Abstract
Neuronal oscillatory activity is generated by a combination of ionic currents, including at least one inward regenerative current that brings the cell towards depolarized voltages and one outward current that repolarizes the cell. Such currents have traditionally been assumed to require voltage-dependence. Here we test the hypothesis that the voltage dependence of the regenerative inward current is not necessary for generating oscillations. Instead, a current INL that is linear in the biological voltage range and has negative conductance is sufficient to produce regenerative activity. The current INL can be considered a linear approximation to the negative-conductance region of the current-voltage relationship of a regenerative inward current. Using a simple conductance-based model, we show that INL, in conjunction with a voltage-gated, noninactivating outward current, can generate oscillatory activity. We use phase-plane and bifurcation analyses to uncover a rich variety of behaviors as the conductance of INL is varied, and show that oscillations emerge as a result of destabilization of the resting state of the model neuron. The model shows the need for well-defined relationships between the inward and outward current conductances, as well as their reversal potentials, in order to produce stable oscillatory activity. Our analysis predicts that a hyperpolarization-activated inward current can play a role in stabilizing oscillatory activity by preventing swings to very negative voltages, which is consistent with what is recorded in biological neurons in general. We confirm this prediction of the model experimentally in neurons from the crab stomatogastric ganglion.
1. Introduction
We are interested in understanding the mechanisms of generation of slow oscillations underlying neuronal bursting. Regenerative inward currents, typically Ca++ or Na+ currents (Jahnsen and Llinas 1984; Kramer and Zucker 1985; McCormick and Huguenard 1992; Dunmyre et al. 2011; Del Negro et al. 2002), are essential for the generation of neuronal oscillations, from spiking to bursting. They are often targets of modulation by neuroactive substances (Tryba et al. 2006; Cantrell and Catterall 2001) as well as by activity (Swensen and Bean 2005; Desai et al. 1999; Turrigiano et al. 1994; Haedo and Golowasch 2006). For example, in the crab stomatogastric nervous system (STNS), a modulatory inward current (IMI), believed to be important in generating slow bursting oscillations (Hooper and Marder 1987), is elicited by the release of several neuromodulators (Golowasch and Marder 1992b; Swensen and Marder 2000).
Regenerative inward currents typically have non-linear current-voltage (I-V) curves consisting of three distinct portions. At very low voltages, the curve is close to zero; at low to intermediate voltages, the curve has negative slope; while at higher voltages the curve has positive slope. The negative-slope portion tends to drive the neuron's voltage away from rest, while the other two regions prevent the voltage from becoming unbounded at either lower or higher voltages, respectively. Oscillations are created by inward currents driving the cell away from rest, subsequently shutting down at higher voltages, after which the outward currents bring the voltage down to lower levels. We refer to these inward currents as regenerative pacemaker currents. IMI is one such current.
Independent of how the inward current is generated, the negative-slope conductance region of the currents primarily allows for continued depolarization of the membrane potential. In a previous experimental study we showed that a linear approximation of this negative conductance region, introduced in a single neuron, is sufficient to generate oscillatory activity in the pyloric network of the crab stomatogastric nervous system (Zhao et al. 2010). The main hypothesis that we examine in the current study is that an inward regenerative current creates the opportunity for oscillations to exist primarily by destabilizing the resting potential of a cell, thereby forcing the membrane potential away from rest.
Linear non-synaptic ionic currents (i.e., leak currents) have been previously shown to be involved in the generation of oscillatory activity (Blethyn et al. 2006; Cymbalyuk et al. 2002; Koizumi and Smith 2008; Lu and Feng 2012; Pang et al. 2009). However, they do so principally by modifying the general excitability of the neurons expressing them (Brickley et al. 2007; Lu and Feng 2012; Rekling et al. 2000) rather than through an independent pacemaker mechanism. In these examples, a reduced leak current typically increases the excitability of the cell, allowing a different current or a synaptic input to drive the membrane potential into a region where a different regenerative (pacemaker) current is activated. In contrast, here we hypothesize that a linear current with negative conductance can act as a regenerative pacemaker current.
Our study has two goals. First, through modeling and experiments, we show that a current that has a negative conductance, but is linear in the biological range, is sufficient to produce the regenerative effect of a pacemaker current. As such, non-linearity in the biological range is not necessary for this purpose. We characterize the minimum set of requirements to obtain stable slow-wave oscillations. We do this by exploring the properties of a simple model consisting of a potassium (recovery) current (IK) and a current with negative conductance that is linear in the biological range (INL). Although INL is not a biological current, we show that it serves as a proxy for a nonlinear inward regenerative current that can function as a pacemaker current.
Second, we use INL as a modeling and experimental tool to reveal the role of ionic currents other than regenerative ones in shaping oscillations. In particular, using the INL protocol, we explore how the addition of an inward hyperpolarization activated current (Ih) may provide oscillation robustness. In fact, we show that in some cases Ih is necessary for the existence of stable oscillations. This minimal model makes several experimental predictions, some of which are verified using experimental data from the crab pyloric dilator (PD) neuron in which INL is introduced using the dynamic clamp technique.
2. Methods
2.1. Model
The equations that describe the full negative linear conductance model involve four currents associated with the negative linear conductance, leak, potassium and h currents:
| (1) |
The parameters g̃NL , gL, gK, and gh are the maximal conductances and ẼNL , EL, EK, and Eh are the reversal potentials for these currents (see the Appendix for specific equations and parameter values). The value g̃NL is negative, while the other conductance values are positive. The terms w∞(v) and h∞(v) are the steady-state activation functions for the potassium and h currents. By letting gNL = g̃NL + gL, and ENL = [g̃L ẼNL + gL EL] / [g̃NL + gL], we can simplify (1) to
| (2) |
Equation (2) makes sense as a model for the negative linear conductance current only if gNL < 0. Thus, we require that −g̃ NL > gL. As discussed below, the introduction of gNL leads, in some cases, to a destabilization of the rest state that drives the cell to extremely hyperpolarized levels. To avoid this possibility, and to restrict our analysis to a biologically relevant voltage range, we define INL = gNL[v − ENL]Heav(v − vcutoff ) where the Heaviside function Heav(x) is a cutoff function that equals 1 when x ≥ 0, and is 0 otherwise. This leads to a corresponding set of equations:
| (3) |
In this study, we analyze mainly the case when vcutoff = ENL, which restricts our analysis to a biologically relevant voltage range. The special case vcutoff = -∞ makes INL strictly linear with voltage and gNL[v − ENL]Heav(v − vcutoff) reduces to gNL [v − ENL].
For much of the analysis, we will simplify Ih to allow us to consider a two-dimensional model. This is achieved by letting h ≡ h∞(v). We realize that the h current need not be fast; the main purpose in making it instantaneous in this context is to focus, in a simplified setting, on the effects INL. We refer to the instantaneous Ih as Ih-fast and the original Ih in equation (3) as Ih-slow. Thus, the simplified equations for the two-dimensional model are
| (4) |
The nullclines of (4) are found by setting the right-hand side of the equations to 0. This yields the v-nullcline explicitly given by
| (5) |
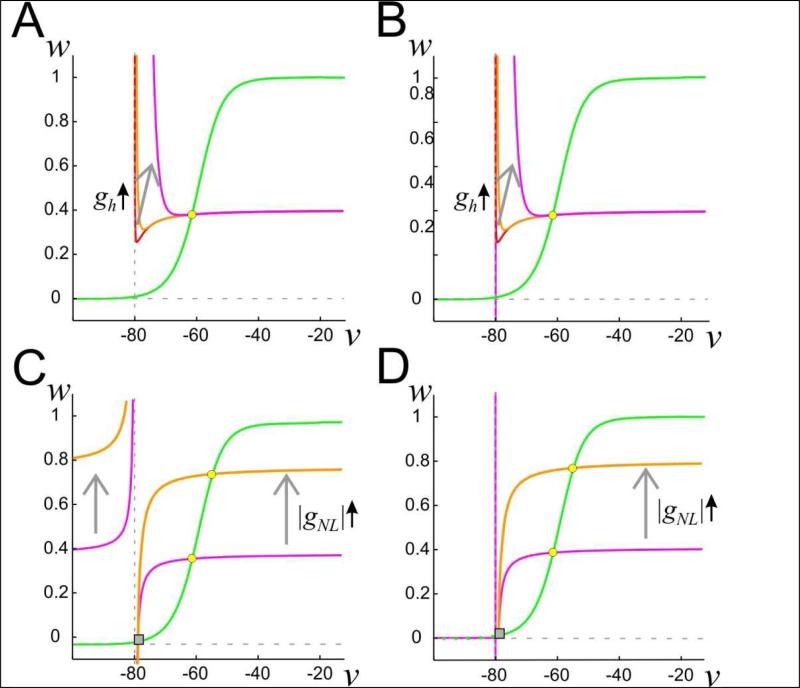
and the w-nullcline given by w = w∞(v) (Fig. 1). Here we have taken the case ENL > EK. For the case in which vcutoff = -∞ (Fig. 1A), the v-nullcline has two pieces that are separated by the vertical asymptote v = EK. For the region v > EK, the v-nullcline has a stable, decreasing, left branch, if gh is large enough. By stable, we mean that trajectories that start in a neighborhood of this left branch tend to transiently approach the nullcline and remain nearby it until they reach a neighborhood of the local minimum. Depending on other parameters, this nullcline can have an unstable middle branch (Fig. 1A, red and gold), as well as a branch that asymptotes to |gNL/gK| as v tends to ∞. By unstable, we mean that nearby trajectories quickly leave a neighborhood of the middle branch. Increases in gh move the v-nullcline up in the v-w phase space. If gh is large enough, the v-nullcline is monotone decreasing for v > EK (Fig. 1A, magenta). For the case in which vcutoff = ENL, the v-nullcline has a stable left branch in the region v > EK if gh > 0, independent of its size (Fig 1B).
Figure 1. Phase plane structure of the linear negative conductance-based neuron model.
A. The nullclines of the model described by equation (5) with vcutoff = -∞. The w-nullcline is in green; the v-nullcline in red, gold and magenta (overlapping for large v). Increasing gh results in a shift up in the v-nullcline for the region v > EK as indicated by the arrow. B. The corresponding set of nullclines for the vcutoff = ENL case. C. The nullclines of the model described by equation (6) and vcutoff = -∞. The w-nullcline is in green, the v-nullcline in magenta and gold; gh = 0. Increasing gNL results in a shift in the v-nullcline as indicated by the arrows. EK = -80 mV, ENL = -79 mV. D. The corresponding set of nullclines described by equation (6) for the vcutoff = ENL case, and gh = 0. The w-nullcline is shown as dashed for v < EK to visualize the horizontal vnullcline at w = 0 in this region. However, in our analysis only the nullclines for v > EK are relevant.
The first part of the analysis will focus on just the effects of IK and INL. In this case, we set gh = 0 in equation (4), which reduces it to
| (6) |
Now the v-nullcline is given by
| (7) |
Figure 1C shows the resulting nullclines with vcutoff = -∞. For v > EK, the v-nullcline is a monotone increasing, concave down curve that asymptotes to |gNL/gK| as v tends to ∞ and towards -∞ as v → E+K (the superscript + denotes limit from above). Increases in |gNL| move the nullcline up in the phase space. For v < EK, the nullcline is increasing and concave up as shown. Finally, Figure 1D shows the nullclines for the equations with vcutoff = ENL. Note that for EK < v <ENL, the v-nullcline is just the line segment w = 0. This produces a stable fixed point at v = EK, w = w∞(EK), since INL = 0 there. The v-nullcline for v < EK is not relevant as the voltage will never reach these values.
2.2. Experimental
Experiments were carried out on identified neurons from the stomatogastric ganglion (STG) of male crabs (Cancer borealis). Animals were obtained from local markets in Newark (NJ) and maintained in seawater tanks at 7–10°C. The entire stomatogastric nervous system, including the anterior commissural and esophageal ganglia, STG and connecting and motor nerves from a crab were dissected out as previously described (Selverston et al. 1976), pinned down on a Sylgard-coated Petri dish, and the STG desheathed to allow electrode penetration of the cell bodies. All preparations were continuously superfused with cooled (10 –13°C) physiological Cancer saline. Saline composition was (in mM) 11 KCl, 440 NaCl, 13 CaCl2, 26 MgCl2, 11.2 Trizma base, 5.1 maleic acid, pH 7.4 –7.5.
Extracellular recordings were performed with pin electrodes placed in petroleum jelly wells built around individual nerves and recorded differentially relative to a distant electrode using an A-M Systems 1700 differential amplifier (A-M Systems). Intracellular recordings and current injections were performed with Axoclamp 2B amplifiers (Molecular Devices) using double impalements with 0.6 M K2SO4 + 20 mM KCl-filled borosilicate electrodes. Low resistance electrodes (15-20 MΩ ) were used for current injection, and high resistance electrodes (30-40 MΩ ) for voltage measurement. Individual neurons were identified by matching intracellularly recorded action potentials to action potentials on identified motor nerves that innervate known muscles (Selverston et al. 1976).
The crab pyloric network was isolated from all neuromodulatory input by decentralization (Zhao et al. 2010), resulting in quiescent preparations and the pyloric dilator (PD) neurons were recorded. When required, all action potentials (and therefore all modulatory inputs) were removed by bath application of 10-7M tetrodotoxin (TTX; Biotium). Current injection was performed using the dynamic clamp technique to emulate the activation of INL and Ih with a variety of values of the two current's parameters (Sharp et al. 1993; Zhao et al. 2010). For this we used software developed in our laboratories (available for download at http://stg.rutgers.edu/software) in the LabWindows/CVI software environment (National Instruments) on a Windows platform, and using a NI PCI-6070-E board (National Instruments, Austin, TX, USA). Data acquisition was performed using a Digidata 1332A data acquisition board and the pClamp 10.3 software (Molecular Devices).
Injections of current in dynamic clamp were performed at 10 kHz and voltage recordings at 5 kHz. The following equations were used:
The parameter values used were ENL = −62 to −58 mV, EMI = 0 mV, Eh = −20 mV, gNL = -0.04 to -0.2 S, gMI = 0.08 to 0.2 μS, gh = 0.1 μS.
3. Results
We will examine, in detail, a minimal model consisting of the biological current IK and the non-biological current INL. We show that as the negative leak conductance gNL is increased in absolute value, various bifurcations occur that give rise to, and subsequently destroy, oscillations. To obtain oscillations, we show that the negative leak current need only be linear in the biological range of voltages. The insight gained from the bifurcation analysis allows us to then explore a second major goal of this paper, namely to identify the role that Ih has in stabilizing neuronal oscillations. In particular, we will show that the existence of Ih allows the cell to display oscillations over a wider range of parameters and increases the size of the basin of attraction of periodic solutions that already exist. Moreover, we will show that there are circumstances where Ih is necessary to produce oscillations. Using the fully linear version of INL, we will show how slow buildup of Ih can explain a curious experimental finding associated with hyperpolarization dips.
Negative linear conductance induces oscillations in a biological system
Figure 2 shows that oscillations in a biological system can result purely from a linear current with negative conductance. Isolated PD neurons were recorded in TTX (initial black trace in Fig. 2B). When an instantaneous regenerative current IMI (Golowasch and Marder 1992b; Swensen and Marder 2000) with an I-V curve as shown in Figure 2A (red) was injected, oscillatory activity resulted (Fig. 2B, red). Very similar activity was activated when only a linear current, with negative conductance that matched the region of negative conductance of IMI (Fig. 2A, INL with vcutoff = -∞ (blue), vcutoff = EK (aqua)) was introduced in the same cell (Fig. 2B, blue; the two cutoff values produce the same output thus aqua not shown). These results build on previous experiments described by Zhao et al (2010) who showed that the expression of INL in the PD neuron in the decentralized pyloric network resulted in the recovery of the full pyloric rhythm. The current experiments show that oscillations can be generated by INL in an isolated PD neuron and in the absence of action potentials. Thus, it appears that the activation of oscillatory activity does not require nonlinear regenerative currents.
Figure 2. Linear negative conductance current elicits oscillations in PD neurons.
A. I-V curve for the modulator-activated inward current IMI (red), INL (blue/aqua) which was matched in slope (at the inflection point) to the negative conductance portion of IMI. The two INL curves have vcutoff = -∞ (solid blue) or vcutoff = ENL (dotted aqua). B. PD neuron membrane potential with added IMI (red), INL (blue; the two vcutoff values produce identical results), showing onset of very similar oscillatory activity immediately after onset of dynamic clamp current (gray traces). Horizontal arrows show –58 mV (top), and 0 nA (bottom).
Oscillations resulting from INL and IK
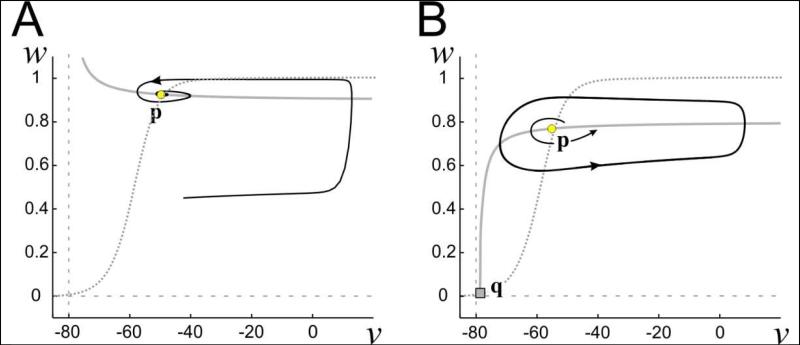
We begin by showing that in certain parameter regimes, INL and IK can together produce stable oscillations. Consider equation (6) in which Ih is not present. When EK > ENL, the v-nullcline is a concave-up decreasing curve on the region v > EK that tends to |gNL/gK| as v → (Fig. 3A). Its intersection with the w-nullcline yields a single fixed point p (yellow circle) that is globally attracting. However, when ENL > EK, the v-nullcline is instead a concave-down monotone-increasing curve in the region v > ENL (Fig. 3B). It now intersects the w- nullcline at a second point q that is an unstable saddle point (gray square). Depending on the value of gNL, a third intersection near w = 1 can also exist (shown and discussed later). In this case, the fixed point p is either unstable or no longer globally attracting. This is the situation on which we will focus, as it is the one that provides the opportunity for oscillations.
Figure 3. Phase plane of the simplified linear negative conductance current model.
Dotted trace is the w-nullcline. Vertical dashed gray line is v=EK. Continuous gray trace is the v-nullcline. vcutoff = ENL. Saddle point q is located at the square, while the circle corresponds to the (spiral) fixed point p. A. ENL<EK yields a stable spiral point p. B. ENL > EK can yield an unstable spiral point p, which is surrounded by a stable limit cycle. v in mV.
Note that in the region EK ≤ v < ENL, the v-nullcline is simply the line segment w = 0, which does not intersect the w-nullcline (Fig. 1D). But at v = EK, w = w∞(EK), there is a stable fixed point, independent of the value of gNL, because INL = 0 there. For clarity, we do not show the v-nullcline in the region v ≤ EK in Fig. 3.
Consider the linearization of (6) about any of the fixed points, denoted as (v*, w*). The eigenvalues of the corresponding Jacobian matrix satisfy
| (8) |
where Fv = –gKw* – gNL, Fw = –gK(v* – EK), Gv = δ[(w∞(v) – w) / τK(v)] / δv, evaluated at (v* , w*), and Gw = −1/τK(v*). The lower fixed point q occurs at w*q ≈ 0 , Gv ≈ 0. The eigenvalues are therefore well approximated by Fv = –gNL > 0 and Gw = –τ(v*q) < 0. Thus, the fixed point q is a saddle. The stable and unstable manifolds of this saddle will play an important role in determining the existence and stability of various solutions. The upper fixed point p = (v*p, w*p) can be stable or unstable. For small values of |gNL|, p is always asymptotically stable. As |gNL| increases, the v-nullcline moves up in the phase space causing p to also move up and w*p to increase. Note that the v-nulllcline asymptotes to -gNL/gK as v → ∞. The w-nullcline asymptotes to 1; thus, when - gNL=gK, a third fixed point, denoted r, is created at v = ∞. As |gNL| increases r moves in from ∞ and eventually collides with p at a bifurcation point as describe below.
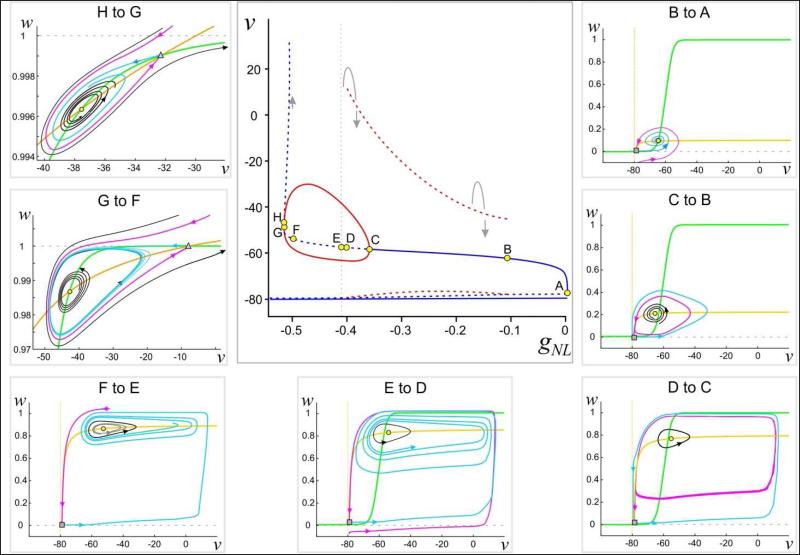
Certain bifurcations may occur as |gNL| increases, as depicted in Figures 4 and 5 (for different parameter sets). Figures 4 and 5A show bifurcations that lead to periodic solutions. The types of bifurcations in each case are different and lead to distinct behaviors in the system given by equation (6). Figure 5B, by contrast, shows the case where there are no stable oscillations, instead just a stable fixed point. Common to all three figures is a bifurcation that occurs for large values of |gNL| where p and r collide (H in Fig. 4, I in Fig. 5A, F in Fig. 5B).
Figure 4. Bifurcation diagrams for system (6) and associated phase planes.
Center: Bifurcation diagram with gNL as parameter. Solid (dashed) lines/curves indicate stable (unstable) fixed points (blue)/periodic orbits (red). Note that the fixed point is stable between H and G. Points marked by letters indicate local or global bifurcation points. Small arrows show the direction of the flow. Horizontal solid line is the fixed point at EK = −80 mV. The dashed horizontal curve above that is the position of the saddle point Surround: Phase plane diagrams in different regions of the bifurcation diagram (as marked in each panel). The v-nullcline is shown in yellow and the w-nullcline in green. Solid curves indicate trajectories with the direction of the flow shown (closed curves are periodic orbits). The two fixed points p (yellow circle) and q (gray square) as in Fig. 3 are marked. Note that the fixed point p is stable in the region H to G. Magenta and cyan curves respectively indicate the relevant branches of the stable and unstable manifolds of the saddle point q (square in panels showing points from F to A) or r (triangle in panels G to F and H to G). v in mV, gNL in μS.
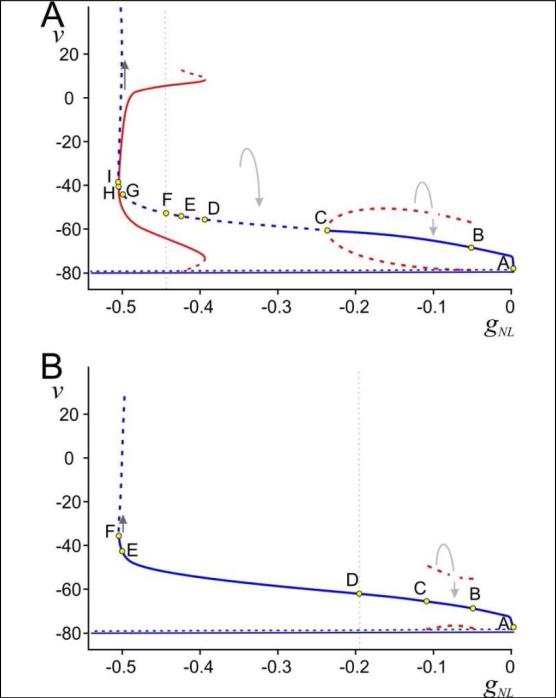
Figure 5. Alternative bifurcation diagrams for system (6).
Bifurcation diagrams with gNL as parameter. Solid (dashed) lines/curves indicate stable (unstable) fixed points (blue)/periodic orbits (red). Points marked by letters indicate local or global bifurcation points. Small arrows show the direction of the flow. Horizontal solid line is EK = −80 mV. The dashed horizontal curve above that is the position of the saddle point. A: Bifurcation diagram with τ1 = 80 ms and k1 = 4 mV. Note that the fixed point is stable in the region I to H.B: Bifurcation diagram with τ1 = 60 ms and k1 = 4 mV. v in mV, gNL in μS.
To understand the different activity patterns that can arise with different values of gNL, we will describe Figure 4 in more detail. We have labeled eight points A-H corresponding to different values of gNL (increasing |gNL|) at which the structure of the phase space changes. We will describe the bifurcation diagram in this order.
The point A corresponds to gNL = 0 where a single fixed point exists at v = EK. As |gNL| is increased, on the interval (B, A), two new fixed points emerge, a saddle point q near v = EK and the point p. Note that when v < ENL , INL = 0, therefore v = EK remains a stable fixed point (this is true for all values of gNL). On the (B, A) interval, p is asymptotically stable, but has a limited basin of attraction in the phase space. The unstable manifold (cyan) of q with v > EK approaches p in forward time, while the stable manifold (magenta) of q encircles p as shown in the inset. The stable manifold separates trajectories that are attracted to p versus v = EK. The stable and unstable manifolds of q meet at a homoclinic bifurcation point B, creating an unstable periodic orbit (dashed red curves) that surrounds p. As |gNL| is increased, this homoclinic orbit gives rise to an unstable periodic orbit (dashed red curves in bifurcation diagram). On the interval (C, B), the stable and unstable manifolds of q switch their orientation relative to those on the interval (B, A). The stable manifold of q now approaches the unstable periodic orbit in backward time, while the unstable manifold of q asymptotes to v = EK.
At the point C, p loses stability through a supercritical Hopf bifurcation. The new asymptotically stable periodic orbit (solid red curves in bifurcation diagram) that is created by this bifurcation lies inside the previously created unstable orbit. In (D, C), the stable periodic solution is not globally attracting on the set v > EK: there still remain trajectories that tend to v = EK. At the value D, another homoclinic bifurcation occurs that destroys the unstable periodic solution. The orientation of the stable and unstable manifolds of q again switch such that the unstable manifold in forward time approaches the asymptotically stable periodic orbit. On the interval (E, D), there still remain trajectories that tend to v = EK as the stable manifold of q lies below the line w = 1. At the point E (vertical dashed gray line in the bifurcation diagram), the stable manifold of q asymptotes to w = 1 in backward time. The stable manifold of q now blocks trajectories with initial conditions lying to the right and below it (roughly v > ENL and w < 1) from converging to v = EK. The asymptotically stable periodic orbit now attracts every initial condition on this set. This remains the case on the interval (F, E).
At the point F, -gNL = gK and as v → ∞, the v-nullcline asymptotes to w = 1. This creates a new fixed point r at +∞ that is a saddle point. As |gNL| increases, the point r (indicated by the triangle in the G to F and H to G panels of Fig. 4) moves in from +∞. Along the way, the unstable manifold of q intersects the stable manifold of r making a heteroclinic connection (not demarcated in the bifurcation diagram). For (G, F), the asymptotically stable periodic orbit continues to exist. However, now there are initial conditions that lie on trajectories that escape to +∞ (black curve). As |gNL| is increased further, the periodic orbit finally disappears through a super-critical Hopf bifurcation at G. The fixed point p now becomes a stable spiral on the interval (H, G). As |gNL| is increased through this interval, the two fixed points p and r move together and collide at H. The point H therefore appears to be a saddle-spiral bifurcation. Such saddle-spiral bifurcations have been shown to exist in the category of co-dimension two Bogdonov-Takens bifurcations (Kuznetsov 2004). A more detailed analysis, beyond the scope of the current discussion, is needed to resolve the exact nature of this bifurcation.
Figure 5A shows a bifurcation structure for a set of parameters different from those in Figure 4. (The time constant τ1 associated with IK is changed from 60 to 80 ms and the parameter k1 associated with the slope of its half-activation from 2 to 4 mV. The effect of these changes is to weaken the effect of IK.) Here we have labeled 9 points A - I. Points A, B and C are the same as in Figure 4 except that, at C, the Hopf bifurcation is subcritical with the unstable branch of periodic orbits bending back towards smaller values of |gNL|. Further, on the interval (D, C) all trajectories, except for the stable manifold of q, tend to v = EK. At D, a saddle node of periodic orbits occurs creating a stable periodic orbit surrounded by an unstable one. At E, a homoclinic bifurcation corresponding to the intersection of the stable and unstable manifolds of q occurs destroying the unstable periodic orbit. The remaining subintervals of (I, E) have the same type of bifurcation structure as the subintervals (H, D) in Figure 4.
Finally, Figure 5B shows that increasing |gNL| does not necessarily lead to stable oscillations. (Here τ1 = 60 ms and k1 = 4 mV). For this set of parameters, p never loses stability. Points B and C are homoclinic bifurcation points of the manifolds of the fixed point q, between which lies an unstable periodic orbit. At D, convergence to v = EK becomes impossible and, at E, escape to +∞ becomes possible.
We can use the eigenvalues at the linearization given by equation (8) to explain how changes in parameters give rise to different bifurcations of p. In Figures 4 and 5A, p undergoes a Hopf bifurcation as |gNL| increases. A necessary condition for this type of bifurcation is that the real part of the eigenvalue Reλ=1/2(Fv + Gw) must pass through zero as gNL changes. From (8),
| (9) |
The second line follows since τK(v*p) is basically constant near p. Note, however, that the value of w*p depends on the location of the fixed point p and therefore on gNL, as seen from Equation (7) and Fig. 1. At the point labeled C in Figures 4 and 5A, a Hopf bifurcation occurs, i.e., the right-hand side of (9) is 0. While in Figure 5B, p remains asymptotically stable, i.e., the right-hand side of (9) remains negative for all |gNL|. The difference between Figures 4 and 5B is simply a change in the parameter k1 from 2 to 4, which makes the w-nullcline less steep. This, in turn, causes an increase in w* as the fixed point shifts up and to the right in the phase plane. As a result, the right-hand side of (9) decreases, thus keeping it negative. Similarly, the difference between Figures 5A and 5B is a change in τ1 from 80 to 60. Again, a smaller τ1 decreases the right-hand side of (9), also keeping it negative. Thus, the eigenvalues in both of these situations remain in the negative half-plane and p remains asymptotically stable.
The above analysis allows us to make several observations. First, in both Figures 4 and 5, there are parameter regimes for which trajectories converge to v = EK. Thus, the existence of a strong enough depolarizing current is not by itself sufficient to produce oscillations. A second observation from the model is that the voltage under the negative-conductance leak protocol can escape to +∞, which can occur when |gNL |> gK. Note that, even when |gNL |> gK , stable oscillations can exist (see G to F region in Fig. 4). However, if |NL|> IK for all v values (to the left of H in Fig. 4), then the destabilizing drive of the negative-conductance leak current exceeds the stabilizing effect of the potassium current and all orbits (above EK) escape to +∞.
The role of Ih in stabilizing oscillations
The addition of the hyperpolarization-activated current Ih qualitatively changes the nature of solutions. Indeed, it largely wipes out the interesting bifurcation structure of the previous section and leads typically to stable oscillations or convergence to a stable fixed point. To better understand the roles of IK and Ih, consider now the simplified model given by equation (4) with vcutoff = ENL where Ih is instantaneous, h ≡ h∞(v), the Ih-fast case (Fig. 6).
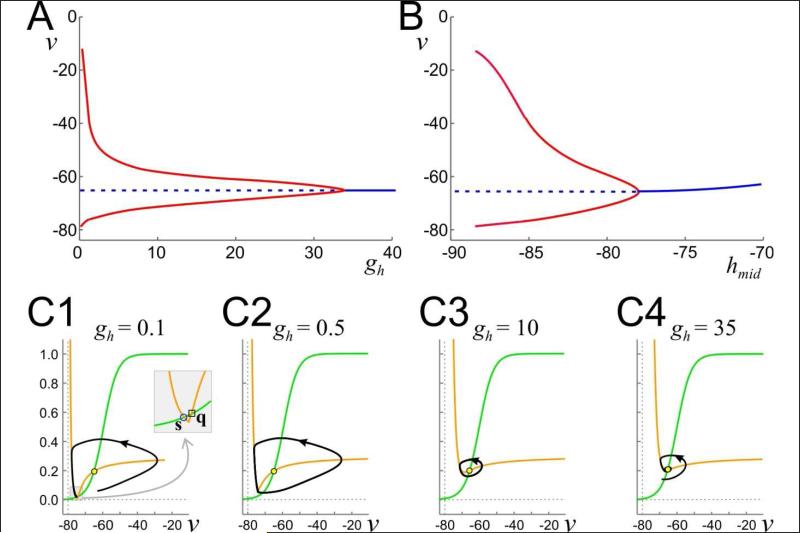
Figure 6. Bifurcation diagrams and phase plane behaviors for system (4) and vcutoff = ENL.
A: Bifurcation diagram with gh as parameter. Solid (dashed) lines/curves indicate stable (unstable) fixed points (blue)/periodic orbits (red). B: Bifurcation diagram with hmid as parameter and gh = 1 μS. C1-C4: Phase planes and limit cycles for various values of gh as noted. v in mV, gh in μS. Inset in C1 highlights the fixed points q and s.
We start with parameter values taken from Figure 5A in the regime (C, B) where trajectories tend to v = EK or to p. We fix gNL = -0.15 μS and increased ENL = −75mV to better illustrate the results. For gh > 0, the phase plane changes qualitatively. In particular, for v > EK, the v-nullcline now has a decreasing left branch, a local minimum and an increasing right branch that asymptotes to |gNL/gK|. For fixed values of w, the left branch is attracting. Note that there is a large change in the v-nullcline on the region EK < v < ENL. Recall for gh = 0, this was just the line segment w = 0 (Fig. 1D). In contrast, for gh > 0, the v-nullcline is a decreasing left branch (Fig.6C). When gh is small, this left branch intersects the w-nullcline in the region EK < v < ENL creating a stable fixed point denoted s (Fig. 6C1). As gh is increased, the v-nullcline moves up in the phase space and this newly created fixed point collides with q at a saddle-node on an invariant circle bifurcation yielding the first requirement for oscillations. It is quite straightforward to derive the condition on gh where this occurs. The saddle-node point occurs when the local minimum of the v-nullcline intersects the w-nullcline. Because vcutoff = ENL, the v- nullcline must have its local minimum at v = ENL (see equation (5)). The w value of the vnullcline at v = ENL is derived from equation (5) while that for the w-nullcline is simply w∞ (ENL). Setting the w values of both equal to one another yields the condition
| (10) |
Let gmin-h denote the right hand side of equation (10). Except for the value of Eh, gmin-h is determined by parameters that are independent of Ih. Moreover, equation (10) is independent of gNL. Thus the existence of the saddle-node bifurcation can be controlled by the left hand side of (10) which is dependent only on parameters associated with Ih. In particular if gh∞(ENL) < gmin-h, then the v-nullcline intersects the w-nullcline twice in a neighborhood of w = 0 and if gh∞ (ENL) > gmin-h then it does not. Note that gh and gh and h∞ (ENL) can be modulated separately. The former is the maximal conductance of Ih, while the latter is the fraction of Ih activation at ENL and is strongly dependent on hmid, the half-activation value.
A second requirement for the existence of oscillations is the instability of p. The location of p can be estimated as the solution v* of w∞(v*) = –gNL / gK, because h∞(v) is small in a neighborhood of the intersection and ENL – EK is also small. Thus, the location of p is largely independent of gh, but its stability will depend on local changes in the slope of the v-nullcline which do depend on gh. Keeping h∞(ENL) = −85 mV fixed, Figure 6A shows a bifurcation diagram of how the stability of p changes as a function of gh. At low values, for gh∞ (ENL) < gmin-h , solutions converge to the fixed point s (Fig. 6C1). Oscillations arise when gh increases so that gh∞ (ENL) > gmin-h and continue to exist for intermediate values (Fig. 6C2-C3). At a larger value of gh , p undergoes a supercritical Hopf bifurcation that destroys oscillations. For sufficiently large values of gh, the v-nullcline becomes monotone decreasing, stabilizing the spiral fixed point p (Fig. 6C4).
Because of the terms on the left hand side of equation (10), a qualitatively similar set of bifurcations can be obtained by changing hmid, the half-activation point of the h current, instead of gh, leading to a limited range of hmid values over which oscillations are possible (Fig. 6B). At more negative values of hmid, oscillatory activity ends because the v-nullcline intersects the wnullcline because h∞(ENL) becomes too small and the stable fixed point s reappears. Note that h∞(ENL) is a monotone-increasing function of hmid. Thus the model makes a prediction that, for oscillations to exist, the h-current half-activation has to sit at relatively hyperpolarized levels. On the other hand, if hmid is too large, then Ih never deactivates and the fixed point p is stable. Furthermore, by analyzing equation (10) it can be seen that, h∞(ENL) and gh are inversely related; increasing one decreases the other. Thus if hmid is relatively large causing h∞(ENL) to be relatively large, then a smaller value of gh is required for oscillatory activity.
As seen in the previous section, the existence of Ih is not necessary to produce oscillations. Indeed, if the model produces stable oscillations when gh = 0 then those oscillations will continue to exist, qualitatively unchanged, when gh > 0 but not too large. However, the basin of attraction for those solutions may become larger as the existence of a large enough Ih prevents convergence to either EK or to s. As a result, stable oscillations become more robust to noise and perturbations. For example, a perturbation introduced to the model consisting of just INL and IK can cause the trajectory to converge to EK. But when Ih is present, the same perturbation will not. Figure 7 shows modeling and experimental verifications of this. In panel A, gh = 0 a stable periodic orbit (black trajectory) is perturbed with the injection of an inhibitory external current pulse. The trajectory (red curve) leaves a neighborhood of the stable orbit, dips below ENL and EK, after which the external current is removed. The trajectory (gray curve) now is attracted to the stable fixed point at EK. In contrast, in panel B where gh > 0, the same type of perturbation prevents the voltage from dipping below ENL. Thus, after the external current is removed, the solution (gray curve) is attracted back to the original periodic orbit. Panels B and D, show the corresponding experimental realization of such. Finally, if parameters are chosen as in Figure 5B where no oscillations exist over the range of gNL values, the inclusion of Ih will not enable the model to produce oscillations (not shown).
Figure 7. Perturbation of the periodic orbit of system (4) without and with Ih.
A. When gh = 0, perturbation (red) of the trajectory along a stable periodic orbit (black) by injecting a hyperpolarizing current pulse causes the trajectory (gray) to converge to the stable fixed point at v = EK. B. When gh > 0, the same type of perturbation cannot remove the trajectory from the basin of attraction of the periodic orbit and the ensuing trajectory (gray) is attracted back to the periodic orbit. C. and D. Corresponding voltage traces from a biological PD neuron with INL injected using dynamic clamp (and vcutoff=ENL). A -5 nA current pulse (red bar) shuts down the oscillations in the absence of Ih (C). In the presence of a dynamic-clamp injected instantaneous Ih, the same current pulse only provides a brief interruption in the oscillations (D).
A fully linear INL: setting vcutoff to -∞
The destabilizing effects of the INL current can exist for voltages both above and below ENL. This will occur if we take the cutoff value of INL in equation (6) (the value below which INL = 0) to lie below ENL. For example, if we set vcutoff = -∞, then INL = -gNL[v - ENL], and the current will drive the voltage away from ENL both above and below this value. Consider equation (6) in such a case. The major change is that v = EK is no longer a stable fixed point. Instead trajectories can now escape to -∞. We recomputed the bifurcation diagrams using the parameter values of Figs. 4 and 5 with vcutoff = -∞. These diagrams would each show only one change: the stable fixed point at v=EK (solid blue line) disappears. As an example of this, choose gNL to have a value between points C and D in Fig. 5A with gh = 0, and vcutoff = -∞. Figure 1C shows the nullcline structure for this case. The v-nullcline has two distinct pieces that are separated by the vertical asymptote at v = EK. There are no stable fixed points or periodic orbits in the phase plane and solutions spiral away from the unstable point p and escape to -∞ (not shown).
An interesting question is what effect the addition of Ih would have for these cases. When Ih-fast is added, the nullcline structure is as in Fig. 1A. Here, the globally destabilizing effect of INL is not strong enough to disrupt the stabilization provided by Ih. However, if we instead use a slower version of the h current (Ih-slow), where the activation occurs over a longer time, our model makes dips in voltage to extremely hyperpolarized values, as shown in the left panel of Fig. 8A, preceded by a sequence of oscillations that grow in size. The voltage, however, does not actually escape to -∞, but instead eventually repolarizes back and resumes the oscillatory pattern. It is reasonably straightforward to explain this result using our prior analysis. In the trace shown in Fig. 8A, the voltage begins at a large value (v > EK) with Ih-slow non-zero and negative. Since the term -Ih-slow appears on the right-hand side of the voltage equation in (3), this current provides a positive contribution which causes the v-nullcline to have a stable left branch (as in Fig. 1A) and oscillations that accompany that nullcline structure. Ih-slow decays towards 0 during this time as the current deactivates. Once it has decayed enough, the left branch of the v-nullcline disappears and the nullcline structure changes to that of Fig. 1C for which escape to -∞ is possible. As the voltage makes its hyperpolarizing dip, Ih-slow activates, eventually pulling the voltage back up and the process repeats. In the right panel of Fig. 8A, Ih-slow was changed to a fast (instantaneous) current Ih-fast in the model and, as expected, the large hyperpolarizing dips disappear because now the nullcline structure of Fig. 1A persists.
Figure 8. Hyperpolarizing dips in system (3) and experimental verification.
A: The model described by equation (3) and vcutoff = -∞ shows hyperpolarizing dips (left) to very negative membrane potentials when |gNL| is large enough. Escape to -∞ is prevented by a slow Ih (τh1 = 20 ms). Making Ih fast (right) prevents the hyperpolarizing dips. B: The hyperpolarizing dips are observed in the biological PD neuron (left) when INL with a large enough conductance (-0.2 μS here) is added with dynamic clamp. As in the model, addition of a fast (instantaneous) Ih (0.1 μS here) via dynamic clamp prevents the hyperpolarizing dips and results in stable oscillations (right). Arrows indicate -60 mV for the voltages and 0 nA for Idclamp.
Figure 8B shows an experimental validation of the model predictions. In the left panel, the voltage trace shows hyperpolarizing dips. This is done by injecting INL with dynamic clamp (see Experimental Methods). The biological PD neuron has a slow h current(Khorkova and Golowasch 2007) that activates by the hyperpolarizing dips that result from INL injection with a sufficiently large conductance value. Subsequent addition of Ih-fast with dynamic clamp removes the hyperpolarization dips. There are some differences between the model and experimental results, for example, the local extremes of the latter are much smoother. Despite these differences in the details, the model captures the mechanisms that underlie this phenomenon.
4. Discussion
In this paper we examine whether neuronal regenerative inward currents need to be voltage-dependent to induce oscillatory activity, i.e., be designated as a pacemaker current. Our hypothesis is that voltage-dependence is not required and that a linear current with negative conductance, representing the negative conductance region of a typical regenerative current, is all that is needed. Furthermore, we hypothesize that such a negative conductance current, INL, acts by destabilizing the resting potential of a cell.
It is important to note that the current we define as INL is not a biological current as such (negative conductance has no physical correlate). Therefore, simply down-regulating leak currents in a cell (Blethyn et al. 2006; Lu and Feng 2012; Pang et al. 2009; Brickley et al. 2007; Rekling et al. 2000) cannot by itself generate oscillations. Here we confirm our hypothesis that a system consisting of only a voltage-gated K+ current and INL is capable of generating oscillatory activity. We show that the linear properties of INL in the biologically relevant voltage range are sufficient to explain the origin of oscillations without the need to resort to any other nonlinearities in the system than those of the recovery outward current. Furthermore, here we describe how a hyperpolarization-activated inward current (Ih) can contribute to stabilize oscillations by preventing excursions of the membrane potential to very negative values. In the case where we allow INL to extend below the biological plausible range (i.e. below EK), the membrane voltage can transiently deflect below EK. However, this is purely an artifact of the linear current approximation being used outside of a biologically relevant domain.
Furthermore, we show that this system expresses a rich behavioral repertoire as parameters, particularly gNL, are varied. Additionally, we show that transitions from stable non-oscillatory states to rhythmic activity, as gNL is varied, occur in phase space through various bifurcations. Interestingly, even in this very simple model, as the value of |gNL| is increased, oscillations may arise through either a supercritical Hopf bifurcation or through a saddle-node of periodic orbits. The rich bifurcation structure of the model arises through an interaction between INL and IK, resulting in the existence of saddle point q in the phase plane. When ENL > EK, the stable and unstable orbits of this saddle point define the boundaries between the basin of attraction of the periodic orbit, the stable fixed points p or EK and the regions where escape to +∞ is possible. As |gNL| is increased, these stable and unstable orbits can coincide (e.g., at points B and D of Fig. 4), to result in homoclinic bifurcations.
When assigning an ionic current as a pacemaker current, it is important to distinguish between a current that is necessary for pacemaker activity and one that shifts the membrane potential into a range where other ionic currents drive the oscillation. In the latter case the current can effectively be substituted with a DC current that shifts the membrane potential to the appropriate range, whereas the former is responsible for the regenerative depolarization in each cycle of oscillation. It has been shown that introducing the linear negative-conductance current INL by dynamic clamp can induce oscillatory activity in biological neurons of the crab STG (Zhao et al. 2010). However, in this experimental study, it was not possible to eliminate the possibility that INL only moves the voltage of the cell into a range in which other currents act as the regenerative pacemaker currents. Our results here, by using a simplified model consisting of only two currents, prove that a linear current with a negative conductance can indeed act as a regenerative, pacemaker current. This does not imply that any current that can be reduced to a negative-conductance current such as INL is necessarily a pacemaker current. For example, both the classical axonal Na+ current (Hodgkin et al. 1952) and other slow as well as non-inactivating Na+ currents (Jahnsen and Llinas 1984) have a region of negative conductance. However, while the axonal Na+ current could be said to underlie periodic activity under certain conditions (and thus be labeled ‘pacemaker’), the slow Na+ current of thalamic neurons does not play such a role during slow oscillations (Jahnsen and Llinas 1984).
In the stomatogastric pyloric network, which inspired the current work, a voltage-gated inward current, IMI, is activated by a number of neuromodulatory substances, each of which can turn on oscillations in the pyloric network (Golowasch and Marder 1992b; Hooper and Marder 1987; Swensen and Marder 2000). The negative-slope conductance region of the I-V curve of IMI can be approximated by the linear INL current. In the present study, we show that the PD neurons can generate oscillations with INL even when regenerative currents carried by Na+ are removed. It is possible that low-threshold Ca++ currents contribute to the generation of oscillations in the pyloric pacemaker neurons. However, the results of the present study, together with those of Zhao et al (2010), suggest that IMI is the pacemaker current of the pyloric network.
Our results indicate a need for a relatively close balance of parameters in order to ensure the generation of oscillatory activity. For example, when ENL < EK, the fixed point p is always stable and there is no possibility of stable oscillations (Fig. 3A). What this implies for biological oscillations is that the slope and position of the negative-conductance region of the I-V curve of a regenerative inward current is important for producing stable oscillations. If the linear approximation of the negative-conductance region (blue or aqua in Fig. 2A) intersects the v-axis below EK (for example, if the I-V curve is not steep enough), oscillations are not possible irrespective of the maximal conductance of this current. The steepness of this region can be changed, however, by increasing the maximal conductance of the regenerative inward current to the point that this linear approximation intersects the v-axis above EK to allow stable oscillations to exist. If the shape of the I-V curve is known, this simple principle can constrain the range of the maximal conductances of the regenerative inward current in a biological neuron in order to produce stable oscillations.
This kind of relationship between two ionic currents in oscillatory neurons has been shown before in neurons of the rat Pre-Bötzinger complex that drive rhythmic inspiratory activity (Del Negro et al. 2002) and in bursting glutamatergic interneurons within lamina I of the rat spinal cord (Li and Baccei 2011). In both cases, pacemaking is attributed to the activity of a slowly inactivating Na+ current, INaP, which drives membrane potential oscillations in close concert with a linear leak current. Our analysis shows that a close relationship between conductances is necessary in order to maintain the dynamical properties that allow oscillatory activity to emerge. Thus, as has been shown previously, we conclude that the relative expression of ionic conductances needs to be constrained to restricted ranges to produce oscillatory activity (see (Del Negro et al. 2002; Prinz et al. 2004; Ball et al. 2010; Goldman et al. 2001). We further show that parameters other than conductances, such as half-activation values (e.g. hmid), may also be subject to such constraints.
An interesting prediction of our model is that the principal role of the hyperpolarization-activated inward current, Ih, in oscillatory neurons is not to generate pacemaker activity as proposed previously (Jahnsen and Llinas 1984; McCormick and Bal 1997; DiFrancesco 2005), but rather to stabilize oscillations. This stabilization is principally achieved by preventing excursions of the membrane potential towards negative voltages (see also (Robinson and Siegelbaum 2003). In this case, we find that a balance between conductances, in this case between gNL and gh, is also required to ensure the ability of the cell to oscillate. A very large conductance gh results in an increase of the input conductance of the cell to a point where the unstable fixed point needed for oscillatory activity becomes stable. On the other hand, too much gNL results in excursions of the membrane potential to negative or positive values. Interestingly, this balance seems to constrain gh to be small relative to most other conductances in neurons, which is confirmed by experimental measurements in a variety of systems (Golowasch and Marder 1992a; Bazhenov et al. 2002; Tobin et al. 2006). We note that a finite non-zero gh value is not necessarily required to ensure oscillations, which can be generated even when gh = 0. However, a non-zero gh can ensure that perturbations (such as inhibitory synaptic inputs) do not push the voltage to a stable rest state and thus destroy the oscillations.
In our model, trajectories cannot escape to +∞ so long as |gNL |> gK , but large depolarizing excursions are possible. Just as Ih can stabilize oscillations by preventing large hyperpolarizing excursions of the membrane potential, one role of the voltage-gated outward currents (such as IK in our model) is to stabilize oscillations by preventing such large depolarizing excursions. The stabilizing effect of IK depends on both its maximal conductance and how fast it activates. Biological neurons typically have a number of different voltage gated outward currents and the conductances of these currents are usually large compared to the regenerative inward pacemaker currents (Golowasch and Marder 1992a; Bazhenov et al. 2002; Tobin et al. 2006). As a result, once the membrane potential is depolarized enough, the pacemaker current is dominated by the outward currents which guarantee the stability of oscillations in the depolarizing direction.
In conclusion, we have shown that a linear current with negative conductance is sufficient to generate oscillatory activity in conjunction with a simple delayed rectifier voltage-dependent K+ current. Our results predict a stabilizing role for hyperpolarization activated inward currents in addition to their known role in determining the rate of oscillations. Our results also suggest that stability of oscillations requires a balance of the conductance levels of specific ionic currents, which is confirmed by recent models and experimental observations in various systems.
Acknowledgments
This work was supported by NSF DMS1122291 (AB), NIH MH064711 (JG) and NIH MH060605 (FN).
5. Appendix
We used the following set of equations for simulations associated with Figures 4-8.
where
The gating functions are
and the time constants are given by τK (v)=τ1 / [1+exp(v/ks)] and τh (v) = τh1 ms. The following parameter values were used in all simulations EK = −80 mV, gK = 0.5, Iext = 0, andCm = 1.0 nF
In Figures 4 and 5 there was no h current, which we modeled simply by setting gh = 0 μS. We let ENL = −79 mV, wmid = -60 mV, ks = 2 mV. For Figure 4, we used k1 = 2 mV and τ1 = 60 ms. For Figure 5A, we used k1 = 4 mV and τ1 = 80 ms. For Figure 5B, we used k1 = 4 mV and τ1 = 60 ms.
In Figure 6, an instantaneous version of the h current was used, i.e., the equation for h’ was replaced with h ≡ h∞ (v). For this figure, parameter values from Figure 5A were used. In addition, Eh = −30 mV, hmid = −85 mV, h1 = 2 mV , ENL = -75 mV and gNL = −0.15 μS. Figure 6B was obtained by varying hmid while gh = 1 μS was fixed.
In Figure 7, we used parameter values from Figure 5A with gNL = −0.45 μS, ENL = -75 mV, gh = 1. The perturbation was achieved be setting Iext = -1 for 50 mS.
In Figure 8, we used Cm = 10.0 nF, τh1 = 200 ms, τ1 = 600 ms to match the biological timescale. Additionally, we set with ks(v) = τ1 / cosh((v – wmid)) / ks) with ks = 5.6 mV, gNL = −0.45 μS, ENL = −65 mV, wmid = −40 mV, gh = 0.8 μS and h1 = 6mV.
References
- Ball JM, Franklin CC, Tobin AE, Schulz DJ, Nair SS. Coregulation of ion channel conductances preserves output in a computational model of a crustacean cardiac motor neuron. J Neurosci. 2010;30(25):8637–8649. doi: 10.1523/JNEUROSCI.6435-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. J Neurosci. 2002;22(19):8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blethyn KL, Hughes SW, Toth TI, Cope DW, Crunelli V. Neuronal basis of the slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J Neurosci. 2006;26(9):2474–2486. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, et al. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J Neurosci. 2007;27(35):9329–9340. doi: 10.1523/JNEUROSCI.1427-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2(6):397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Cymbalyuk GS, Gaudry Q, Masino MA, Calabrese RL. Bursting in leech heart interneurons: cell-autonomous and network-based mechanisms. J Neurosci. 2002;22(24):10580–10592. doi: 10.1523/JNEUROSCI.22-24-10580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr., Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88(5):2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2(6):515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Cardiac pacemaker I(f) current and its inhibition by heart rate-reducing agents. Curr Med Res Opin. 2005;21(7):1115–1122. doi: 10.1185/030079905x50543. [DOI] [PubMed] [Google Scholar]
- Dunmyre JR, Del Negro CA, Rubin JE. Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. J Comput Neurosci. 2011;31(2):305–328. doi: 10.1007/s10827-010-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Golowasch J, Marder E, Abbott LF. Global structure, robustness, and modulation of neuronal models. J Neurosci. 2001;21(14):5229–5238. doi: 10.1523/JNEUROSCI.21-14-05229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Marder E. Ionic currents of the lateral pyloric neuron of the stomatogastric ganglion of the crab. J Neurophysiol. 1992a;67(2):318–331. doi: 10.1152/jn.1992.67.2.318. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Marder E. Proctolin activates an inward current whose voltage dependence is modified by extracellular Ca2+. J Neurosci. 1992b;12(3):810–817. doi: 10.1523/JNEUROSCI.12-03-00810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedo RJ, Golowasch J. Ionic mechanism underlying recovery of rhythmic activity in adult isolated neurons. J Neurophysiol. 2006;96(4):1860–1876. doi: 10.1152/jn.00385.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL, Marder E. Modulation of the lobster pyloric rhythm by the peptide proctolin. J Neurosci. 1987;7(7):2097–2112. doi: 10.1523/JNEUROSCI.07-07-02097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27(32):8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Botzinger complex in vitro. J Neurosci. 2008;28(7):1773–1785. doi: 10.1523/JNEUROSCI.3916-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH, Zucker RS. Calcium-dependent inward current in Aplysia bursting pace-maker neurones. J Physiol. 1985;362:107–130. doi: 10.1113/jphysiol.1985.sp015666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov IUA. Applied mathematical sciences. 3rd ed. Vol. 112. Springer; New York: 2004. Elements of applied bifurcation theory. [Google Scholar]
- Li J, Baccei ML. Pacemaker neurons within newborn spinal pain circuits. J Neurosci. 2011;31(24):9010–9022. doi: 10.1523/JNEUROSCI.6555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TZ, Feng ZP. NALCN: a regulator of pacemaker activity. Mol Neurobiol. 2012;45(3):415–423. doi: 10.1007/s12035-012-8260-2. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68(4):1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- Pang DS, Robledo CJ, Carr DR, Gent TC, Vyssotski AL, Caley A, et al. An unexpected role for TASK-3 potassium channels in network oscillations with implications for sleep mechanisms and anesthetic action. Proc Natl Acad Sci U S A. 2009;106(41):17546–17551. doi: 10.1073/pnas.0907228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7(12):1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80(2):767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activaterd cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Selverston AI, Russell DF, Miller JP. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol. 1976;7(3):215–290. doi: 10.1016/0301-0082(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Sharp AA, O'Neil MB, Abbott LF, Marder E. Dynamic clamp: computer-generated conductances in real neurons. J Neurophysiol. 1993;69(3):992–995. doi: 10.1152/jn.1993.69.3.992. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance. J Neurosci. 2005;25(14):3509–3520. doi: 10.1523/JNEUROSCI.3929-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20(18):6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AE, Van Hooser SD, Calabrese RL. Creation and reduction of a morphologically detailed model of a leech heart interneuron. J Neurophysiol. 2006;96(4):2107–2120. doi: 10.1152/jn.00026.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26(10):2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264(5161):974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Zhao S, Golowasch J, Nadim F. Pacemaker neuron and network oscillations depend on a neuromodulator-regulated linear current. Front Behav Neurosci. 2010;4:21. doi: 10.3389/fnbeh.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]