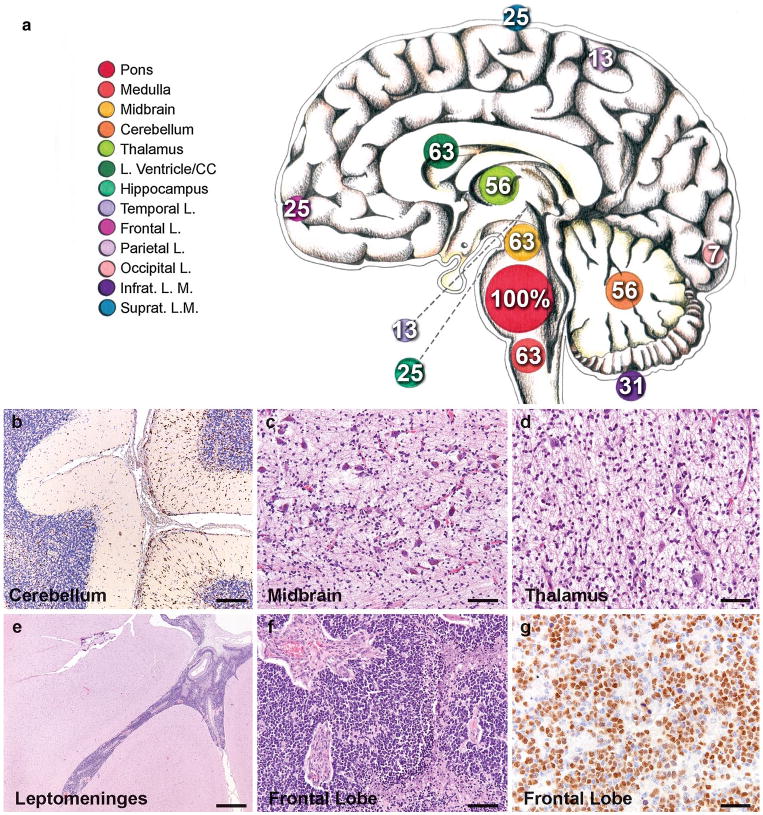
Extent of spread in DIPG. a Neuroanatomical sites and frequency of tumor invasion. Numbers indicate the percentage of cases that exhibit tumor invasion at the indicated anatomical location. The size of the circles marking each anatomical site (color key to the left) illustrates the frequency. CC corpus callosum, infrat. L.M. infratentorial leptomeninges, Suprat. L.M. supratentorial leptomeninges. photomicrographs illustrating b Olig2 + tumor cells infiltrating the cerebellum, c tumor infiltrating the substantia nigra in the midbrain (H&E), d tumor infiltrating the thalamus (H&E), e leptomeningeal spread affecting the temporal lobe (H&E), f tumor in the frontal cortex (H&E) and g Olig2 + tumor cells in the frontal lobe. Immunostains DAB with hematoxylin counterstain. Scale bars 100 μm (b–d, f), 1 mm (e) and 50 μm (g)
A previously under-recognized pattern of subventricular spread was noted in 10/16 cases, with infiltration of the subventricular zone (SVZ) and tumor nodules in the frontal horns of the lateral ventricles. In three cases lateral ventricular disease was noted on pre-mortem MRI (Fig. 2a), but subclinical tumor invasion in the SVZ of the lateral ventricles was found in six additional cases; subventricular spread was found in the third ventricle of one additional case (Fig. 2). The observed pattern of ventricular/subventricular involvement could be due to direct invasion along the SVZ corridor, intraventricular cerebrospinal fluid (CSF) seeding of the SVZ, or an as yet undescribed mechanism. The postnatal SVZ is a neural stem cell niche in the human brain [8] and DIPG cells express an immunophenotype reminiscent of neural precursor cells (Fig. S1 and [9]). Whether DIPG cells exhibit a particular tropism for this niche remains to be explored.
Fig. 2.
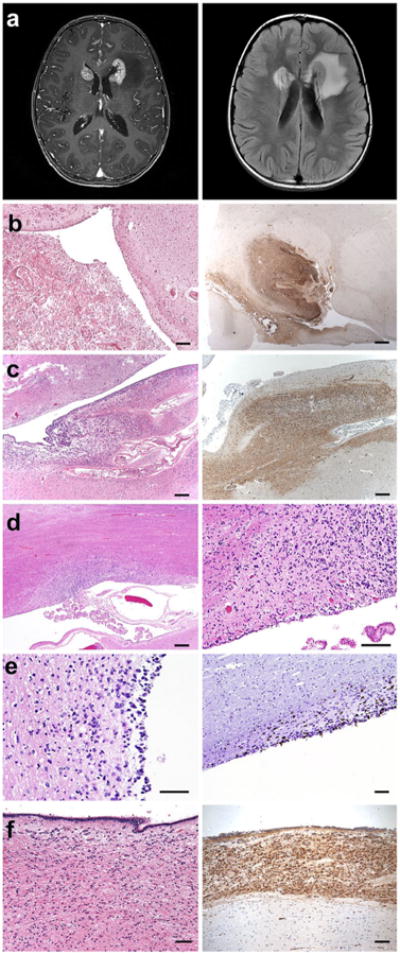
Invasion of the subventricular zone in DIPG (a). MRI images illustrating enhancing lesions (T1 post gadolinium, left image) at the frontal horns of the lateral ventricles with associated edema (FLAIR, right image) in case SU-DIPG-XIII. b H&E (left) and nestin immunostaining (right) of DIPG tumor at the frontal horn of the lateral ventricle in case SU-DIPG-III. c Similar to b in SU-DIPG-V. d H&E demonstrating DIPG tumor at the frontal horn of the lateral ventricle in SU-DIPG-XIII. e H&E stain (left) and Ki67 immunostaining (right image) illustrating tumor infiltration in the lateral ventricle SVZ of SU-DIPG-VI. f H&E (left) and nestin immunostaining (right) of DIPG tumor in the third ventricle of SU-DIPG-IV. Immunostains DAB with hematoxylin counterstain. Scale bars 1.25 mm (b–c, d, left panel), 250 μm (d, right panel; e, f)
Following standard brainstem radiotherapy, disease progression typically occurs locally in the brainstem. However, in three of sixteen cases the subventricular frontal lobe disease contributed substantially to morbidity and mortality and preceded pontine recurrence in two cases. As therapies improve and patients survive longer in the natural history of their cancer, new patterns of regional relapse often appear (e.g. sanctuary disease in childhood leukemia). Our data show subventricular tumor spread in the majority of patients, typically later in the course of their disease. Thus as future therapies evolve to control local disease, strategies including extended or whole brain irradiation may become crucial. The patterns of widespread dissemination, including leptomeningeal, direct extension and subventricular spread, suggest that the extent of the optimal radiation field should be re-examined.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support from the National Institute of Neurological Disorders and stroke (NINDS K08NS070926 to M.M.), Alex's Lemonade stand Foundation (M.M.), McKenna Claire Foundation (M.M.), The Cure starts Now (M.M.), Lyla Nsouli Foundation (M.M.), Semmy Foundation (V.C.), the Dylan Jewett, Connor Johnson, Zoey Ganesh, Dylan Frick, Abigail Jensen, Wayland Villars, and Jennifer Kranz Memorial Funds (M.M.), Matthew Larson Foundation (M.M.), Ludwig Foundation (M.M.), Price Family Charitable Fund (M.M.), Child Health research Institute at Stanford Anne T. and Robert M. Bass Endowed Faculty Scholarship in Pediatric Cancer and Blood Diseases (M.M.), Child Health Research Institute, Lucile Packard Foundation for Children's Health, as well as the Stanford CTSA, award number UL1 TR000093- (V.C.) and the Stanford University School of Medicine Dean's Fellowship (V.C.). The authors thank Terri Haddix, Marilyn Masek, Beth Hoyte and Norm Cyr for their expert assistance.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-014-1307-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Viola Caretti, Departments of Neurology, pediatrics and Neurosurgery, Stanford University school of Medicine, Stanford, USA; Department of Pediatric Oncology, VU University Medical Center, Amsterdam, The Netherlands; Department of Neurosurgery, VU University Medical Center, Amsterdam, The Netherlands; Department of Neuro-Oncology research Group, VU University Medical Center, Amsterdam, The Netherlands.
Marianna Bugiani, Department of Pathology, VU University Medical Center, Amsterdam, The Netherlands.
Morgan Freret, Departments of Neurology, pediatrics and Neurosurgery, Stanford University school of Medicine, Stanford, USA.
Pepijn Schellen, Department of Pediatric Oncology, VU University Medical Center, Amsterdam, The Netherlands; Department of Neurosurgery, VU University Medical Center, Amsterdam, The Netherlands; Department of Neuro-Oncology research Group, VU University Medical Center, Amsterdam, The Netherlands.
Marc Jansen, Department of Pediatric Oncology, VU University Medical Center, Amsterdam, The Netherlands; Department of Neuro-Oncology research Group, VU University Medical Center, Amsterdam, The Netherlands.
Dannis van Vuurden, Department of Pediatric Oncology, VU University Medical Center, Amsterdam, The Netherlands; Department of Neuro-Oncology research Group, VU University Medical Center, Amsterdam, The Netherlands.
Gertjan Kaspers, Department of Pediatric Oncology, VU University Medical Center, Amsterdam, The Netherlands.
Paul G. Fisher, Departments of Neurology, pediatrics and Neurosurgery, Stanford University school of Medicine, Stanford, USA
Esther Hulleman, Department of Pediatric Oncology, VU University Medical Center, Amsterdam, The Netherlands; Department of Neurosurgery, VU University Medical Center, Amsterdam, The Netherlands; Department of Neuro-Oncology research Group, VU University Medical Center, Amsterdam, The Netherlands.
Pieter Wesseling, Department of Neuro-Oncology research Group, VU University Medical Center, Amsterdam, The Netherlands; Department of Pathology, VU University Medical Center, Amsterdam, The Netherlands; Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands.
Hannes Vogel, Email: hvogel@stanford.edu, Departments of Neurology, Pediatrics and Neurosurgery, Stanford University school of Medicine, Stanford, USA; Department of Pathology, Stanford University school of Medicine, Stanford, USA.
Michelle Monje, Email: mmonje@stanford.edu, Departments of Neurology, Pediatrics and Neurosurgery, Stanford University school of Medicine, Stanford, USA.
References
- 1.Donaldson SS, et al. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24(8):1266–1272. doi: 10.1200/JCO.2005.04.6599. [DOI] [PubMed] [Google Scholar]
- 2.Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 3.Wu G, et al. Somatic Histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantravadi RV, et al. Brain stem gliomas: an autopsy study of 25 cases. Cancer. 1982;49(6):1294–1296. doi: 10.1002/1097-0142(19820315)49:6<1294::aid-cncr2820490636>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Gururangan S, et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J Neurooncol. 2006;77(2):207–212. doi: 10.1007/s11060-005-9029-5. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura J, et al. Clinicopathological study of diffuse type brainstem gliomas: analysis of 40 autopsy cases. Neurol Med Chir. 2003;43(8):375–382. doi: 10.2176/nmc.43.375. [DOI] [PubMed] [Google Scholar]
- 7.Caretti V, et al. Implementation of a multi-institutional diffuse intrinsic pontine glioma autopsy protocol and characterization of a primary cell culture. Neuropathol Appl Neurobiol. 2013;39(4):426–436. doi: 10.1111/j.1365-2990.2012.01294.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 9.Monje M, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. PNAS. 2011;108(11):4453–4458. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


