Abstract
Background
Youth with family histories of substance use disorders (FH+) are at increased risk for developing substance use disorders relative to those without such histories (FH−). FH+ individuals show deficits in impulse control that parallel those in individuals with substance use disorders. Elucidating how specific components of impulse control are affected in FH+ pre-adolescents would advance our understanding of how deficits in impulse control relate to risk of substance use disorders.
Method
A total of 386 children (305 FH+, 81 FH−; ages 10-12) with no histories of regular alcohol or other drug use were compared on measures of delay discounting (Kirby), response inhibition (GoStop Impulsivity Paradigm), and response initiation impulsivity (Immediate Memory Task). The independent associations between these three behavioral measures of impulsivity and FH status were analyzed using logistic regression models.
Result
FH+ pre-adolescents performed more impulsively on measures of delay discounting and response inhibition impulsivity, but there were no significant group differences on response initiation impulsivity. When the behavioral impulsivity measures were examined simultaneously, delay discounting was most robustly associated with FH status.
Conclusions
These results identify deficits in impulse control present in FH+ pre-adolescents before the onset of regular substance use, and suggest that increased delay discounting may be an important behavioral phenotype for pre-adolescents at risk for substance use involvement.
Keywords: Family History of Substance Use Disorders, Risk, Impulsivity, Pre-Adolescent
1. INTRODUCTION
Across a variety of measures and drug classes, substance use disorders are associated with increased impulsivity (de Wit, 2009; Rogers et al., 2010). However, it is not clear if these deficits result from substance use or are due to pre-existing risk factors that may contribute to problem substance use. To address this question, some researchers have studied individuals with a family history of substance use disorders (FH+), who are at increased risk for developing alcohol and other drug use disorders relative to those without such histories (FH−; Finn et al., 1990; Lieb et al., 2002; McCaul et al., 1990; Merikangas et al., 1998). This risk has a significant genetic basis (Cloninger et al., 1981; Merikangas, 1990; Reich et al., 1998; Slutske et al., 2002), and influences behavior prior to the onset of substance use. In particular, FH+ youth may display greater impulsivity in childhood, which may in turn increase their likelihood of substance use involvement. A better understanding of impulsivity in FH+ youth would help clarify its association with problem substance use.
Impulsivity is a multifaceted construct and different approaches to assessment yield distinct information about impulsivity (de Wit, 2009; Evenden, 1999; Winstanley et al., 2006). One important distinction in impulsivity assessment is that of personality versus behavioral approaches to measurement, which tend to have little association with one another (Cyders and Coskunpinar, 2011; Dougherty et al., 2003b; Lane et al., 2003; Reynolds et al., 2006). This is not surprising, given that personality approaches focus on subjective report of impulsive traits expressed across situations and time, while behavioral approaches examine momentary impulsive performance under particular task demands (any one behavioral task reflecting a narrow, specific impulsive process; Reynolds et al., 2006). Personality measures of impulsivity have been reliably associated with substance use onset and risk; however, there has been less research examining underlying behavioral mechanisms in youth at risk for substance use/misuse (de Wit, 2009). Therefore, the focus of this study is an examination of behavioral impulsivity, using multiple behavioral measures, in children at risk for substance use disorders based on their family history.
It is generally recognized there are at least three core behavioral impulsivity processes: delay discounting, response initiation impulsivity, and response inhibition impulsivity (Dougherty et al., 2009a, 2005). FH+ adults without substance use disorders show increases in each facet of impulsivity that parallel those seen among individuals with substance use disorders (Acheson et al., 2011b; de Wit, 2009; MacKillop, 2013). For example, Delay discounting, or devaluing delayed relative to immediate rewards, is elevated in non-affected FH+ adults as well as individuals with substance use disorders (Acheson et al., 2011b; Kirby and Petry, 2004). Similarly, response initiation impulsivity, or the rapid responding that occurs before complete processing and evaluation of a stimulus, is also elevated both in non-affected FH+ adults and individuals with substance use disorders (Acheson et al., 2011a; Finn et al., 2002; Verdejo-Garcia and Perez-Garcia, 2007). Finally, response inhibition impulsivity, or the failure to inhibit an already-initiated response, is elevated in both populations (Acheson et al., 2011a; Li et al., 2009, 2006; Nigg et al., 2004; Saunders et al., 2008).
Collectively, this research indicates increases in different forms of impulsivity are present in individuals with family histories of substance use disorders, and these deficits parallel those in substance users. However, it is not clear to what extent these processes may be differentially related to FH status, since earlier studies did not typically compare all three measures in the same subjects. As a result, it is not clear which forms of impulsivity are most affected in FH+ subjects and thus may make the greatest contributions to their enhanced risk for developing substance use disorders. Additionally, much of this research has focused on young adults, and typically excluded FH+ individuals with past or present substance use disorders. To improve understanding of the contribution of behavioral impulsivity to the development of substance use disorders, it is necessary to first examine impulsivity among FH+ youth prior to the increase in impulsive and sensation-seeking behavior, including substance use, that occurs during adolescence.
To address these issues, we used a battery of behavioral impulsivity measures in pre-adolescents with and without family histories of substance use disorders before the onset of regular substance use. Previous studies of behavioral impulsivity in FH+ individuals have relied on adult samples (Acheson et al., 2011a) or more general measures of cognitive functioning rather than focused assessments of impulsivity (Nigg et al., 2004). This study is the first to comprehensively assess behavioral impulsivity in FH+ youths prior to adolescence. FH+ and FH− children (10 to 12 years old) were tested with laboratory measures that index three distinct forms of behavioral impulsivity: delay discounting, response inhibition, and response initiation impulsivity. We hypothesized that FH+ pre-adolescents would be more impulsive than FH− children across all measures. Additionally, we sought to examine the magnitude of group differences across the different dimensions of impulsivity to determine which measures are most robustly associated with FH status.
2. METHOD
2.1. Participants
A total of 386 children participated: 305 children with a family history of substance use disorders (FH+; 152 boys, 153 girls) and 81 children with no family history of substance use disorders (FH−; 35 boys, 46 girls). These children and their parents were enrolled in a longitudinal study assessing impulse control development and substance use during adolescence (Ryan et al., Under Review). Family history was established using the Family History Assessment Module (Rice et al., 1995) based on parent report. FH+ participants had at least a biological father with a past or present substance use disorder. FH− participants had no history of substance use disorders among parents or grandparents. Children and their parents were recruited from the community through internet, radio, newspaper, and television advertisements. Exclusion criteria were: regular substance use (defined as substance use at least once per month for 6 consecutive months; Clark et al., 2005), positive urine test at time of screening, low IQ (< 70), or physical/developmental disabilities that would interfere with the ability to understand or complete study requirements. Oppositional Defiant Disorder, Conduct Disorder, ADHD, Dysthymia, or Anxiety Disorders were not exclusionary for the FH+ group because these disorders are commonly co-morbid with substance use involvement and are an expression of the traits that may underlie inherited risk for substance misuse (Iacono et al., 2008). Written informed assent/consent was obtained from children and their parent/guardian before study participation, and the experimental protocol was approved by the Institutional Review Board of The University of Texas Health Science Center at San Antonio.
2.2 Procedure
Potential participants completed a screening visit to determine eligibility and an initial baseline study visit where the impulsivity measures (described below) were completed. Participants provided breath and urine samples to screen for recent substance use upon arrival at the laboratory. No subjects tested positive for alcohol or drug use. Parents and children completed interviews, questionnaires, and behavioral measures separately. The administration of laboratory behavioral measures of impulsivity was counterbalanced, with standardized instructions given before each task. Children and one of their parents were paid approximately $100 each for completing the on-site screening visit and the initial baseline study visit.
2.3. Measures
2.3.1. Screening measures
Participants provided expired-air samples to screen for recent alcohol use (AlcoTest® 7110 MKIII C, Draeger Safety Inc., Durango, CO) and urine samples to screen for recent drug use (THC, cocaine, benzodiazepines, opiates, and amphetamines; Panel/Dip Drugs of Abuse Testing Device, Redwood Biotech, Santa Rosa, CA). Family history classification was based on information collected from the participating parent using the Family History Assessment Module (Janca et al., 1992; Rice et al., 1995). Psychiatric symptoms and diagnoses were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997). Tests were administered by trained research assistants and results reviewed by a staff psychiatrist who is board-certified in child and adolescent psychiatry. Intelligence was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999). Family socioeconomic status was measured using the Four Factor Index of Socioeconomic Status (FFISS; Hollingshead, 1975). Child health was assessed during a physical exam that included a measure of pubertal development (Brooks-Gunn et al., 1987; Petersen et al., 1988). Self-reported impulsivity was assessed using the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995; Stanford et al., 2009).
2.3.2. Delay discounting
The delay discounting procedure has been described previously (Kirby, 2009; Kirby et al., 1999) and consisted of 27 choices between smaller, immediate and larger, delayed amounts of money such as, “Would you prefer (a) $34 today or (b) $50 in 30 days?” The magnitude of the delayed choice varied; 9 of the choices offered small amounts of delayed money ($25–$35), 9 offered medium amounts of delayed money ($50–$60), and 9 offered large amounts of delayed money ($75–$85). Typically, delay discounting increases as reward magnitude increases. Discount rate estimates (k) based on the hyperbolic discounting function of Mazur (1987) were estimated for each participant based on the pattern of choices across the small, medium, and large amount of money. An average value for all items was also calculated. Possible values of k ranged from 0.00016 (choosing all delayed options) to 0.25 (choosing all immediate options).
2.3.3. Immediate Memory Task (IMT)
The IMT (Dougherty et al., 2003a, 2002) is a go/no go task used to measure response initiation impulsivity. In this procedure, a series of 5-digit numbers appear on a computer monitor in black text on a white background. Numbers are randomly generated and appear for 500 msec at a rate of one per second. The children were instructed to respond when the 5-digit number they saw was identical to the one that preceded it. This task yielded two variables of interest: (1) Correct Detections, how often children correctly responded to a 5-digit number identical to the preceding number; and (2) Commission Errors, how often children responded to a 5-digit number that differed from the preceding number by only one digit (its position and value determined randomly). In this 11 minute session, 600 trials were delivered, there were equal numbers of trials where Correct Detections and Commission Errors were possible (i.e., 33% of nonconsecutive trials). The primary dependent measure for this task was the IMT Ratio (proportion of Commission Errors relative to Correct Detections).
2.3.4. GoStop impulsivity paradigm (GoStop)
The GoStop (Dougherty et al., 2005) is a stop signal task used to measure response inhibition impulsivity. Similar to the procedure for the IMT, a series of 5-digit numbers are displayed for 500 msec with a 1,500 msec inter-stimulus interval. The children were instructed to respond (mouse click) when a 5-digit number matched the previously presented 5-digit number and remained black. Children were instructed to withhold responding to numbers that did not match the previous stimuli, or a consecutive matching number that changed from black to red after presentation (i.e., the “stop signal” cue). The stop signal (color of the 5-digit number changes from black to red) occurred after 50, 150, 250, or 350 msec. There were two dependent measures of interest: (1) Go Responses, how often children responded to a 5-digit number that matched the preceding number (and the number remained black); and (2) Inhibition Failures, how often children failed to withhold responding to a matching 5-digit number when a stop signal appeared. The primary dependent measure for this task was the GoStop Ratio (proportion of Inhibition Failures relative to Go Responses). Inhibition Failures from the 150 ms stop delay best discriminate between impulsive and control groups (e.g., Dougherty et al., 2003a, 2009b), so responses to the 150 ms trials are the primary focus of analyses.
2.4. Data analyses
Due to right skewed distributions for k values, differences between FH+ and FH− children were evaluated using the nonparametric Mann-Whitney U test for continuous variables. Categorical variables were compared using the Fisher's exact test or chi-square test. Because there is no suitable nonparametric test to examine within-subject effects, repeated measures ANOVA was used to examine the effect of magnitude (small, medium, large), group (FH+, FH−), and their interaction on average k values for delay discounting. The k values were given a natural log transformation before the repeated measures ANOVA analysis to correct for skewness.
Impulsivity is a multi-dimensional construct that requires multiple assessment approaches to adequately describe it. To better differentiate which aspects of behavioral impulsivity are most strongly associated with FH status, we first examined the magnitude of group differences across the three primary impulsivity measures (average k for delay discounting, IMT ratio, and GoStop ratio) was determined by calculating standardized mean differences (Higgins and Green, 2011). We next tested whether these measures of impulsivity were independently associated with FH status by adding them sequentially to logistic regression models, with FH group as the dependent variable, after adjusting for IQ, socioeconomic status, and ethnicity. The impulsivity measure with the largest standardized mean difference (i.e., that differed the most between FH+ and FH− children) was entered into the logistic model first, followed by impulsivity measures with smaller standardized mean differences. Appropriate data transformations were applied when needed. The area under the receiver operating characteristic (ROC) curve, known as the AUC, was used to assess the accuracy of each fitted logistic regression model in terms of discriminating from FH+ to FH−. Differences between AUCs were evaluated using a nonparametric U-statistic method (DeLong et al., 1988).
We then analyzed the effects of psychiatric comorbidity on impulsive performance within the FH+ group by inspecting distributions of impulsive performance, group comparisons of FH+ children with and without DSM-IV diagnoses made with Mann-Whitney U tests, and logistic regressions comparing presence or absence of diagnoses.
All analyses were performed using R 2.15.3 (The R Foundation for Statistical Computing, Vienna, Austria). A small percentage of participants (ranging from 1-3% of the sample per measure) provided invalid or incomplete data and are excluded from analyses of those variables.
3. RESULTS
3.1. Participants
Participant demographic characteristics, current DSM-IV-TR (APA, 2000) diagnoses, and history of substance use are reported in Table 1. The FH+ and FH− groups did not differ in age, pubertal development, gender, or race, but FH+ participants did have lower socioeconomic status and were more likely to be of Hispanic ethnicity. FH+ children self-reported significantly more attentional impulsivity, non-planning, and total impulsivity on the BIS-11 than did FH− children. FH+ participants also had lower IQs, although both groups were well within the average range, and some FH+ participants met criteria for DSM-IV-TR disorders.
Table 1.
Demographic information for the cohort.
| FH- (N= 81) | FH+ (N= 305) | ||
|---|---|---|---|
| Median [Q1, Q3] | Median [Q1, Q3] | p-value | |
| Age | 11.62 [10.71, 12.36] | 11.35 [10.7, 12.28] | 0.441 |
| Pubertal development | 2.0 [1.6, 2.6]2 | 2.0 [1.6, 2.4] | .901 |
| Intelligence | 101 [94, 111] | 95 [86, 101] | < 0.0011 |
| Socioeconomic Status | 43.5 [35, 50] | 31.5 [22, 40] | < 0.0011 |
| BIS-11 | |||
| Attentional | 14 [11, 17] | 15 [13, 18]2 | .02 |
| Motor | 21 [19, 23] | 22 [19, 24]3 | .15 |
| Non-planning | 25 [21, 28] | 26 [23, 30] | .03 |
| Total | 60 [55, 66] | 64 [57, 70]4 | .005 |
| n (%) | n (%) | ||
|---|---|---|---|
| Gender | 0.295 | ||
| Boys | 35 (43.3) | 152 (49.8) | |
| Girls | 46 (56.7) | 153 (50.2) | |
| Race | 0.35 | ||
| African-American | 5 (6.1) | 37 (12.1) | |
| Caucasian | 74 (91.4) | 261 (85.6) | |
| Other | 2 (2.5) | 7 (2.3) | |
| Ethnicity | 0.0455 | ||
| Hispanic/Latino | 57 (70.4) | 246 (80.7) | |
| Non-Hispanic/Latino | 24 (29.6) | 59 (19.3) | |
| Current Psychiatric Disorders | |||
| ADHD | 0 (0) | 89 (29.2) | < 0.0016 |
| ODD | 0 (0) | 29 (9.5) | 0.0016 |
| Conduct Disorder | 0 (0) | 2 (0.7) | 16 |
| GAD | 0 (0) | 16 (5.3) | 0.036 |
| SAD | 0 (0) | 12 (3.9) | 0.086 |
| Specific Phobia | 0 (0) | 9 (3.0) | 0.216 |
| Alcohol & Drug | |||
| Alcohol | 2 (2.5) | 10 (3.3) | 16 |
| Marijuana | 0 (0) | 2 (0.7) | 16 |
| Cigarettes | 1 (1.2) | 4 (1.3) | 16 |
| Other | 0 (0) | 0 (0) | 16 |
ADHD = Attention Deficit Hyperactivity Disorder: FH+= family history of substance use disorders (SUDs); FH-= no family history of SUDs; GAD = Generalized Anxiety Disorder; ODD = Oppositional Defiant Disorder; SAD = Separation Anxiety Disorder; Q1=first quartile; Q3=third quartile
p values were computed based on Mann-Whitney U test for testing the differences between FH+ and FH-.
Missing 1 observation
Missing 3 observations
Missing 4 observations
p values were computed based on Chi-squared test for testing the differences between FH+ and FH-.
p values were computed based on Fisher's exact test for testing the differences between FH+ and FH-.
3.2 Parental history of substance use
FH+ parents reported substance use disorders associated with a range of substances, including: alcohol, amphetamines, cannabis, cocaine, hallucinogens, inhalants, opioids, and sedatives/hypnotics. On average, FH+ fathers had two substance use diagnoses. A substantial minority (28.7%) of FH+ families had both a father and a mother with a substance use disorder. Alcohol dependence was the most prevalent diagnosis for both fathers (69.8%) and mothers (14.5%), followed by cocaine (Fathers: 42.9%; Mothers: 9.5%) and cannabis (Fathers: 32.0%; Mothers: 6.5%) dependence. Due to our exclusion criteria, no FH− fathers or mothers had a current or past substance use disorder diagnosis.
3.3. Group differences on behavioral impulsivity measures
Summary statistics (median, first quartile, and third quartile) of all impulsivity measures by family history status and p values (based on Mann-Whitney U test for group comparisons) are shown in Table 2.
Table 2.
Group differences on behavioral impulsivity measures.
| FH– (N=81) | FH+ (N=305) | ||
|---|---|---|---|
| Median [Q1, Q3] | Median [Q1, Q3] | p-value1 | |
| Average k | 0.02 [0.005, 0.04] | 0.03 [0.01, 0.08] | .003 |
| IMT | |||
| Correct Detections | 75.52 [67.5, 85.9]2 | 70.18 [61.0, 80.5]4 | .002 |
| Commission Errors | 50.85 [43.8, 58.8] | 48.31 [39.1, 57.4]5 | .07 |
| Ratio | 69.3 [62.0, 74.3]2 | 69.32 [60.23, 77.4]6 | .34 |
| GoStop | |||
| Go Responses | 69.5 [65, 74]2 | 66.5 [60, 73]3 | .004 |
| Inhibition Failures | 8 [6, 11] | 9 [6, 11]3 | .19 |
| Ratio | 44.44 [32.8, 61.7]2 | 53.73 [37.4, 72.7]3 | .04 |
P values were computed based on Mann-Whitney U test for testing the differences between FH+ and FH-.
Missing 1 observations
Missing 7 observations
Missing 10 observations
Missing 2 observations
Missing 11 observations
3.3.1. Delay discounting
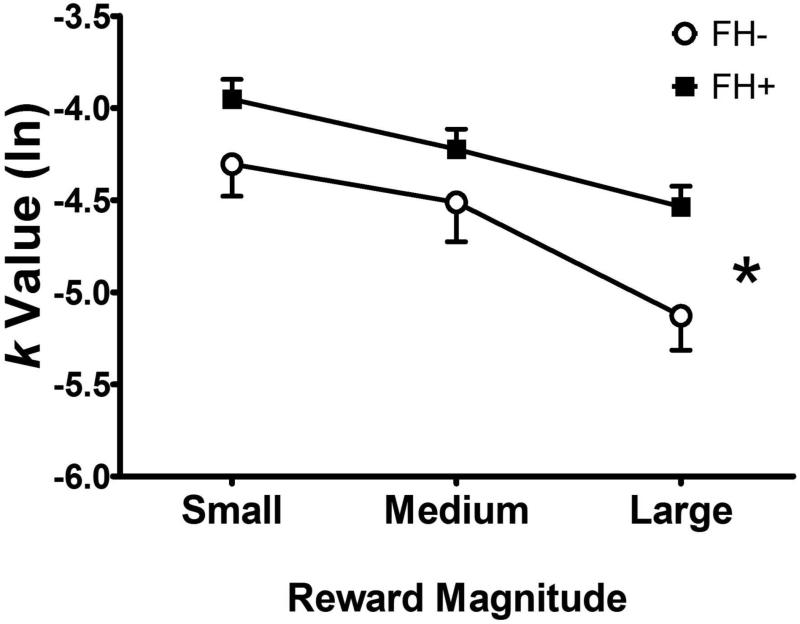
FH+ children had higher average k values than FH− children (p = 0.003, Table 2). Similarly, repeated ANOVA showed that FH+ children had higher overall k values than FH− children [F (1,384) = 4.36, p = 0.04], indicating greater discounting of delayed rewards (see Figure 1). As expected, k values declined with increasing amounts of delayed money [F(2, 768) = 21.68, p <.001], indicating smaller delayed rewards were discounted more steeply than larger delayed rewards. There was no significant interaction between FH status and reward size (F(2, 278)=1.09, p=0.34).
Figure 1.
Comparison of children with family histories of substance use disorders (FH+) or without such histories (FH−) on delay discounting (indexed by k values). FH+ children discounted delayed rewards more steeply than FH− children [F (1,384) = 4.36, p = 0.04], and all children discounted less with increasing reward magnitude [F(2, 768) = 21.68, p <.001].
3.3.2. IMT
FH+ children had fewer Correct Detections (p = 0.002, Table 2), and a trend towards decreased Commission Errors (p = 0.07). However, there were no significant group differences in the ratio of errors to correct detections (IMT Ratio; p=0.34).
3.3.3. GoStop
FH+ children had significantly higher GoStop Ratios for the primary outcome variable of interest, the 150 msec trials (p = 0.04, Table 2), indicating more failures to inhibit responses following a stop signal relative to correct responses. They also had fewer Go Responses on these trials (p = 0.004), but there were no significant group differences in Inhibition Failures (p=0.19; Table 2). FH+ children had similarly increased ratios for the 50 msec trials (FH−: median: 24.1; interquartile range: 10.6, 35.2; FH+: median: 31.3; interquartile range: 13.8, 51.4; p, = .006). As expected, the groups did not differ on ratios of inhibited vs. correct responses for the 250 msec (FH−: median: 71.7; interquartile range: 58.4, 80.9; FH+: median: 75.0; interquartile range: 58.1, 87.7; p, = .18) or 350 msec trials (FH−: median: 84.5; interquartile range: 76.9, 97.0; FH+: median: 88.9; interquartile range: 76.9, 97.0; p, = .29).
3.4. Comparisons of Behavioral Impulsivity Measures
GoStop Ratios and IMT Ratios were modestly correlated (r = .29, p < .001; Table 3), none of the other behavioral impulsivity tasks were significantly associated with one another (all ps > .09). Scores for average k, GoStop ratio, and IMT ratio were standardized and compared using standardized mean differences (SMD; Table 4). The largest SMD was for average k and GoStop ratio. IMT ratio did not differ significantly between groups, and showed the smallest group difference compared to the other measures.
Table 3.
Pearson's Correlations Among Behavioral Impulsivity and Demographic Variables.
| Delay Discounting | GoStop Ratio | IMT Ratio | IQ | SES | |
|---|---|---|---|---|---|
| GoStop Ratio | .061 | ||||
| IMT Ratio | .087 | .292* | |||
| IQ | −.113* | −.043 | −.119* | ||
| SES | −.046 | −.071 | −.081 | .424* | |
| Ethnicity | −.012 | −.014 | .028 | −.150* | −.280* |
= p ≤ 0.05
Table 4.
Comparison of behavioral impulsivity measures.
| Variable | SMD | (95% CI) |
|---|---|---|
| Delay Discounting | 0.34 | (0.10, 0.59) |
| GoStop Ratio | 0.25 | (0.004, 0.50) |
| IMT Ratio | 0.15 | (−0.10, 0.40) |
SMD = standardized mean differences
3.5. Impulsivity Association with FH group
Based on the results in Table 3, after controlling for IQ, socioeconomic status, and ethnicity, sequential models were fitted adjusting additively for average k, GoStop ratio, and IMT ratio. After adjusting for IQ and demographic variables, delay discounting was associated with family history status, whereas GoStop ratio and IMT ratio did not have significant independent associations with family history of substance use disorders (Table 5). The model with delay discounting and IQ (Model 1 in Table 5) had an AUC of 0.780, as good as the full model including all three impulsivity measures (Model 3) in terms of discriminating from FH+ from FH−.
Table 5.
Independent prediction of FH status using behavioral impulsivity measures.
| Model | Covariates | Coefficient | SE | 95% CI | P value | AUC |
|---|---|---|---|---|---|---|
| 1 | Delay Discounting | 0.191 | 0.084 | [0.026, 0.356] | 0.023 | 0.780 |
| IQ | −0.025 | 0.013 | [−0.049, −0.0002] | 0.048 | ||
| SES | −0.074 | 0.014 | [−0.101, −0.048] | <0.001 | ||
| Ethnicity | −0.041 | 0.326 | [−0.679, 0.597] | 0.899 | ||
| 2 | Delay Discounting | 0.19 | 0.084 | [0.025, 0.355] | 0.024 | 0.780 |
| GoStop Ratio | 0.002 | 0.004 | [−0.006, 0.01] | 0.659 | ||
| IQ | −0.025 | 0.013 | [−0.05, −0.0049] | 0.046 | ||
| SES | −0.073 | 0.014 | [−0.1, −0.047] | <0.001 | ||
| Ethnicity | −0.031 | 0.327 | [−0.672, 0.609] | 0.923 | ||
| 3 | Delay Discounting | 0.191 | 0.084 | [0.026, 0.356] | 0.023 | 0.781 |
| GoStop Ratio | 0.002 | 0.004 | [−0.006, 0.01] | 0.628 | ||
| IMT Ratio | −0.002 | 0.005 | [−0.013, 0.008] | 0.668 | ||
| IQ | −0.025 | 0.013 | [−0.05, −0.0001] | 0.049 | ||
| SES | −0.073 | 0.014 | [−0.1, −0.047] | <0.001 | ||
| Ethnicity | −0.035 | 0.327 | [−0.676, 0.606] | 0.915 | ||
3.5.1. FH+ and psychiatric diagnoses
Visual inspections indicated that performance distributions on the delay discounting and GoStop tasks were very similar between FH+ children with and without DSM-IV psychiatric diagnoses. Direct comparisons between FH+ children with and without DSM-IV diagnoses using Mann-Whitney U-tests found no significant differences on k values or GoStop performance (p values > 0.1). Finally, we repeated our regression model with psychiatric status in place of FH status and found no significant association between delay discounting and having a DSM-IV disorder (p = 0.4).
4. DISCUSSION
To our knowledge, this is the first report comparing multiple behavioral measures of impulsivity in FH+ and FH− pre-adolescents. FH+ pre-adolescents performed more impulsively on a measure of delay discounting and, to a lesser degree, response inhibition impulsivity. However, there were no significant group differences on response initiation impulsivity between FH+ and FH− pre-adolescents. Similarly, among behavioral impulsivity measures, delay discounting was most robustly associated with FH status. The results of this study indicate deficits in impulse control are present in some FH+ individuals before regular substance use begins. Further, the evidence suggests that increased delay discounting may be an important component of the behavioral phenotype of pre-adolescents who are at risk for substance use involvement.
Increased discounting of delayed rewards has been observed among individuals with alcohol, stimulant, tobacco, and opiate use disorders (Coffey et al., 2003; Hoffman et al., 2006; Madden et al., 1997; Mitchell et al., 2005; Petry, 2001; Reynolds et al., 2004), and in FH+ adults without substance use disorders (Acheson et al., 2011b). Three previous studies using similar delay discounting measures failed to find significant differences between adults with and without family histories of alcohol use disorders (Crean et al., 2002; Herting et al., 2010; Petry et al., 2002), however these studies used small sample sizes and/or did not rule out family histories of other substance use disorders in the FH− groups (MacKillop, 2013). In contrast, the Acheson et al. (2011b) study had a large sample size and did rule out other substance use disorders in the FH− group, similar to our approach in the present study. Similar to the present findings, we previously observed that among a battery of cognitive and behavioral laboratory measures, increased impulsivity on a delayed reward choice procedure was among the best identifiers of heavy adolescent marijuana use (Dougherty et al., 2013), and increased delay discounting in children and adolescents is associated with later development of problem substance use (Audrain-McGovern et al., 2009; Ayduk et al., 2000). Collectively, these results indicate that increased delay discounting may precede regular substance use and could be a risk factor for later development of substance use disorders.
In contrast to the findings for delay discounting, FH+ pre-adolescents showed only modest increases in response inhibition impulsivity and no group differences in response initiation impulsivity. Furthermore, neither response initiation nor response inhibition impulsivity were significantly associated with FH status. These results contrast with previous findings of increased response initiation and response inhibition impulsivity in FH+ adults using the same tasks (Acheson et al., 2011a) and in FH+ youths tested with a potentially less cognitively demanding response inhibition task (Nigg et al., 2004). The low rates of correct detections on the GoStop and the IMT in the present study suggests there may have been a floor effect in performance. Although we have previously demonstrated a relationship of substance misuse with performance on these tasks among adolescents (i.e., 13+ years; Dougherty et al., 2007, 2013), this is the first study we are aware of to use these tasks in children as young as 10 years of age. The task parameters might have been difficult for children in this age range; however, they will be appropriate for testing developmental changes in performance as the cohort matures into adolescence and even adulthood. Consequently, more robust deficits in FH+ individuals may emerge as overall performance on these measures improves. Given previous research demonstrating that poor response inhibition in mid-adolescence is predictive of later substance use problems (Nigg et al., 2006), the performance of FH+ youth as they progress through mid-adolescence may be particularly relevant for their substance abuse risk.
This study had important strengths and limitations. Strengths include a large, well-characterized sample of FH+ and FH− pre-adolescents evaluated at an important developmental period immediately preceding increases in impulsive and risk-taking behavior that can include the initiation of regular substance use. We did not exclude FH+ individuals with Oppositional Defiant Disorder, Conduct Disorder, ADHD, Dysthymia, or Anxiety Disorders because these disorders are common in families with substance use disorders, and are associated with increased risk for developing problem substance use. Even FH+ individuals who do not meet criteria for these disorders still tend to have greater externalizing and internalizing disorder symptoms (Iacono et al., 2008; Tarter, 2002). As others have noted, excluding co morbidities in studies of impulsivity and substance use risks biasing sample selection in a manner that omits the very behavior of interest (Verdejo-Garcia et al., 2008). Additionally, our follow-up analyses indicated the presence of these disorders did not drive the FH group effects on behavioral impulsivity. Limitations include a lack of substance use outcome data for the participants. However, we are currently monitoring these same individuals in an ongoing longitudinal study.
The delay discounting outcomes of this study are supportive of the Common Liability Model of substance use risk, which proposes children enter adolescence with varying levels of predisposition, or liability, for behavioral undercontrol (Iacono et al., 2008; Vanyukov et al., 2009, 2003). Our findings potentially inform this model by providing more detail on which impulsive processes are most robustly affected in children at risk based on family history. Specifically, increased delay discounting may be part of the mechanism of family transmission of substance use disorder liability. Because we are monitoring these children prospectively, we will be able to examine to what extent delay discounting, response inhibition, and response initiation impulsivity predict the initiation and progression of substance use and substance use disorders across adolescence, and how these processes are affected by substance use.
If delay discounting does contribute to problem substance use, it may be beneficial to screen and target pre-adolescents who score high on these measures for preventive interventions, and there are potential means to do so. Previous work has demonstrated that delay discounting can be reduced by training individuals to consider their reward choices as a linked rather than individual serial selections (Kirby and Guastello, 2001). This method involved training to view a current reward-delay choice as setting a precedent that predicts future choices. The training then shifts the focus to overall preference across many choices, rather just the current smaller-sooner versus larger-later selection (Kirby and Guastello, 2001). Thus delay discounting may be a feasible and effective target for interventions to reduce the likelihood of future substance use involvement.
Highlights.
Preadolescents with family histories of substance use disorders are more impulsive
Delay discounting was most robustly associated with family history
Increased impulsivity is present before the onset of regular substance use
Increased delay discounting may be a marker for substance use risk
Acknowledgements
Allison Ford, Marika Vela-Gude, David Hernandez, Anran Xu, Jessica Gutierrez-Barr, and Amanda Paley performed data collection. Martin Goros provided technical assistance to Dr. Liang.
Role of funding source
Research reported in this publication was supported by NIDA of the National Institutes of Health under award numbers R01-DA026868, R01-DA033997, and T32-DA031115. Dr. Dougherty is also supported by the William and Marguerite Wurzbach Distinguished Professorship. The content is solely the view of the authors and does not necessarily represent the official view of any of the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Donald Dougherty, Charles Mathias, and Ashley Acheson designed the study and wrote the protocol. Drs. Nora Charles and Ashley Acheson managed the literature searches and summaries of previous related work. Drs. Ryan and Rene Olvera made final determination of psychiatric diagnoses. Dr. Liang managed the statistical analyses. Drs. Acheson and Charles wrote the initial draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors have no conflict of interests to declare.
REFERENCES
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug Alcohol Depend. 2011a;117:198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcohol. Clin. Exp. Res. 2011b;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: strategic self-regulation for coping with rejection sensitivity. J. Person. Soc. Psychol. 2000;79:776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls' pubertal status. Child Dev. 1987;58:829–841. [PubMed] [Google Scholar]
- Clark DB, Cornelius JR, Kirisci L, Tarter RE. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 2005;77:13–21. doi: 10.1016/j.drugalcdep.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross fostering analysis of adopted men. Arch. Gen. Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav. Brain Res. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: is there overlap in nomothetic span and construct representation for impulsivity? Clin. Psychol. Rev. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC. Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J. Child Psychol. Psychiatry. 2003a;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behav. Res. Methods Instrum. Comput. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, Shannon EE, Acheson A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology (Berl.) 2013;226:307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Liguori A, Marsh DM, Dawes MA, Moeller FG. Behavioral impulsivity in adolescents with conduct disorder who use marijuana. Addict. Disord. Their Treat. 2007;6:43–50. [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM, Furr RM, Nouvion SO, Dawes MA. Distinctions in behavioral impulsivity: implications for substance abuse research. Addict. Disord. Their Treat. 2009a;8:61–73. doi: 10.1097/ADT.0b013e318172e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM, Prevette KN, Dawes MA, Hatzis ES, Palmes G, Nouvion SO. Impulsivity and clinical symptoms among adolescents with non-suicidal self-injury with or without attempted suicide. Psychiatry Res. 2009b;169:22–27. doi: 10.1016/j.psychres.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM. Laboratory measures of impulsivity. In: Coccaro EF, editor. Aggression: Psychiatric Assessment and Treatment Medical Psychiatric Series. Marcel Dekker Publishers; New York: 2003b. pp. 247–265. [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav. Res. Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Impulsivity: a discussion of clinical and experimental findings. J. Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kleinman I, Pihl RO. The lifetime prevalence of psychopathology in men with multigenerational family histories of alcoholism. J. Nerv. Ment. Dis. 1990;178:500–504. [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol. Clin. Exp. Res. 2002;26:186–206. [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol. Clin. Exp. Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Wiley; Chichester: 2011. [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl.) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven, CT.: 1975. [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu. Rev. Clin. Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Janca A, Bucholz K, Janca I. Family History Assessment Module. Washington University School of Medicine; St. Louis, MO.: 1992. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychon. B. Rev. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Guastello B. Making choices in anticipation of similar future choices can increase self-control. J. Exp. Psychol. Appl. 2001;7:154–164. doi: 10.1037//1076-898x.7.2.154. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhoades HM, Pietras CJ, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: implications in substance abuse and dependence. Addict. Disord. Their Treat. 2003;2:33–40. [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcohol. Clin. Exp. Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol. Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- MacKillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J. Exp. Anal. Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp. Clin. Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons JE, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analysis Of Behavior: Vol 5 The Effect Of Delay And Of Intervening Events On Reinforcement Value. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE, Cromwell CC. Alcohol and drug use by college males as a function of family alcoholism history. Alcohol. Clin. Exp. Res. 1990;14:467–471. doi: 10.1111/j.1530-0277.1990.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR. The genetic epidemiology of alcoholism. Psychol. Med. 1990;20:11–22. doi: 10.1017/s0033291700013192. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch. Gen. Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol. Clin. Exp. Res. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J. Abnorm. Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J. Abnorm. Psychol. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J. Stud. Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Wechsler Abbreviated Scale of Intelligence (WASI) Manual. Harcourt Brace and Company; San Antonio, TX.: 1999. Psychological. [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, et al. Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. Am. J. Med. Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers. Individ. Dif. 2006;40:305–315. [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav. Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol. Clin. Exp. Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: implications for alcoholism. Alcohol. Clin. Exp. Res. 2010;34:1319–1333. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Sorocco KH, Lovallo WR. Impulsive errors on a go-nogo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol. Clin. Exp. Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J. Abnorm. Psychol. 2002;111:124–133. [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers. Individ. Dif. 2009;47:385–395. [Google Scholar]
- Tarter RE. Etiology of adolescent substance abuse: a developmental perspective. Am. J. Addict. 2002;11:171–191. doi: 10.1080/10550490290087965. [DOI] [PubMed] [Google Scholar]
- Vanyukov M, Kirisci L, Moss L, Tarter R, Reynolds M, Maher B, Kirillova G, Ridenour T, Clark D. Measurement of the risk for substance use disorders: phenotypic and genetic analysis of an index of common liability. Behav. Genet. 2009;39:233–244. doi: 10.1007/s10519-009-9269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci. Biobehav. Rev. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl.) 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin. Psychol. Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]