Abstract
Alteration in normal hippocampal (HPC) function attributed to reduced parvalbumin (PV) expression has been consistently reported in schizophrenia patients and in animal models of schizophrenia. However, it is unclear whether there is an overall loss of interneurons as opposed to a reduction in activity-dependent PV content. Co-expression of PV and the constitutively-expressed substance P (SP)-receptor protein has been utilized in other models to ascertain the degree of cell survival, as opposed to reduction in activity-dependent PV content, in the HPC. The present study measured the co-expression of PV and SP-receptors in the dentate and dorsal and ventral CA3 subregions of the HPC in the methylazoymethanol acetate (MAM) rat neurodevelopmental model of schizophrenia. In addition, these changes were compared at the postnatal day 27 (PND27) and post-natal day 240 (PND240) time points.
Brains from PND27 and PND240 MAM (n=8) and saline (SAL, n=8) treated offspring were immunohistochemically processed for the co-expression of PV and SP-receptors. The dorsal dentate, dorsal CA3 and ventral CA3 subregions of PND27 and PND>240 MAM rats demonstrated significant reductions in PV but not SP-receptor expression, signifying a loss of PV-content. In contrast, in the ventral dentate the co-expression of PV and SP-receptors was significantly reduced only in PND>240 MAM animals, suggesting a reduction in cell number. While MAM-induced reduction of PV content occurs in CA3 of dorsal and ventral HPC, the most substantial loss of interneuron number is localized to the ventral dentate of PND>240 animals. The disparate loss of PV in HPC subregions likely impacts intra-HPC network activity in MAM rats.
Keywords: substance P, parvalbumin, schizophrenia, ventral hippocampus, periadolescence, dentate gyrus
Introduction
The most consistently reported cellular alteration observed in the brains of schizophrenia patients is the reduction in the expression of the calcium-binding protein, parvalbumin (PV) (Lewis et al., 2001; Beasley et al., 2002; Kalus et al., 2002; Zhang et al., 2002c; Hashimoto et al., 2003; Sakai et al., 2008; Fung et al., 2010; Konradi et al., 2011; Curley et al., 2013) in GABAergic interneurons. Disruptions in inhibitory networks resulting from reductions in PV expressing interneurons are proposed to contribute to the hyperactivity and loss of evoked gamma rhythms observed in the prefrontal cortex and HPC (Lewis and Moghaddam, 2006; Lisman et al., 2008; Lodge et al., 2009; Konradi et al., 2011). The expression of PV is dependent on neural activity (Patz et al., 2004; Jiang and Swann, 2005; Sun, 2009). Recently, it has been shown that reductions of PV measured post-mortem in the HPC of schizophrenia patients are not likely to involve an overall loss of cells as opposed to a decrease in protein content (Zhang et al., 2002c; Konradi et al., 2011).
As observed in patients, in the methylazoxymethanol acetate (MAM) developmental rodent model of schizophrenia there is a reduction in parvalbumin (PV)-expressing interneurons within the ventral HPC, which is proposed to lead to an aberrant increase in dopamine activity in the ventral tegmental area (Lodge et al., 2009). Aberrant increases in hippocampal (HPC) activity have been implicated in the emergence of symptoms in schizophrenia (Schobel et al., 2013; Schobel et al., 2009; Small et al., 2011). While a decrease in PV expression in the ventral subiculum of the HPC of MAM rats has been reported previously, there is support for functional heterogeneity between the different HPC subregions, suggesting that the alterations resulting from MAM treatment may not be uniform. In particular, the dentate and CA3 regions of the HPC are important for the disambiguation of similar contexts, or pattern separation (Leutgeb et al., 2007; Tamminga et al., 2010; Schmidt et al., 2012). There is evidence that normal context discrimination is impaired in patients with schizophrenia, perhaps resulting from alterations in normal activity within the dentate and CA3 subregions. Consequently, assessing PV expression in the dentate and CA3 subregions in the MAM model would confirm pathological changes specific to these areas and potential resultant behavioural abnormalities reported in schizophrenia patients.
The substance P (SP) receptor protein is expressed exclusively on nonprincipal, GABAergic interneurons in the hippocampus (Sloviter et al., 2001). The co-expression of SP receptors and PV has previously been reported for the dentate, CA1, and CA3 subregions of the dorsal HPC (Acsady et al., 1997; Sloviter et al., 2001) and alterations in the co-expression has been used to quantify changes in HPC interneuron number in animal models of epilepsy (Sloviter, 1991; Sloviter et al., 2001; Sloviter et al., 2003), in that substance P receptors are continuously expressed even when activity-dependent PV content is lost. Verifying the extent of PV-expressing interneuron cell survival by measuring the co-expression of SP and PV in the HPC in the MAM model would be consistent with the pathological findings reported in schizophrenia patients. Recent studies have shown in normal rats that there is a progressive increase in PV expression in the vental subiculum of the HPC as well as PFC when comparing post-natal days (PND) 25–40 and PND 60–85 (Caballero et al., 2013; Caballero et al., 2014). Consequently, there are likely alterations in the normal developmental expression of PV in the MAM rat.
For the present experiment, offspring of dams treated with MAM or saline ( on post-natal day 27 (PND27) or post-natal day 240 (PND>240). Immunofluorescent double-labeling was used to assess the expression of PV and SP receptors in the dentate/CA3 subregion of the dorsal and ventral HPC. By examining PV expression at these time points, it could be determined whether there is an initial decrease in PV-expression during periadolescence that persists to adulthood, or alternatively, a progressive decrease in PV expression that is not observed until adulthood.
METHODS
Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. All immunohistochemical experiments were conducted in PND27 (n=8) and PND>240 (n=8) offspring of pregnant dams treated with either saline or methylazoxymethanol acetate (details provided below). Animals were housed in a temperature (22°C) and humidity (47%) controlled environment with a 12-hour light/dark cycle (lights on at 7 a.m.) with ad libitum access to both food and water.
Methazoxymethanol Treatment
MAM administration was performed as described previously (Moore et al., 2006; Lodge and Grace, 2007; Lodge et al., 2009; Gill et al., 2011). Briefly, timed pregnant female Sprague Dawley rats (Hilltop) were obtained on gestational day (GD) 15 and individually housed in ventilated plastic breeding tubs. MAM (20 mg/kg, i.p.) was administered on GD 17. Control dams received injections of saline (1 ml/kg, i.p.). Male pups were weaned on day 21 and pair-housed with litter mates for either 27 days or 8 months, after which time they were used immunohistochemical experiments.
Immunohistochemistry
PND27 and PND>240 animals were deeply anesthetized with sodium pentobarbital and perfused transcardially with .85% saline followed by 4% paraformaldehyde. Two time points were selected based on previous work in the laboratory examining the impact of early pharmaceutical interventions (PND 31–40), e.g. diazepam treatment, on subsequent dopamine system pathology in adult MAM animals (>PND60) (Du and Grace, 2013). We have also shown that this early time point is also associated with altered stress responses, e.g. increased vocalizations and blunted HPA axis activation in response to stressful stimuli, in MAM animals (Zimmerman et al., 2013). In addition, PND27 has been shown to coincide with an early developmental time point (PND25–45) associated with decreased PV expression in the ventral subiculum of the HPC and PFC, increased risk taking behaviors, reduced behavioral inhibition, and perseverative errors during instrumental learning compared to adult animals (>60PND) (Sturman et al., 2010; Andrzejewski et al., 2011; Caballero et al., 2013; Simon et al., 2013). Brains were removed and subsequently cryoprotected in 25% sucrose for 24 to 48 hours prior to being frozen and sliced on a freezing microtome (35μm/slice). Free floating sections were then processed for their expression of parvalbumin and substance p via immunofluorescence using techniques described previously (Cano et al., 2001; Card et al., 2006). Sections were incubated in a combination of donkey serum, 0.1% Triton X-100, and mouse anti-PV antibody (1:1000 dilution, Sigma) with rabbit anti-substance P receptor antibody (1:100 dilution, Millipore) for 24 hours at room temperature. After washing 3 × 10 min in phosphate buffered saline (PBS), the sections were then incubated in a mixture of Cy3 and Alexa conjugated donkey anti-rabbit/mouse antiserum (Jackson Immunoresearch Laboratories) for 2 hours. Subsequently, sections were washed 3 × 5 min in PBS prior to being mounted on microscope slides. Prior to cover slipping, microscope slides were rinsed in a series of increasing concentrations of EtOH followed by xylene (2 min/wash).
Immunofluorescence Quantification
Slides were viewed under two different filter conditions for Cy3 and Alexa Fluor 488 on an OlympusBX51 microscope with a Hamamatsu Orca-ER camera. For cell counts, focus was set on PV-positive neurons and digital images were obtained using SimplePCI6 software (Hamamatsu Corporation). Sequential images (from both left and right hemispheres over region of interest obtained from 3 slices separated by 210 μm; 6 samples total per subject) were taken at 10x and 20x magnification through equivalent areas of the CA3 and dentate subregions of the ventral and dorsal HPC of SAL and MAM treated animals. Sections were selected from equivalent locations (same anterior-posterior position relative to bregma) in MAM and SAL rats. Only neurons within the hilus region of the dentate gyrus and pyramidal cell layer of CA3 were quantified (Fig. 1). Image-editing software (Adobe Photoshop) was used to combine the obtained images into plates prior to manual quantification of immunopositive neurons (immunopositive neurons = signal to background>3:1). Statistical comparisons were made on the average cell counts of the 6 samples from each region of interest.
Figure 1.
Schematic of coronal sections of the rat brain with quantification boundaries indicated in dorsal (A) and ventral (B) dentate and CA3 (shaded areas) subregions of HPC. (modified from (Paxinos and Watson, 1996).
Statistical Analysis
Two-way analysis of variance was used to compare differences between the MAM and SAL groups, PND>240 and PND27 groups, and the possible interaction between age group and MAM treatment. Tukey’s post-hoc comparisons were conducted for significant main effects. All statistics were calculated using SigmaPlot.
RESULTS
Ventral HPC subfield-specific loss of PV and SP-receptor expression in PND>240 MAM animals
We reported previously that MAM rats demonstrate reduced PV expression in the mPFC and ventral subiculum of the HPC (Lodge et al., 2009). The aberrant activation of the dopamine system in MAM rats has been attributed to alterations in HPC output resulting from the loss of the normal functionality of PV-expressing interneurons (Lodge and Grace, 2007; Lodge et al., 2009). However, potential subregional changes, in either content or cell number, within the ventral HPC of MAM rats have not been described. Given that SP receptors are expressed in PV neurons in an activity-independent manner, the coexpression of PV and the SP-receptor has been utilized to determine whether the loss of PV represented a reduction in cell number or a loss of PV-content (Sloviter et al., 2003). Unlike the PFC where there is a low-level of co-expression of PV and SP-receptors (Matute et al., 1993), the HPC is especially amenable for the quantification of both proteins. Consequently, in the present study we assessed changes in PV and SP-receptor expression in HPC subregions known to exhibit a high-degree of co-expression of the two proteins, the dentate and both ventral and dorsal CA3 subfields (Leranth and Nitsch, 1994; Sloviter et al., 2001) of MAM (N=8) and SAL (N=8) rats (Fig. 2).. By measuring the co-expression of the two proteins, we determined whether PV changes represented a loss of protein content within intact neurons or an overall reduction in cell number.
Figure 2.
Representative images of PV and SP- receptor expression in the dentate and CA3 subregions of the ventral (A) and dorsal (B) HPC of PND27 and adult SAL- and MAM-treated offspring. Yellow arrows indicated neurons that were double-labeled for SP-receptors and PV.
Individual 2-way ANOVAs were performed to assess differences in PV, SP and % of neurons co-expressing both proteins. Unless otherwise stated, Tukey’s post-hoc comparisons were used. In the hilus of the dentate gyrus of the ventral HPC, MAM rats exhibited significantly fewer PV-expressing neurons than SAL rats that was dependent on age (Fig. 3A;treatment x age interaction, F(1,15) = 7.41, p < .01; post-hoc, p< .05). PND27 SAL rats demonstrated significantly more PV-expressing neurons than PND>240 MAM animals (individual Student’s t-test, t(6) = 2.25, p< .05). In contrast, there was no difference in the number of PV-expressing neurons between PND27 SAL rats and PND27 MAM rats (p = 0.13). This would suggest that there was a progressive loss of PV in MAM rats from PND27 to PND>240. In comparison, the dentate of PND27 SAL rats contained more SP receptor-expressing neurons than both PND27 and PND>240 MAM animals (Fig. 3B; main effect of treatment, F(1,12) = 13.09, p<.01; post-hoc, p<.05). However, when considering the percent of cells co-expressing both PV and SP-receptors, only PND>240 MAM rats exhibited significantly fewer co-expressing neurons than PND27 SAL rats (Fig. 3C; main effect of treatment, F(1,12) = 6.33, p<.05; individual Student’s t(6) = 2.63, p <.05). This would suggest that the loss of PV in PND>240 MAM animals represents a loss of cell numbers as opposed to loss of PV content.
Figure 3.
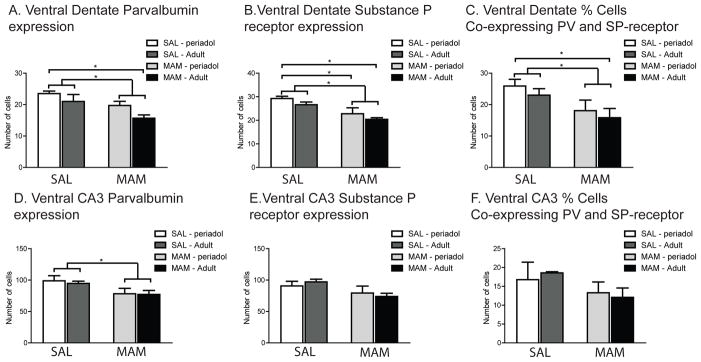
MAM-treated offspring displayed significant reductions in the average number of PV-expressing neurons in the dentate region of the ventral HPC compared to SAL-treated offspring. In ventral CA3 subregion of the HPC, there is a reduction in PV neurons but no change in SP-receptor-expressing neurons. (A) The dentate from PND>240 MAM animals had significantly fewer PV-expressing neurons than PND>240 SAL animals. (B) Similarly, MAM-treated offspring exhibited significantly fewer SP receptor-expressing neurons in the ventral dentate compared to SAL rats at both the PND27 and PND>240 time points. (C) The dentate of PND>240 MAM animals contained significantly fewer neurons double-labeled for both SP receptors and PV than PND27 SAL animals, which is indicative of a loss of cell number rather than level of expression. (D) MAM-treated offspring displayed significant reductions in the average number of PV-expressing neurons in the CA3 region of the ventral HPC compared to SAL-treated offspring. (E) In contrast, there was no difference between SAL and MAM rats in the number of SP-receptor expressing neurons in ventral CA3. (F) In addition, there was no difference between SAL and MAM rats in the number of neurons double-labeled for both SP-receptors and PV, indicative of a preservation of cell number but reduction in PV content. (* denotes p<.05 following Tukey’s post-hoc comparisons)
Similar to the dentate, MAM rats demonstrated significantly fewer PV-expressing neurons than SAL rats in the ventral CA3 subregion (Fig. 3D; 2-way main effect of treatment, F(1,12) = 5.67, p< .01; post-hoc, p< .05). However, unlike the pattern observed in the dentate, there was no interaction between PV expression at the PND27 and PND>240 time points and MAM treatment (F(1,15) = 0.04, p = 0.85). Subsequent post-hoc comparisons failed to reveal any significant differences amongst the individual age groups, but there was a significant difference between the combined MAM and SAL groups (post-hoc, p = .03). This would suggest a general reduction of PV expression in MAM animals independent of age. In contrast to the reductions in SP-receptor expression observed in the ventral dentate, there were no changes in SP-receptor expression in ventral CA3 in the MAM animals (Fig. 3E; main effect of treatment, F(1,12) = 3.97, p = 0.07), nor were there any differences in SP-receptor expression in ventral CA3 between the PND27 and PND>240 time points (main effect of age, F(1,12) = 0.46, p = 0.51). As expected from the pattern of reduced PV-expression, but unaltered SP-receptor expression in ventral CA3, the percent of cells co-expressing the two proteins did not significantly vary between MAM and SAL animals (Fig. 3F;main effect of treatment, F(1,12) = 0.94, p = 0.35). Consequently, the loss of PV in ventral CA3 may represent a reduction in PV content and not necessarily a loss of PV neurons in MAM rats.
Dorsal HPC reduction in PV but not SP-receptor expression in MAM animals
There are distinct behavioral functions attributed to the dorsal and ventral subregions of the HPC (Anagnostaras et al., 2001; Peleg-Raibstein and Feldon, 2006; Fanselow and Dong, 2010; Kesner et al., 2011). In addition, increased activation of the ventral, but not dorsal, HPC has been associated with increased activation of the dopamine system (Peleg-Raibstein and Feldon, 2006). We sought to determine whether reductions of PV-expression reported for the ventral HPC of MAM rats (Lodge et al., 2009) extended to the dorsal HPC as well.
In the hilus of the dentate gyrus of the dorsal HPC, MAM rats exhibited significantly fewer PV-expressing neurons than SAL rats (Fig. 4A; main effect of treatment, F(1,12) = 23.09, p < .01; post-hoc, p< .05). In addition, there were age-dependent differences in PV expression between SAL and MAM rats. (main effect of age, F(1,12) = 7.95, p<.05). At the PND27 time point, MAM rats already showed reduced PV-expression compared to PND27 SAL rats (p<.05). PND>240 SAL rats exhibited fewer PV-expressing neurons than PND27 SAL rats (p<.05). However, PND>240 MAM rats exhibited significantly reduced PV expression in the dorsal dentate than PND>240 SAL rats (p<.05). This would suggest that although there was already an age-dependent reduction in PV expression in the dorsal dentate of normal rats, MAM rats demonstrated an even greater decrease. In contrast, there was no difference between SAL and MAM rats in the number of SP-receptor-expressing neurons in the dorsal dentate (Fig. 4B;main effect of treatment, F(1,12) = 2.99, p=.11). In addition, when considering the percent of cells co-expressing both PV and SP-receptors, there was no difference between SAL and MAM rats (Fig. 4C; main effect of treatment, F(1,12) = 0.67, p=.43). Similar to what was observed in the ventral CA3 subregion, these data support a reduction of PV-content in neurons in the dorsal dentate of PND27 and PND>240 MAM rats, as well as PND>240 SAL rats. This is in contrast to the overall reduction in cell number observed in the ventral dentate of MAM rats. These data suggest that the decrease in PV in dorsal dentate in MAM rats may be due to decreased PV neuron drive, whereas the decrease in the ventral dentate is due to PV neuron loss, suggesting a greater vulnerability of neurons in the ventral dentate to MAM treatment.
Figure 4.
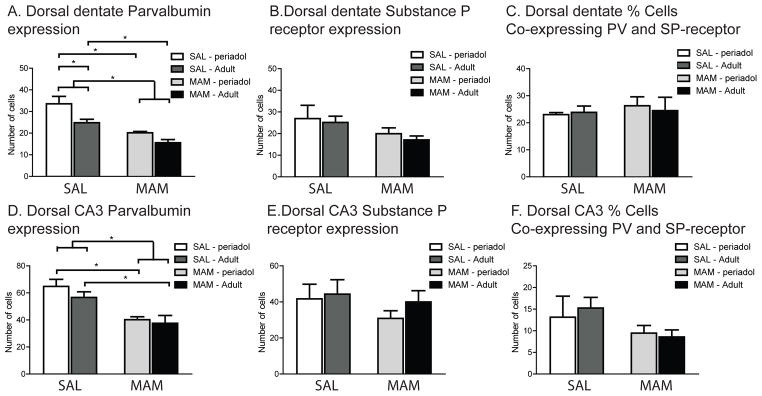
MAM-treated rats exhibit decreased PV-expressing neurons without changes in SP-receptor expressing neurons in dorsal dentate gyrus and CA3. (A) In the dentate subregion of the dorsal HPC, MAM-treated offspring, at both the PND27 and PND>240 time points, displayed significant reductions in the average number of PV-expressing neurons compared to SAL-treated offspring at the corresponding time points. In addition, adult SAL rats demonstrated significantly fewer PV-expressing neurons in the dorsal dentate than PND27 SAL rats. (B) In contrast, there was no difference between SAL and MAM rats in the number of SP-receptor expressing neurons in dorsal dentate. (C) In addition, there was no difference between SAL and MAM rats in the number of neurons double-labeled for both SP-receptors and PV. (D) MAM-treated offspring, at both the PND27 and PND>240 time points, displayed significant reductions in the average number of PV-expressing neurons in the CA3 subregion of the dorsal HPC compared to SAL-treated offspring at the corresponding time points. (E) In contrast, there was no difference between SAL and MAM rats in the number of SP-receptor expressing neurons in dorsal CA3. (F) In addition, there was no difference between SAL and MAM rats in the number of neurons double-labeled for both SP-receptors and PV. (* denotes p<.05 following Tukey’s post-hoc comparisons)
Similar to the dorsal dentate, MAM rats demonstrated significantly fewer PV-expressing neurons than SAL rats in the dorsal CA3 subregion that was not dependent on age (Fig. 4D;main effect of treatment, F(1,12) = 17.43, p< .001; post-hoc, p< .05;main effect of age, F(1,12) = 1.09, p = 0.32). Both PND27 and PND>240 MAM rats displayed significantly fewer PV-expressing neurons in dorsal CA3 compared to SAL rats (post-hoc comparisons, p<.05, respectively). In addition, there were no changes in SP-receptor expression in dorsal CA3 in the MAM animals (Fig. 4E;main effect of treatment, F(1,12) = 0.92, p = 0.36), nor were there any differences in SP-receptor expression in dorsal CA3 between the PND27 and PND>240 time points (main effect of age, F(1,12) = 0.55, p = 0.47). As expected from the pattern of reduced PV-expression but unaltered SP-receptor expression in dorsal CA3, the percent of cells co-expressing the two proteins did not significantly vary between MAM and SAL animals (Fig. 4F;main effect of treatment, F(1,12) = 0.65, p = 0.44). Consequently, the loss of PV in dorsal CA3 likely represented a reduction in PV content in neurons in MAM rats.
DISCUSSION
This is the first report of HPC subregional differences in PV and SP-receptor expression in the MAM model of schizophrenia. Previously, we demonstrated a reduction in PV expression in the ventral subiculum of the HPC (Lodge et al., 2009). We extend these findings of diminished PV expression to the dentate gyrus and both dorsal and ventral CA3 subregions of the HPC. Even though there is not complete overlap in those neurons expressing both SP and PV, there is a sufficient proportion of neurons co-expressing the two proteins. As a consequence, reductions or increases in the co-expression would be indicative of a loss of cell number, as opposed to mere changes in an individual protein without alterations in their co-expression. As such, by evaluating the co-expression of SP-receptors and PV, we show that the loss of PV in the CA3, but not dentate, is likely a reduction of PV-content in surviving neurons. These data are consistent with studies from schizophrenia patients demonstrating HPC reduction in the relative density of PV-expressing neurons without any change in overall neuron number (Zhang et al., 2002c; Konradi et al., 2011). In contrast, the ventral dentate exhibited a significant decrease in neurons co-expressing SP receptors and PV, indicative of a loss of cell number. This would suggest that the impact of MAM on PV-expressing interneurons is not uniform across all HPC subregions and could have a broad impact on intra-HPC network activity. Importantly, the reduction in PV and SP-receptors was most prominent in the PND>240 MAM animals. A progressive loss of these proteins into adulthood could signify a potential time point during periadolescence for therapeutic intervention that could potentially prevent the transition to schizophrenia in the adult (Du and Grace, 2013).
Implications for pathological changes in ventral HPC and cognitive disturbances in schizophrenia
The dorsal and ventral subdivisions of the HPC in the rat are homologous to the posterior and anterior portions, respectively, of the HPC in humans (Strange and Dolan, 1999; Sasaki et al., 2004). While some studies have reported simultaneous alterations in both anterior and posterior HPC areas of schizophrenia patients (Herold et al., 2013; Zierhut et al., 2013), there have also been reports of exclusive volumetric and activity-related changes in the anterior, but not posterior, HPC (Szeszko et al., 2003; Narr et al., 2004; Velakoulis et al., 2006; Schobel et al., 2009). Evidence from rodents also suggests that PV expression in the ventral, but not dorsal, subiculum undergoes protracted changes during development (Caballero et al., 2013).
Numerous morphological and neurochemical alterations specific to the dentate gyrus region of the HPC have been observed in the postmortem brain from schizophrenia patients (reviewed in (Kobayashi, 2009). It has been suggested that the dentate gyrus may be especially vulnerable to developmental alterations due to continuous postnatal neurogenesis. Mossy fibers from the dentate gyrus convey processed entorhinal cortical input representing current contextual stimuli to CA3 (Treves and Rolls, 1992; O’Reilly and McClelland, 1994). Consequently, developmental abnormalities observed in the dentate can have downstream effects on the normal plasticity at the mossy fiber-CA3 synapses. Altered connectivity between the dentate gyrus and CA3 has been proposed to contribute to errors of pattern completion in schizophrenia patients and an inability to disambiguate between present and past experiences in memory (Tamminga et al., 2010). In addition, electrophysiological recordings from and assessment of immediate early gene activation in the dentate gyrus have substantiated a role for this HPC subregion in disambiguating between similar behavioral contexts (reviewed in (Schmidt et al., 2012). Close examination of the mossy fiber-CA3 projection in schizophrenia patients reveals a reduction in spine number as well as spines forming synapses (Kolomeets et al., 2005). These data indicate a decreased efficacy of projections from the dentate to CA3 and an association with the presence of positive symptoms. Data from the current study confirm a significant loss of interneurons in the ventral dentate of PND>240 MAM rats. Consequently, alterations in the normal connectivity between the dentate and CA3 resulting from this cell loss in MAM rats could lead to inadequate conveyance of current contextual stimuli to CA3, and may play a role in sensory processing or cognitive disturbances characteristic of schizophrenia.
There is evidence that alteration in the normal functionality of ventral, but not dorsal, HPC is more involved in some of the behavioral disturbances associated with schizophrenia. Prepulse inhibition (PPI) of the startle reflex, a measure of sensorimotor gating, is commonly disrupted in schizophrenia. Both electrical and chemical activation via NMDA of the ventral, but not dorsal, HPC can disrupt PPI in rodents (Klarner et al., 1998; Bast et al., 2001; Swerdlow et al., 2001; Zhang et al., 2002a, b; Howland et al., 2004). Schizophrenia patients also exhibit impaired performance during attentional set-shifting tasks (Pantelis et al., 1999). Temporary inactivation of the ventral, but not dorsal, HPC in rodents early in development can lead to impaired attentional set-shifting performance in adulthood (Brooks et al., 2012). Furthermore, there are indications that regionally-specific alterations in PV in the HPC could underlie both the behavioral and oscillatory disturbances observed in schizophrenia. Genetic disruption of normal antioxidant activity, a known susceptibility factor for schizophrenia, by interfering with glutathione synthesis results in selective decreases in PV expression in the dentate and CA3 subregions of the ventral, but not dorsal, HPC (Steullet et al., 2010). The reduction in PV coincides with altered beta and gamma oscillatory activity as well as impaired object recognition and delayed fear conditioning (Lodge et al., 2009; Steullet et al., 2010). Consequently, the differential contribution of the ventral and dorsal HPC in generating the pathological behaviors observed in schizophrenia is compatible with the disparity in reduced co-expression of SP-receptors and PV observed in the present study.
It is important to note that there is a diverse array of interneuron subtypes, expressing either PV, somatostatin, cholecystokinin, neuropeptide Y, or vasopressin in both the dorsal and ventral HPC. While there are reports of reductions in other interneuronal markers, including somatostatin (Morris et al., 2008; Fung et al., 2010; Konradi et al., 2011) and cholecystokinin (Curley and Lewis, 2012), in schizophrenia patients, corresponding experiments in the MAM model have not been conducted. In addition, PV-expressing interneurons represent the largest population of interneurons in the HPC along with previously reported high co-localization with the SP-receptor (Sloviter et al., 2001). Reductions in PV have previously been reported in the MAM model in both the mPFC and ventral subiculum (Lodge et al., 2009). Importantly, these reductions were associated with alterations in task-induced gamma oscillations during the latent inhibition paradigm, similar to what has been reported in schizophrenia patients (Spencer et al., 2003; Symond et al., 2005; Bucci et al., 2007; Williams et al., 2009; Koenig et al., 2012). Consequently, PV is the most suitable target when assessing immunohistochemical changes in the MAM model.
Therapeutic implications for age-related changes in PV-expression in SZ
The loss of PV- and SP-receptor expressing neurons in the dentate gyrus of MAM rats was most pronounced in the PND>240 animals. There is evidence from rodents and nonhuman primates that PV expression in the PFC as well as the ventral subiculum of the HPC increases across development (Fung et al., 2010; Caballero et al., 2013; Fish et al., 2013; Caballero et al., 2014). It should be noted that in the present study the reduction of PV in the dorsal dentate in normal rats PND>240 might indicate that this region is more susceptible during normal aging. PND>240 is at the early stage of observed decreases in neurogenesis in the dentate gyrus in adult rats, with the largest decreases occurring >360 (Kuhn et al., 1996; Rao et al., 2006). However, in the present study additional decreases in PV in the dentate were observed in the MAM animals at the same time point as SAL rats. Typically, postmortem assessment of regional changes of PV expression in schizophrenia involves the comparison with age-matched control subjects. Currently, there is no definitive study examining the temporal pattern of PV expression changes in schizophrenia patients. However, a compelling recent study reported that reductions in PFC PV expression were not associated with the age or duration of illness of schizophrenia patients (Hoftman et al., 2013). When considered with the normal expected developmental increase in PV, this would suggest that there is a dampening of PV expression in schizophrenia. The data from the present study are consistent with the therapeutic potential of interventions introduced during adolescence in schizophrenia. Indeed, our lab has shown that treatment with diazepam during adolescence in MAM rats can prevent the abnormal increase in dopamine system activation observed in PND>240 animals (Du and Grace, 2013). Alternative animal models of schizophrenia have reported a similar benefit of antipsychotic drug treatment during adolescence in reversing various morphological and behavioral correlates of schizophrenia in adult animals (Piontkewitz et al., 2011; Piontkewitz et al., 2012). Of note, risperidone treatment can prevent the reduction in HPC PV-expression observed in the polyriboinosinic–polyribocytidilic acid model of schizophrenia (Piontkewitz et al., 2012). Perhaps by preventing the cell loss observed in the dentate gyrus of MAM rats, potential therapies could preserve the normal connectivity with CA3 and enable the subject to retain normal pattern separation behavioral functions.
Conclusions
While MAM-induced reduction of PV content occurs in the CA3 subregion of dorsal and ventral HPC, the most devastating loss of interneuron number is localized to the ventral dentate of PND>240 animals. These data suggest that PV loss in dorsal HPC may be due to decreased PV neuron drive, whereas in the ventral HPC it is due to PV neuron loss. Given that the pathology is more prevalent in the PND>240, these data suggest that early intervention may prevent the transition to psychosis later in life (Thompson et al., 2004; Du and Grace, 2013).
Acknowledgments
This work was supported by United States Public Health Service Grants MH57440 (A.A.G.)
We thank Dr. J. Patrick Card and Dr. Linda Rinaman for their assistance with the immunohistochemical protocol. This work was funded by a United States Public Health Service Grant MH57440 (A.A.G.)
Footnotes
Statement of Interest
Competing financial interests. Johnson and Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio (A.A.G.). All other authors have no biomedical financial interests or potential conflicts of interest to report.
References
- Acsady L, Katona I, Gulyas AI, Shigemoto R, Freund TF. Immunostaining for substance P receptor labels GABAergic cells with distinct termination patterns in the hippocampus. J Comp Neurol. 1997;378:320–336. [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Heidbreder C, Feldon J. Hyperactivity and disruption of prepulse inhibition induced by N-methyl-D-aspartate stimulation of the ventral hippocampus and the effects of pretreatment with haloperidol and clozapine. Neuroscience. 2001;103:325–335. doi: 10.1016/s0306-4522(00)00589-3. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Brooks JM, Pershing ML, Thomsen MS, Mikkelsen JD, Sarter M, Bruno JP. Transient inactivation of the neonatal ventral hippocampus impairs attentional set-shifting behavior: reversal with an alpha7 nicotinic agonist. Neuropsychopharmacology. 2012;37:2476–2486. doi: 10.1038/npp.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci P, Mucci A, Merlotti E, Volpe U, Galderisi S. Induced gamma activity and event-related coherence in schizophrenia. Clin EEG Neurosci. 2007;38:96–104. doi: 10.1177/155005940703800212. [DOI] [PubMed] [Google Scholar]
- Caballero A, Diah KC, Tseng KY. Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus. 2013;23:1331–1336. doi: 10.1002/hipo.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2014;219:395–406. doi: 10.1007/s00429-013-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1– 18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012 doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiol Dis. 2013;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. Peripubertal Diazepam Administration Prevents the Emergence of Dopamine System Hyperresponsivity in the MAM Developmental Disruption Model of Schizophrenia. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 2013;33:8352–8358. doi: 10.1523/JNEUROSCI.0306-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold CJ, Lasser MM, Schmid LA, Seidl U, Kong L, Fellhauer I, Thomann PA, Essig M, Schroder J. Hippocampal volume reduction and autobiographical memory deficits in chronic schizophrenia. Psychiatry Res. 2013;211:189–194. doi: 10.1016/j.pscychresns.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered Cortical Expression of GABA-Related Genes in Schizophrenia: Illness Progression vs Developmental Disturbance. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, MacKenzie EM, Yim TT, Taepavarapruk P, Phillips AG. Electrical stimulation of the hippocampus disrupts prepulse inhibition in rats: frequency- and site-dependent effects. Behav Brain Res. 2004;152:187–197. doi: 10.1016/j.bbr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Jiang M, Swann JW. A role for L-type calcium channels in the maturation of parvalbumincontaining hippocampal interneurons. Neuroscience. 2005;135:839–850. doi: 10.1016/j.neuroscience.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Kalus P, Bondzio J, Federspiel A, Muller TJ, Zuschratter W. Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport. 2002;13:713–717. doi: 10.1097/00001756-200204160-00035. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Ziegler W. The role of the dorsal and ventral hippocampus in olfactory working memory. Neurobiol Learn Mem. 2011;96:361–366. doi: 10.1016/j.nlm.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Klarner A, Koch M, Schnitzler HU. Induction of Fos-protein in the forebrain and disruption of sensorimotor gating following N-methyl-D-aspartate infusion into the ventral hippocampus of the rat. Neuroscience. 1998;84:443–452. doi: 10.1016/s0306-4522(97)00475-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- Koenig T, van Swam C, Dierks T, Hubl D. Is gamma band EEG synchronization reduced during auditory driving in schizophrenia patients with auditory verbal hallucinations? Schizophr Res. 2012;141:266–270. doi: 10.1016/j.schres.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165– 173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, DickinsonAnson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. Journal of Neuroscience. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Nitsch R. Morphological evidence that hypothalamic substance P-containing afferents are capable of filtering the signal flow in the monkey hippocampal formation. J Neurosci. 1994;14:4079–4094. doi: 10.1523/JNEUROSCI.14-07-04079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gammaaminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Wahle P, Gutierrez-Igarza K, Albus K. Distribution of neurons expressing substance P receptor messenger RNA in immature and adult cat visual cortex. Exp Brain Res. 1993;97:295– 300. doi: 10.1007/BF00228697. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of setshifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. San Diego: Academic; 1996. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2011;37:1257–1269. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Bernstein HG, Dobrowolny H, Bogerts B, Weiner I, Keilhoff G. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun. 2012;26:353–363. doi: 10.1016/j.bbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Sakai T, Oshima A, Nozaki Y, Ida I, Haga C, Akiyama H, Nakazato Y, Mikuni M. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150. doi: 10.1111/j.1440-1789.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Tohyama K, Matsunaga S, Nakamura M, Tomizawa N, Inoue T, Ogawa H, Ehara S, Ogawa A. MRI identification of dorsal hippocampus homologue in human brain. Neuroreport. 2004;15:2173–2176. doi: 10.1097/00001756-200410050-00005. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behav Brain Res. 2012;226:56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, Harkavy-Friedman J, Malaspina D. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Horvath KD, Menkens KA. Substance P receptor expression by inhibitory interneurons of the rat hippocampus: enhanced detection using improved immunocytochemical methods for the preservation and colocalization of GABA and other neuronal markers. J Comp Neurol. 2001;430:283–305. [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B, Dolan R. Functional segregation within the human hippocampus. Mol Psychiatry. 1999;4:508–511. doi: 10.1038/sj.mp.4000593. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J Neurophysiol. 2009;102:2955–2973. doi: 10.1152/jn.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Hanlon FM, Henning L, Kim YK, Gaudet I, Halim ND. Regulation of sensorimotor gating in rats by hippocampal NMDA: anatomical localization. Brain Res. 2001;898:195–203. doi: 10.1016/s0006-8993(01)02143-6. [DOI] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, Lencz T, Bates J, Crandall DT, Kane JM, Bilder RM. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190– 2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Williams LM, Whitford TJ, Nagy M, Flynn G, Harris AW, Silverstein SM, Gordon E. Emotion-elicited gamma synchrony in patients with first-episode schizophrenia: a neural correlate of social cognition outcomes. J Psychiatry Neurosci. 2009;34:303–313. [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. Prepulse inhibition in rats with temporary inhibition/inactivation of ventral or dorsal hippocampus. Pharmacol Biochem Behav. 2002a;73:929–940. doi: 10.1016/s0091-3057(02)00936-x. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. Effects of hippocampal N-methyl-D-aspartate infusion on locomotor activity and prepulse inhibition: differences between the dorsal and ventral hippocampus. Behav Neurosci. 2002b;116:72–84. doi: 10.1037//0735-7044.116.1.72. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun J, Reynolds GP. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin Med J (Engl) 2002c;115:819–823. [PubMed] [Google Scholar]
- Zierhut KC, Grassmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136:804–814. doi: 10.1093/brain/aws335. [DOI] [PubMed] [Google Scholar]
- Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal Stress Responsivity in a Rodent Developmental Disruption Model of Schizophrenia. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]