Abstract
BACKGROUND
The clinical epidemiology of venous thromboembolism has changed recently due to advances in identification, prophylaxis, and treatment. We sought to describe secular trends in occurrence of venous thromboembolism among residents of the Worcester, Massachusetts, metropolitan statistical area (WMSA).
METHODS
Population-based methods were used to monitor trends in event rates of first-time or recurrent venous thromboembolism in 5025 WMSA residents diagnosed with acute pulmonary embolism and/or lower-extremity deep vein thrombosis during 9 annual periods between 1985 and 2009. Medical records were reviewed by abstractors and validated by clinicians.
RESULTS
Age- and sex-adjusted annual event rates for first-time venous thromboembolism increased from 73 (95% CI 64–82) per 100,000 in 1985/1986 to 133 (122–143) in 2009, due mostly to an increase in pulmonary embolism. The rate of recurrent venous thromboembolism decreased from 39 (32–45) in 1985/1986 to 19 (15–23) in 2003, and then increased to 35 (29–40) in 2009. There was an increasing trend in using non-invasive diagnostic testing, with about half of tests being invasive in 1985/1986 and almost all non-invasive by 2009.
CONCLUSIONS
Despite advances in identification, prophylaxis, and treatment between 1985 and 2009, the annual event rate of venous thromboembolism has increased and remains high. While these increases may be partially due to increased sensitivity of diagnostic methods, especially for pulmonary embolism, it may also imply that current prevention and treatment strategies are less than optimal.
Keywords: venous thromboembolism, venous thrombosis, pulmonary embolism, incidence, outcomes research
Venous thromboembolism, comprising deep vein thrombosis and pulmonary embolism (, is associated with increased long-term morbidity, functional disability, and all-cause mortality.1 Over three decades ago, venous thromboembolism was estimated to be the third most common acute cardiovascular event after the acute coronary syndromes and ischemic stroke.2 Recent data on the clinical epidemiology of venous thromboembolism are, however, limited.3
Considerable variation exists in estimates of the annual incidence rates of venous thromboembolism, derived from population-based studies and hospital discharge or health-insurance claims databases.3 Major advances have occurred in identifying patients at increased risk for venous thromboembolism, in thromboprophylaxis, and in diagnostic methods and treatments.3–9 Growing awareness of venous thromboembolism as an important public-health problem became the impetus for evidence-based guidelines for appropriate prevention and treatment, which have been revised over time.10–11 These advances have likely influenced the reported frequency of venous thromboembolism.
Using data from the Worcester venous thromboembolism study (1985 to 2009), we describe 25-year trends in event rates, patient characteristics, and use of different diagnostic approaches among residents of the Worcester, Massachusetts, metropolitan statistical area (WMSA) diagnosed with clinically recognized acute venous thromboembolism.
METHODS
The Worcester venous thromboembolism study employed population-based surveillance methods to monitor trends in event rates of first-time or recurrent episodes of pulmonary embolism and/or deep vein thrombosis, including management strategies, case-fatality rates, and recurrences after the index event among WMSA residents.12–15 Reflecting the evolution of the standard care of acute venous thromboembolism, Cohort-I included all hospital inpatients discharged with a primary/secondary diagnosis of venous thromboembolism during two 18-month periods, July 1985 to December 1986, and July 1988 to December 1989. Cohort-II included hospitalized patients and outpatients diagnosed with venous thromboembolism based on outpatient, emergency department, radiology department, or diagnostic laboratory encounter during 1999, 2001, 2003, 2005, 2007, and 2009. Medical records were reviewed by trained abstractors and validated by clinicians.
This study was approved by the institutional review committee at participating hospitals.
Venous thromboembolism Definition
Both cohorts used International Classification of Disease, 9th revision, codes to identify eligible acute cases of pulmonary embolism and/or deep vein thrombosis (Table S1). There were slight differences in our study populations due to the refining of these codes over the years. In addition, Cohort-II included patients diagnosed with upper-extremity deep vein thrombosis alone. These were excluded in the present analyses due to important differences in the natural history of upper-extremity and lower-extremity deep vein thrombosis.16–17
Patients were classified as either ‘first-time’ if the index event was a first-time episode, or as ‘recurrent’ at index visit if the patient had a prior episode of venous thromboembolism noted in their medical records.
Data Analysis
Annual event rates of venous thromboembolism are reported per 100,000 population. The number of first-time episodes served as the numerator for calculation of event rates of first-time venous thromboembolism (incidence rate), while the number of recurrent episodes served as the numerator for calculation of the event rates of recurrent venous thromboembolism. The 1985 United States (US) Census data of the WMSA (n=379,953) were used as the denominator for calculation of 1985–1989 annual crude event rates and 2000 Census data of the WMSA (n=477,598) were used as the denominator for calculation of 1999–2009 annual crude event rates.12, 14 These crude rates were used to calculate age- and sex-adjusted rates by the direct adjustment method.18 Since the WMSA population was approximately 90% white during the years under study, the age and sex distribution of the 2000 United States white (reference) population was used to calculate age- and sex-adjusted rates.19 Confidence interval (CI) estimates were based on the Poisson distribution. Trends in rates during the years under study were assessed by Poisson regression. Annual case counts were modeled using the SAS procedure GENMOD with a logarithmic link function and a log (population) offset term. A main effects model included a term for sex, age group (<40, 40–49, 50–59, 60–69, 70–79, ≥80 years), and study period. Separate models were constructed for episodes of first-time or recurrent venous thromboembolism, overall, and separated into pulmonary embolism (with or without deep vein thrombosis) and deep vein thrombosis alone.
Patient characteristics and diagnostic tests are reported as frequencies and percentages for categorical variables, and as means (standard deviations) or medians (interquartile ranges) for continuous variables. The Cochran-Armitage tests and linear regression models were used to test for linear trends over time among categorical variables and continuous variables, respectively. Comparison of the 2009 cohort with the 1985/86 cohort was performed using the chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC) and statistical significance level was pre-specified as α=.05 (two-sided).
RESULTS
During the study period (1985–2009), 5487 WMSA residents were diagnosed with acute venous thromboembolism (1235 from Cohort-I, 4252 from Cohort-II). After excluding 462 (10.9%) patients diagnosed with upper-extremity deep vein thrombosis alone in Cohort-II, 5025 patients with a diagnosis of acute pulmonary embolism or lower-extremity deep vein thrombosis alone were examined in the present analyses. This included 3887 (77.4%) first-time venous thromboembolism and 1138 (22.6%) recurrent venous thromboembolism. Increases in the proportion of first-time venous thromboembolism were observed over time from approximately two-thirds in the initial cohort to nearly 80% in the 2009 cohort (trend P<.001, Table 1).
Table 1.
Characteristics of patients with venous thromboembolism in the Worcester venous thromboembolism study (1985–2009)
| Study year | P value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1985/1986 (n=617) |
1988/1989 (n=618) |
1999 (n=539) |
2001 (n=597) |
2003 (n=554) |
2005 (n=608) |
2007 (n=698) |
2009 (n=794) |
Trend test | 1985/86 vs 2009 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First-time venous thromboembolism at index visit, n (% of total venous thromboembolism) | 405 (65.6) | 442 (71.5) | 435 (80.7) | 482 (82.4) | 466 (84.1) | 482 (79.3) | 542 (77.7) | 633 (79.7) | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Demographic characteristics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years) | .001 | .04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean (standard deviation) | 65.9 (17.2) | 66.4 (17.4) | 66.4 (17.7) | 65.8 (17.3) | 63.0 (17.9) | 62.7 (18.9) | 65.0 (18.1) | 63.7 (17.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (interquartile range) | 69 (60–78) | 70 (60–78) | 72 (54–80) | 69 (54–81) | 65 (50–78) | 65 (48–79) | 68 (52–80) | 65 (50–80) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men (%) | 48.6 | 45.5 | 43.4 | 41.9 | 46.8 | 41.3 | 47.6 | 44.6 | .34 | .75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| White (%) | 97.5 | 98.2 | 95.4 | 93.5 | 93.6 | 94.8 | 95.7 | 92.5 | <.001 | .001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recent* medical characteristics (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body mass index class | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| <25 kg/m2 | 42.1 | 44.2 | 35.3 | 32.9 | 26.0 | 36.5 | 28.9 | 26.9 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25–30 kg/m2 | 35.1 | 28.8 | 27.4 | 31.4 | 33.3 | 28.4 | 35.2 | 29.5 | .80 | .11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| >30 kg/m2 | 22.8 | 27.1 | 37.3 | 35.6 | 4.7 | 35.1 | 35.9 | 43.6 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Congestive heart failure | 20.2 | 11.5 | 13.1 | 14.9 | 11.2 | 10.0 | 9.2 | 7.4 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Myocardial infarction | 4.9 | 3.4 | 5.1 | 7.3 | 6.4 | 4.1 | 3.0 | 2.5 | .25 | .04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stroke | 4.2 | 6.6 | 6.4 | 7.1 | 5.6 | 2.7 | 2.2 | 2.2 | .001 | .07 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chronic obstructive pulmonary disease | 23.2 | 15.8 | 17.0 | 21.4 | 16.7 | 21.8 | 24.7 | 23.7 | .04 | .86 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diabetes | 14.3 | 16.5 | 17.0 | 22.4 | 18.0 | 17.2 | 23.4 | 18.8 | .005 | .06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Malignancy | 30.4 | 23.3 | 19.3 | 19.9 | 12.7 | 15.6 | 16.8 | 19.0 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trauma/fracture | 12.8 | 13.8 | 17.7 | 21.0 | 13.5 | 9.8 | 8.9 | 9.5 | .006 | .09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hormone replacement therapy/oral contraceptives† | 4.3 | 4.6 | 21.1 | 21.4 | 14.9 | 10.6 | 7.4 | 10.9 | .008 | .01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post partum† | 3.8 | 1.7 | 2.4 | 1.1 | 1.2 | 2.5 | 1.1 | 1.4 | .08 | .07 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Any surgery, including index admission | 34.6 | 30.5 | 29.2 | 30.5 | 25.8 | 28.2 | 23.2 | 22.7 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Venous thromboembolism characteristics (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of venous thromboembolism event | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pulmonary embolism alone | 24.2 | 22.2 | 15.9 | 20.5 | 18.9 | 28.4 | 29.9 | 28.8 | <.001 | .11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pulmonary embolism and deep vein thrombosis | 8.2 | 6.6 | 15.6 | 14.7 | 15.2 | 18.9 | 19.6 | 19.7 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lower-extremity deep vein thrombosis alone | 67.7 | 71.3 | 68.5 | 64.7 | 65.9 | 52.7 | 50.5 | 51.5 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Community acquired | 78.0 | 80.1 | 73.6 | 75.1 | 75.8 | 78.8 | 79.5 | 77.1 | .68 | .73 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hospital encounter, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Admitted to hospital§ (%) | 100 | 100 | 77.7 | 77.2 | 70.8 | 74.3 | 73.1 | 71.1 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| If admitted, length of stay, days | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean (standard deviation) | 15.3 (24.1) | 15.1 (15.5) | 9.0 (10.0) | 10.1 (11.5) | 9.6 (10.8) | 8.5 (11.0) | 7.2 (8.6) | 6.9 (8.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (interquartile range) | 10 (8–19) | 10 (7–16) | 6 (4–9) | 6 (4–10) | 6 (4–10) | 5 (3–9) | 5 (3–8) | 5 (3–8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrent venous thromboembolism at index visit, n (% of total venous thromboembolism) | 212 (34.4) | 176 (28.5) | 104 (19.3) | 115 (19.3) | 88 (15.9) | 126 (20.7) | 156 (22.4) | 161 (20.3) | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient characteristics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years) | .10 | .17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean (standard deviation) | 62.6 (17.3) | 61.1 (19.0) | 64.1 (16.6) | 64.7 (17.2) | 65.4 (17.7) | 63.8 (18.0) | 66.9 (15.5) | 64.9 (16.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (interquartile range) | 66 (49–75) | 67 (48–75) | 69 (51–77) | 67 (51–80) | 71 (54–79) | 64 (49–80) | 69 (57–80) | 67 (53–79) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men (%) | 45.8 | 55.1 | 47.1 | 47.0 | 50.0 | 48.4 | 50.0 | 51.6 | .83 | .27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| White (%) | 96.2 | 97.2 | 90.7 | 93.6 | 95.4 | 96.0 | 94.7 | 92.4 | .08 | .10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recent* medical characteristics (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body mass index class | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| <25 kg/m2 | 39.0 | 38.6 | 33.8 | 30.9 | 28.1 | 34.8 | 28.9 | 27.6 | .01 | .04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25–30 kg/m2 | 30.8 | 36.2 | 36.4 | 35.8 | 35.1 | 28.1 | 31.6 | 30.6 | .60 | .97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| >30 kg/m2 | 30.1 | 25.2 | 29.9 | 33.3 | 36.8 | 37.1 | 39.5 | 41.8 | .002 | .04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Congestive heart failure | 18.9 | 9.7 | 8.7 | 13.9 | 6.8 | 4.0 | 10.7 | 8.1 | <.001 | .003 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Myocardial infarction | 2.8 | 0.6 | 3.8 | 3.5 | 6.8 | 1.6 | 3.8 | 3.7 | .14 | .63 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stroke | 4.2 | 1.7 | 9.6 | 5.2 | 6.8 | 1.6 | 0.6 | 1.2 | .26 | .12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chronic obstructive pulmonary disease | 25.9 | 19.3 | 19.2 | 20.9 | 10.2 | 26.2 | 32.7 | 29.8 | .13 | .41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diabetes | 15.6 | 11.4 | 17.3 | 16.5 | 13.6 | 19.0 | 19.2 | 26.7 | .003 | .01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Malignancy | 25.9 | 25.0 | 11.5 | 13.9 | 9.1 | 15.1 | 19.2 | 16.8 | <.001 | .03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trauma/fracture | 4.2 | 5.1 | 12.5 | 12.2 | 12.5 | 8.7 | 4.5 | 8.1 | .07 | .12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hormone replacement therapy/oral contraceptives† | 3.5 | 6.3 | 36.4 | 26.2 | 6.8 | 9.2 | 2.6 | 6.4 | .32 | .45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post partum† | 2.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .01 | .27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Any surgery, including index admission | 15.6 | 16.5 | 17.3 | 15.7 | 10.2 | 21.4 | 16.7 | 17.4 | .61 | .64 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Venous thromboembolism characteristics (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of venous thromboembolism event | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pulmonary embolism alone | 16.5 | 15.3 | 12.5 | 20.0 | 13.6 | 16.7 | 17.3 | 23.6 | .10 | .09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pulmonary embolism and deep vein thrombosis | 9.4 | 10.2 | 13.5 | 9.6 | 8.0 | 11.1 | 12.8 | 13.7 | .20 | .20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lower-extremity deep vein thrombosis alone | 74.1 | 74.4 | 74.0 | 70.4 | 78.4 | 72.2 | 69.9 | 62.7 | .02 | .02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Community acquired | 91.0 | 91.5 | 86.5 | 85.2 | 86.4 | 82.5 | 82.7 | 77.6 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hospital encounter§ (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Admitted to hospital | 100 | 100 | 78.8 | 63.5 | 68.2 | 70.6 | 65.4 | 73.3 | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| If admitted, length of stay, days | <.001 | <.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean (standard deviation) | 11.9 (11.3) | 10.9 (7.8) | 7.4 (8.4) | 7.0 (9.3) | 6.4 (7.0) | 6.7 (5.9) | 9.5 (10.4) | 6.3 (8.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (interquartile range) | 9 (7–13) | 9 (7–13) | 5 (4–8) | 5 (3–8) | 4 (2–7) | 5 (3–8) | 6 (3–10) | 3 (2–7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Recent defined as <6 months in 1980s cohort, <3 months in 1999–2007 cohort.
Among women.
Reflecting the standard care for the treatment of acute venous thromboembolism in 1980s, cohorts 85/86 and 88/89 included only inpatients diagnosed with acute venous thromboembolism.
Patient Characteristics at Index Visit
Among the 5025 patients diagnosed with venous thromboembolism, 46% were men, 95% were white, and mean age was 64.6±17.8 years. Patients diagnosed with first-time venous thromboembolism tended to be younger and include an increasing proportion of ethnic minorities over the study period, but no changes were apparent in the recurrent venous thromboembolism group (Table 1). Increases in body mass index and diabetes, with a decline in the frequency of prior congestive heart failure, stroke, and malignancy, were observed in patients with first-time and recurrent venous thromboembolism. Among patients with first-time venous thromboembolism, there was a decrease in the proportion who had recent surgery, trauma, or major fracture.
The proportion of patients with community-accquired venous thromboembolism remained approximately 80% over time among patients presenting with first-time venous thromboembolism, but decreased from approximately 91% to 78% among those diagnosed with recurrent venous thromboembolism at the time of their index visit (trend P=.001). Overall, the proportion of patients admitted to the hospital for treatment of venous thromboembolism, or who developed index venous thromboembolism during hospitalization for another diagnosis, decreased from 100% to approximately 70% during the years under study. Among hospitalized patients, the mean length of stay during the index hospitalization decreased markedly over time in all patient groups.
Annual Event Rates
Among residents of the WMSA during the period 1985 to 2009, the overall age- and sex-adjusted annual event rate (per 100,000) was 108 (95% CI, 98 to 118) for first-time venous thromboembolism and 34 (95% CI, 28 to 40) for recurrent venous thromboembolism. Further stratifying venous thromboembolism into pulmonary embolism and deep vein thrombosis alone, the overall age- and sex-adjusted annual event rate (per 100,000) was 41 (95% CI, 35 to 47) for first-time pulmonary embolism, 66 (95% CI, 59 to 74) for first-time deep vein thrombosis alone, 9.6 (95% CI, 6.6–12.6) for recurrent pulmonary embolism, and 25 (95% CI, 20 to 29) for recurrent deep vein thrombosis alone.
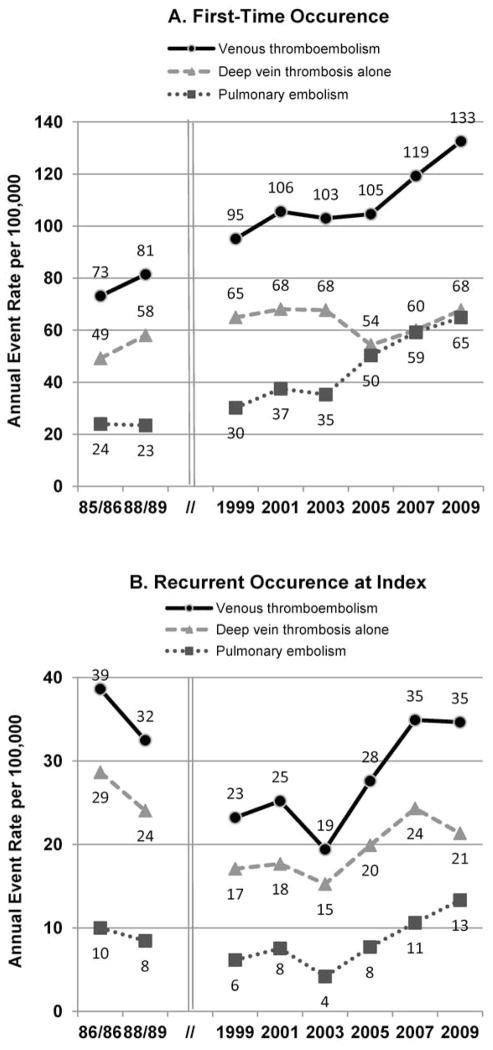
There were increases over time in the age- and sex-adjusted annual event rates of first-time venous thromboembolism from 73/100,000 to 133/100,000 (P<.001, Figure 1A). Poisson regression indicated an approximate 40% increase in first-time venous thromboembolism from 1985 to 2001, which remained essentially unchanged in the early 2000s, followed by an additional 50% increase by 2009 (Table 2). Although the pattern of first-time venous thromboembolism observed in WMSA between the late 1980s and early 2000s was similar in pulmonary embolism and deep vein thrombosis alone groups, the increasing trend in the late 2000s predominantly reflected an increase in the age- and sex-adjusted annual event rate of first-time pulmonary embolism, which increased from 35/100,000 in 2003 to 65/100,000 in 2009 (P<.001, Figure 1A).
Figure 1.
Age- and sex-adjusted annual event rates of (a) first-time and (b) recurrent clinical recognized acute venous thromboembolism among residents of Worcester, Massachusetts, metropolitan statistical area (1985 to 2009).
Table 2.
Annual event rates (per 100,000) of clinically recognized acute venous thromboembolism among Worcester metropolitan statistical area residents (1985–2009)
| Rate (95% confidence interval) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1985/1986 | 1988/1989 | 1999 | 2001 | 2003 | 2005 | 2007 | 2009 | |
| First-time venous thromboembolism at index visit | ||||||||
|
| ||||||||
| Crude | 71 (63–80) | 78 (69–87) | 91 (83–100) | 101 (92–110) | 98 (89–107) | 101 (92–110) | 113 (104–123) | 133 (123–143) |
| Adjusted* | 73 (64–82) | 81 (72–91) | 95 (86–104) | 106 (96–115) | 103 (94–112) | 105 (95–114) | 119 (109–129) | 133 (122–143) |
| Incidence rate ratio † | Reference group | 1.1 (0.9–1.3) | 1.3 (1.1–1.5) | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | 1.6 (1.4–1.8) | 1.9 (1.6–2.2) |
| First-time pulmonary embolism ± deep vein thrombosis at index visit | ||||||||
| Crude | 23 (19–28) | 22 (18–27) | 29 (24–34) | 36 (31–41) | 33 (28–39) | 48 (42–54) | 56 (50–63) | 64 (57–72) |
| Adjusted* | 24 (19–29) | 23 (18–28) | 30 (25–35) | 37 (32–43) | 35 (30–41) | 50 (44–57) | 59 (52–66) | 65 (58–72) |
| Incidence rate ratio † | Reference group | 1.0 (0.7–1.3) | 1.2 (1.0–1.6) | 1.5 (1.2–2.0) | 1.4 (1.1–1.9) | 2.1 (1.6–2.7) | 2.4 (1.9–3.1) | 2.8 (2.2–3.5) |
| First-time deep vein thrombosis alone at index | ||||||||
| Crude | 48 (41–55) | 55 (48–63) | 62 (56–70) | 65 (58–73) | 64 (57–72) | 53 (47–60) | 57 (51–64) | 68 (61–76) |
| Adjusted* | 49 (42–56) | 58 (50–66) | 65 (58–72) | 68 (61–76) | 68 (60–75) | 54 (48–61) | 60 (53–67) | 68 (60–75) |
| Incidence rate ratio † | Reference group | 1.1 (0.9–1.4) | 1.3 (1.1–1.6) | 1.4 (1.1–1.6) | 1.3 (1.1–1.6) | 1.1 (0.9–1.3) | 1.2 (1.0–1.4) | 1.4 (1.2–1.7) |
|
| ||||||||
| Recurrent venous thromboembolism at index visit | ||||||||
|
| ||||||||
| Crude | 37 (31–44) | 31 (26–37) | 22 (18–26) | 24 (20–29) | 18 (15–23) | 26 (22–31) | 33 (28–38) | 34 (29–39) |
| Adjusted* | 39 (32–45) | 32 (27–38) | 23 (19–28) | 25 (21–30) | 19 (15–23) | 28 (23–32) | 35 (29–40) | 35 (29–40) |
| Incidence rate ratio † | Reference group | 0.8 (0.6–1.1) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.5 (0.4–0.6) | 0.7 (0.6–0.9) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) |
| Recurrent pulmonary embolism ± deep vein thrombosis at index visit | ||||||||
| Crude | 10 (7–13) | 8 (5–11) | 6 (4–8) | 7 (5–10) | 4 (2–6) | 7 (5–10) | 10 (7–13) | 13 (10–16) |
| Adjusted* | 10 (7–13) | 8 (5–11) | 6 (4–8) | 8 (5–10) | 4 (2–6) | 8 (5–10) | 11 (8–14) | 13 (10–17) |
| Incidence rate ratio † | Reference group | 0.8 (0.5–1.3) | 0.6 (0.4–1.0) | 0.7 (0.5–1.2) | 0.4 (0.2–0.7) | 0.8 (0.5–1.2) | 1.0 (0.7–1.6) | 1.3 (0.9–2.0) |
| Recurrent deep vein thrombosis alone at index visit | ||||||||
| Crude | 28 (23–33) | 23 (19–28) | 16 (13–20) | 17 (14–21) | 14 (11–18) | 19 (15–23) | 23 (19–27) | 21 (17–26) |
| Adjusted* | 29 (23–34) | 24 (19–29) | 17 (13–21) | 18 (14–22) | 15 (12–19) | 20 (16–24) | 24 (20–29) | 21 (17–25) |
| Incidence rate ratio† | Reference group | 0.8 (0.6–1.1) | 0.6 (0.4–0.8) | 0.6 (0.5–0.8) | 0.5 (0.4–0.7) | 0.7 (0.5–0.9) | 0.8 (0.6–1.1) | 0.8 (0.6–1.0) |
Directly age- and sex-adjusted to the 2000 United States white population.
Based on Poisson regression adjusted by age and sex.
Trends in the age- and sex-adjusted annual event rates of recurrent venous thromboembolism were U-shaped (Figure 1B). Poisson regression indicated an approximate 40% decrease in the event rates of recurrent venous thromboembolism between the mid-1980s and 1999, which remained relatively unchanged in the early 2000s, then increased in the late 2000s (Table 2). Similar trends were found for those with pulmonary embolismand deep vein thrombosis alone (Figure 1B).
Crude and adjusted annual event rates for each study period, as well as by venous thromboembolism type, are shown in Table 2. These rates increased markedly with age regardless of venous thromboembolism type, sex, or study period (Table S2).
Objective Diagnostic Tests
The proportion of patients undergoing at least one objective (either invasive or non-invasive) diagnostic test rose, with rates of non-invasive testing increasing from 60–70% in 1985/86 to nearly 100% in 2009, while rates of invasive testing plunged from over 50% to near zero (Table 3). In particular, there was a marked increase in the use of computed tomography and magnetic resonance imaging scans in the late 2000s (Table 3).
Table 3.
Diagnostic methods for venous thromboembolism: the Worcester venous thromboembolism study (1985–2009)
| Study year | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1985/1986 (n=617) |
1988/1989 (n=618) |
1999 (n=539) |
2001 (n=597) |
2003 (n=554) |
2005 (n=608) |
2007 (n=698) |
2009 (n=794) |
Trend Test | 1985/86 vs 2009 | |
| First-time event at index, n (%) | n=405 | n=442 | n=435 | n=482 | n=466 | n=482 | n=542 | n=633 | ||
|
| ||||||||||
| Any invasive* diagnostic method | 237 (58.5) | 206 (46.6) | 6 (1.4) | 18 (3.7) | 9 (1.9) | 14 (2.9) | 11 (2.0) | 15 (2.4) | <.001 | <.001 |
| Any non-invasive diagnostic method | 285 (70.4) | 335 (75.8) | 406 (93.3) | 456 (94.6) | 453 (97.2) | 477 (99.0) | 535 (98.7) | 615 (97.2) | <.001 | <.001 |
| Diagnostic tests in patients with deep vein thrombosis (%) | n=307 | n=344 | n=366 | n=383 | n=378 | n=345 | n=380 | n=451 | ||
| Venogram | 67.8 | 54.9 | 0.5 | 1.6 | 0.8 | 2.0 | 1.1 | 0.7 | <.001 | <.001 |
| Impedance plethysmography | 57.7 | 53.2 | 0 | 0 | 0 | 0 | 0 | 0 | <.001 | <.001 |
| Duplex/ultrasound scan | 0.3 | 36.3 | 92.9 | 91.6 | 94.4 | 93.0 | 91.3 | 97.8 | <.001 | <.001 |
| Computed tomography | 0 | 0 | 3.3 | 7.0 | 6.9 | 4.9 | 10.3 | 2.9 | <.001 | .003 |
| Magnetic resonance imaging | 0 | 0 | 0 | 0 | 0.3 | 7.8 | 9.2 | 0.7 | <.001 | .28 |
| Any of above tests | 94.8 | 96.8 | 95.4 | 95.6 | 97.6 | 99.4 | 100 | 99.1 | <.001 | <.001 |
| Diagnostic tests in patients with pulmonary embolism (%) | n=131 | n=127 | n=137 | n=170 | n=159 | n=228 | n=268 | n=307 | ||
| Pulmonary angiogram | 16.8 | 11.0 | 2.2 | 2.4 | 2.5 | 2.6 | 2.6 | 3.9 | <.001 | <.001 |
| Lung scan | 80.9 | 88.2 | 59.1 | 40.0 | 19.5 | 24.1 | 11.2 | 7.5 | <.001 | <.001 |
| Spiral computed tomography | 0 | 0 | 24.8 | 60.6 | 79.2 | 80.7 | 86.9 | 87.3 | <.001 | <.001 |
| Magnetic resonance imaging | 0 | 0 | 0 | 0 | 0 | 0.4 | 0 | 0.3 | .33 | 1.0 |
| Any of above tests | 90.1 | 93.7 | 92.7 | 95.3 | 97.5 | 98.7 | 97.8 | 96.4 | <.001 | .008 |
|
| ||||||||||
| Recurrent event at index, n (%) | n=212 | n=176 | n=104 | n=115 | n=88 | n=126 | n=156 | n=161 | ||
|
| ||||||||||
| Any invasive* diagnostic method | 108 (50.9) | 63 (35.8) | 0 | 1 (0.9) | 1 (1.1) | 6 (4.8) | 6 (3.8) | 1 (0.6) | <.001 | <.001 |
| Any non-invasive diagnostic method | 126 (59.4) | 122 (69.3) | 97 (93.3) | 107 (93.0) | 85 (96.6) | 124 (98.4) | 153 (98.1) | 158 (98.1) | <.001 | <.001 |
| Diagnostic tests in patients with deep vein thrombosis (%) | n=177 | n=149 | n=91 | n=92 | n=76 | n=105 | n=129 | n=123 | ||
| Venogram | 49.7 | 39.6 | 0 | 1.1 | 1.3 | 3.8 | 2.3 | 0 | <.001 | <.001 |
| Impedance plethysmography | 47.5 | 49.7 | 0 | 0 | 0 | 0 | 0 | 0 | <.001 | <.001 |
| Duplex/ultrasound scan | 0 | 32.2 | 94.5 | 92.4 | 93.4 | 96.2 | 89.1 | 97.6 | <.001 | <.001 |
| Computed tomography | 0 | 0 | 1.1 | 1.1 | 6.6 | 2.9 | 7.0 | 4.1 | <.001 | .01 |
| Magnetic resonance imaging | 0 | 0 | 0 | 0 | 0 | 6.7 | 11.6 | 4.1 | <.001 | .01 |
| Any of above tests | 83.6 | 87.9 | 94.5 | 94.6 | 98.7 | 99.0 | 99.2 | 99.2 | <.001 | <.001 |
| Diagnostic tests in patients with pulmonary embolism (%) | n=55 | n=45 | n=27 | n=34 | n=19 | n=35 | n=47 | n=60 | ||
| Pulmonary angiogram | 12.7 | 4.4 | 0 | 0 | 0 | 2.9 | 6.4 | 0 | .004 | .005 |
| Lung scan | 65.5 | 88.9 | 66.7 | 44.1 | 31.6 | 22.9 | 8.5 | 13.3 | <.001 | <.001 |
| Spiral computed tomography | 0 | 0 | 25.9 | 58.8 | 63.2 | 74.3 | 85.1 | 78.3 | <.001 | <.001 |
| Magnetic resonance imaging | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Not applicable | Not applicable |
| Any of above tests | 76.4 | 95.6 | 92.6 | 94.1 | 89.5 | 97.1 | 97.9 | 96.7 | .001 | .001 |
Invasive tests including venogram and pulmonary angiogram.
DISCUSSION
The Worcester venous thromboembolism study provides a unique opportunity to examine 25-year trends in the magnitude, characteristics, and diagnostic workup for venous thromboembolism from the perspective of a well-characterized population. The disease burden from venous thromboembolism in this central Massachusetts population remains high, with a trend towards increasing annual event rates as well as substantial changes in patient characteristics and methods used to diagnose venous thromboembolism between 1985 and 2009.
Disease Burden
The age- and sex-adjusted annual event rates of clinically recognized acute first-time and recurrent venous thromboembolism was 142/100,000 during the entire study period, increasing from 112/100,000 in 1985/86 to 168/100,000 in 2009. It is higher than the incidence of the leading two cancers (126/100,000 for prostate cancer and 124/100,000 for breast cancer) and >15 times higher than the incidence rate of HIV (8.3/100,000) in white Americans.20 The age- and sex-adjusted annual event rate of clinically recognized acute pulmonary embolism was 78/100,000 in 2009, nearly equivalent to the annual incidence of ischemic stroke (88/100,000) in white individuals reported by the American Heart Association during that period.18
With increased long-term morbidity and functional disability, and high rates of recurrence and mortality among venous thromboembolism patients,1 this disorder remains a major national health problem with a substantial disease burden.
Time Trends in Occurrence
Between 1985 and 2009, the annual event rates of first-time venous thromboembolism nearly doubled, and first-time pulmonary embolism nearly tripled, with inconsistent patterns noted among patients with recurrent venous thromboembolism.
Our study is the first population-based surveillance project of venous thromboembolism to provide data about trends in annual event rates of first-time and recurrent venous thromboembolism between 1985 and 2009. Data from the Rochester Epidemiology Project (REP), the study with the most similar design to ours, indicated a 23% increase in age- and sex-adjusted annual event rate of first-time venous thromboembolism from 96/100,000 in 1986–1990 to 118/100,000 in 1991 to 1997.19, 21 These results are similar to the 30% increase in the age- and sex-adjusted annual event rate of first-time venous thromboembolism observed in our study between 1985 and 1999. A study based on the US Nationwide Inpatient Sample demonstrated that the number of patients with first-time and recurrent pulmonary embolism discharged from US acute care hospitals approximately doubled between 1998 and 2005,22 consistent with our findings. A retrospective study based on estimates derived from commercial insurance and Medicare databases of insured US residents observed a 33% increase in annual event rates of first-time and recurrent venous thromboembolism between 2002 and 2006.23 This increase was larger than the approximate 20% increase in the age- and sex-adjusted annual event rates of first-time and recurrent venous thromboembolism observed in the present study (from 131/100,000 in 2001 to 154/100,000 in 2007). These results, based on commercial insurance and Medicare databases, may be limited due to their reliance on administrative databases without actual chart review and independent diagnostic validation.
Both the REP and our study indicated that the frequency of venous thromboembolism increased markedly with age regardless of study year, venous thromboembolism type, or sex. Given the aging of the United States population,24 the projected disease burden of venous thromboembolism is expected to more than double between 2006 and 2050.23
Possible Contributory Factors to Observed Trends
Observed increases in the frequency of venous thromboembolism during the study period are likely to be multifactorial. First, the use of one or more non-invasive diagnostic methods for the detection of venous thromboembolism increased from approximately two-thirds of patients in 1985 to nearly all in 2009. In particular, the introduction of computed tomography pulmonary angiography (CTPA) closely parallels the observed increases in the annual event rate of first-time and recurrent pulmonary embolism observed in our study. The proportion of pulmonary embolism patients who underwent a CTPA test increased from approximately 25% in 1999 to 85% in 2009. During this period, the annual event rate of first-time and recurrent pulmonary embolism more than doubled. A time-trend analysis using the Nationwide Inpatient Sample and Multiple Cause-of-Death databases demonstrated that the introduction of CTPA was associated with changes consistent with a rising frequency of pulmonary embolism in the US 25. Clearly we are detecting more cases of venous thromboembolism during recent years than were detected (or were detectable) in the decades before the introduction of newer technology. A more difficult question to address is how much of this increase represents small, clinically insignificant pulmonary embolisms? Following the introduction of high-resolution multiple-detector CTPA, systematic reviews and meta-analyses have suggested an increase in the diagnosis of subsegmental pulmonary embolisms of unclear clinical significance through the use of these newer testing modalities.26 Because most of these subsegmental pulmonary embolisms are treated, their importance remains a key healthcare and resource dilemma. Further study of this issue is warranted.
With expanded access to higher resolution diagnostic imaging, a growing awareness of venous thromboembolism as an important public-health problem may have led clinicians to refer additional patients for evaluation.27 Our findings indicate that the proportion of patients who received any form of objective diagnostic testing has increased over time.
In addition to changes in the diagnosis of venous thromboembolism, the increases in annual event rates of venous thromboembolism observed in WMSA residents could also be related to changes in population characteristics over time. As the US population ages and becomes less active and more obese,20, 24 it may lead to further increases in risk of developing venous thromboembolism.
Although the development and implementation of evidence-based practice guidelines for venous thromboembolism prevention and treatment may have reduced the annual event rate of venous thromboembolism among ‘high-risk’ patients, including those with a recent history of a surgical procedure, pregnancy, trauma, fracture, and hospitalization,28 this would not be expected to impact patients without obvious recent provocations who would be less likely to have received enhanced venous thromboembolism prophylaxis compared with high-risk patients.
Our findings demonstrate that the proportion of patients with community-accquired first-time venous thromboembolism has remained relatively constant, at approximately 80%, during the 25-year period under study. A prior publication from our study suggests that approximately 40% of patients with community-acquired venous thromboembolism had a hospitalization or surgery in the 3 months before their index visit.29 Further work identifying and providing prophylaxis to high-risk patients being discharged from hospital is needed. With respect to the remaining 60% (with unprovoked venous thromboembolism), research aimed at better understanding such patients by risk and identifying possible “minor” triggers for a venous thromboembolism event may provide additional opportunities for prophylaxis.
Interestingly, although the annual event rate of recurrent venous thromboembolism remained relatively unchanged between 1985 and 2009, the trend was U-shaped. The decreasing trend observed between the late 1980s to 1999 could be related to development of improved treatment strategies.30–36 However, given increases in the annual event rates of first-time venous thromboembolism and high recurrence of this thromboembolic disorder,1, 7 it was not surprising that the annual event rates of recurrent venous thromboembolism also increased in residents of the WMSA during the late 2000s.
Study Strengths and Limitations
The Worcester venous thromboembolism study employed rigorous population-based surveillance methods to describe the clinical epidemiology of acute venous thromboembolism in the WMSA. Although we conducted broad screening for cases of venous thromboembolism using multiple databases, validated each potential case of venous thromboembolism, and performed regular chart audits, it is possible that this study may have missed some cases. Owing to low autopsy rates in the WMSA, and the limited validity of death-certificate data, only clinically recognized cases of acute venous thromboembolism were described and some cases of fatal pulmonary embolism could be missed. Further, regional differences may exist in the diagnostic workup of patients presenting with signs and symptoms of venous thromboembolism. Since the WMSA is predominantly a white population, additional population-based studies in minority and economically disadvantaged populations are needed. Owing to lack of funding, we did not collect data between 1990 and 1998, which may not provide a comprehensive view of 25-year trend between 1985 and 2009.
CONCLUSION
Despite advances in identification, prophylaxis, and treatment between 1985 and 2009, the annual event rate of venous thromboembolism has increased and remains high. While these increases may be partially due to increased sensitivity of diagnostic methods, especially for pulmonary embolism, it may also imply that current prevention and treatment strategies are less than optimal.
Supplementary Material
Clinical Significance.
Despite the substantial evolution in methods for venous thromboembolism prevention, diagnosis, and treatment between 1985 and 2009, the disease burden from venous thromboembolism remains high.
Observed increases in the annual event rates of venous thromboembolism may have been due partially to improved detection of venous thromboembolism, especially pulmonary embolism, with more sensitive imaging modalities.
Increases in the incidence of pulmonary embolism may be the result of small emboli detected by computed tomography.
Acknowledgments
Funding: The project described was supported by grants from the National Heart, Lung, and Blood Institute (R01-HL35862, R01-HL70283), and National Institute of Aging (R01AG031083).
Arlene Ash, PhD (Department of Quantitative Health Sciences) and Joel Gore, MD (Department of Medicine) at UMass Medical School for helpful comments. Sophie Rushton-Smith, PhD (Center for Outcomes Research, UMass Medical School) for editorial support.
Footnotes
Conflicts of Interest: FAA has received research grants from Sanofi and The Medicines Company. He has served as a consultant to GlaxoSmithKline and Millennium on the design of outcomes studies. Others have no conflict of interest.
Authorship: All authors had access to the data and played a role in writing this manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institute of Aging.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber sZ. Hemostasis and Thrombosis Basic Principles and Clinical Practice. 5. Vol. 1. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 2.Gillum RF. Pulmonary embolism and thrombophlebitis in the United States, 1970–1985. Am Heart J. 1987 Nov;114(5):1262–1264. doi: 10.1016/0002-8703(87)90212-2. [DOI] [PubMed] [Google Scholar]
- 3.Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010 Apr;38(4 Suppl):S502–509. doi: 10.1016/j.amepre.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Emadi A, Streiff M. Diagnosis and management of venous thromboembolism: an update a decade into the new millennium. Arch Iran Med. 2011 Sep;14(5):341–351. [PubMed] [Google Scholar]
- 5.Martinez-Murillo C, Aguilar-Arteaga ML, Velasco-Ortega E, et al. Clinical guideline for diagnosis and treatment of the thromboembolic venous disease. Rev Med Inst Mex Seguro Soc. 2011 Jul-Aug;49(4):437–449. [PubMed] [Google Scholar]
- 6.Riopel C, Bounameaux H. Doppler ultrasound and D-dimer. Friend or foe? Hamostaseologie. 2012 Jan;32(1):28–36. doi: 10.5482/ha-1182. [DOI] [PubMed] [Google Scholar]
- 7.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 8.Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence) Int Angiol. 2006 Jun;25(2):101–161. [PubMed] [Google Scholar]
- 9.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 10.Hirsh J, Guyatt G, Lewis SZ. Reflecting on eight editions of the American College of Chest Physicians antithrombotic guidelines. Chest. 2008 Jun;133(6):1293–1295. doi: 10.1378/chest.08-0782. [DOI] [PubMed] [Google Scholar]
- 11.Forum TJCaNQ. Specifications Manual for National Hospital Inpatient Quality Measures. Vol. The Joint Commission and Nationla Quality Forum; 2011. http://www.jointcommission.org/venous_thromboembolism/ [Google Scholar]
- 12.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Archives of Internal Medicine. 1991 May;151(5):933–938. [PubMed] [Google Scholar]
- 13.Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis. 2009 Nov;28(4):401–409. doi: 10.1007/s11239-009-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006 Jul;21(7):722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson FA, Jr, Wheeler HB. Physician practices in the management of venous thromboembolism: a community-wide survey. J Vasc Surg. 1992 Nov;16(5):707–714. doi: 10.1067/mva.1992.41080. [DOI] [PubMed] [Google Scholar]
- 16.Spencer FA, Emery C, Lessard D, Goldberg RJ. Upper extremity deep vein thrombosis: a community-based perspective. Am J Med. 2007 Aug;120(8):678–684. doi: 10.1016/j.amjmed.2006.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spyropoulos AC. Upper vs. lower extremity deep vein thrombosis: outcome definitions of venous thromboembolism for clinical predictor rules or risk factor analyses in hospitalized patients. J Thromb Haemost. 2009 Jun;7(6):1041–1042. doi: 10.1111/j.1538-7836.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 19.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998 Mar 23;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 20.Data and Statistics. Centers for Disease Control and Prevention; http://www.cdc.gov/datastatistics/ [Google Scholar]
- 21.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005 Aug;3(8):1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 22.Park B, Messina L, Dargon P, Huang W, Ciocca R, Anderson FA. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009 Oct;136(4):983–990. doi: 10.1378/chest.08-2258. [DOI] [PubMed] [Google Scholar]
- 23.Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011 Feb;86(2):217–220. doi: 10.1002/ajh.21917. [DOI] [PubMed] [Google Scholar]
- 24.From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA. 2003 Mar 19;289(11):1371–1373. [PubMed] [Google Scholar]
- 25.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011 May 9;171(9):831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010 Aug;8(8):1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Chung J, Ju MH, et al. Evaluation of Surveillance Bias and the Validity of the Venous Thromboembolism Quality Measure. JAMA. 2013 Oct 7;310(14):1482–1489. doi: 10.1001/jama.2013.280048. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Akl EA, Crowther M, Schunemann HJ, Gutterman DD, Zelman Lewis S. Introduction to the Ninth Edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):48S–52S. doi: 10.1378/chest.11-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007 Jul 23;167(14):1471–1475. doi: 10.1001/archinte.167.14.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999 Mar 25;340(12):901–907. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003 Apr 10;348(15):1425–1434. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 32.Albada J, Nieuwenhuis HK, Sixma JJ. Treatment of acute venous thromboembolism with low molecular weight heparin (Fragmin). Results of a double-blind randomized study. Circulation. 1989 Oct;80(4):935–940. doi: 10.1161/01.cir.80.4.935. [DOI] [PubMed] [Google Scholar]
- 33.Hull RD, Raskob GE, Pineo GF, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med. 1992 Apr 9;326(15):975–982. doi: 10.1056/NEJM199204093261502. [DOI] [PubMed] [Google Scholar]
- 34.Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med. 1996 Mar 14;334(11):677–681. doi: 10.1056/NEJM199603143341101. [DOI] [PubMed] [Google Scholar]
- 35.Prandoni P, Lensing AW, Buller HR, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992 Feb 22;339(8791):441–445. doi: 10.1016/0140-6736(92)91054-c. [DOI] [PubMed] [Google Scholar]
- 36.Wells PS, Kovacs MJ, Bormanis J, et al. Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low-molecular-weight heparin: a comparison of patient self-injection with homecare injection. Arch Intern Med. 1998 Sep 14;158(16):1809–1812. doi: 10.1001/archinte.158.16.1809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.