Abstract
Background
Although the visual cortex does not typically receive much attention in addiction literature, neuroimaging studies often report significant activity in visual areas when drug users are exposed to drug cues. The purpose of this meta-analysis was to investigate the frequency with which occipital cortex activity is observed during drug cue exposure and to determine its spatial distribution.
Methods
A comprehensive literature search was performed of human functional neuroimaging studies of drug cue-reactivity. Fifty-five studies were used to determine the frequency with which clusters of significant visual cortex activity during visual drug cues versus non-drug cues were reported. The spatial distribution of visual cortex activations was determined via activation likelihood estimation (ALE; FDR corrected, p<0.01) in a subset of these studies (n=24).
Results
Eighty-six percent of studies that reported fMRI results for drug versus neutral visual cues within a substance-dependent group showed significant drug-elicited activity in the visual cortex. ALE revealed clusters in the left secondary visual cortex (BA 19) and clusters in the primary visual cortex (BA 17) that were consistently activated by drug cues.
Conclusions
These data demonstrate that the visual cortex, often overlooked in our discussions of the neural circuitry of addiction, consistently discriminates drug cues from neutral cues in substance dependent populations. While it remains unclear whether drug cue-elicited activation in occipital cortex is related to the rewarding properties of the drug and/or attentional mechanisms, these data support further exploration.
Keywords: visual cortex, addiction, cue-induced craving, fMRI
1. INTRODUCTION
Drug cue-reactivity—the array of psychological, physiological, and behavioral effects elicited by drug-related stimuli—has been utilized for more than two decades in an attempt to understand drug craving and dependence (Rohsenow et al., 1991; Drummond, 2001; Carter and Tiffany, 1999). As of 2014, there were over 100 functional neuroimaging studies that investigated cue-reactivity in a range of drug using populations. These studies have provided substantial knowledge regarding the neural response to drug cues, revealing a common set of brain regions which are now classically considered a part of the network engaged in response to drug cues and craving. These oft-cited brain regions—up-regulated in the presence of visual drug cues—include the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, insula, and the striatum (Kühn and Gallinat, 2011; Schacht et al., 2013). These established regions are similarly reported in non-human primate studies of drug self-administration (Porrino et al., 2004), and rodent studies of drug reinstatement (McFarland et al., 2003; Dayas et al., 2007).
In addition to frontal and striatal brain regions, human neuroimaging studies of drug-cue reactivity often observe activation in another fundamental brain region that is given much less emphasis—the visual cortex. Although significant drug cue-elicited activity in the occipital cortex is commonly demonstrated and has been found in previous meta-analyses of drug cue-reactivity (Chase et al., 2011; Engelmann et al., 2012; Schacht et al., 2013), it is rarely reported as a primary finding and has historically not been given very much consideration in the context of addiction. More recently however, numerous investigations have described significant drug cue-elicited activity in visual cortex that directly relates to a host of clinical factors such as cigarette craving during 24-hour abstinence but not satiety (McClernon et al., 2009), resisting craving for cigarettes (Brody et al., 2007), as well as measures of self-recognition of problematic cocaine use and desire to change (Prisciandaro et al., 2014). Given these associations, and the fact that visual cortex activity specific to drug cues is compatible with emerging literature regarding the role for primary visual cortex in reward processing (Schuler et al., 2006; Yalachkov et al., 2010), we suggest that occipital cortex, including primary visual cortex, activation in response to drug cues deserves much more consideration.
To this end, the primary goal of this proof of concept study was to document and localize the involvement of the visual cortex during functional magnetic resonance imaging (fMRI) studies of drug cue-reactivity. Specifically, the aims were to quantify the frequency with which occipital cortex activity is observed across drug classes and, through activation likelihood estimation (ALE; Turkeltaub et al., 2002; Eickhoff et al., 2012), determine the spatial locations in occipital cortex that are most frequently activated.
2. METHODS
2.1. Inclusion criteria and identification of articles
We conducted a comprehensive PubMed electronic database search of all English language, addiction-related studies published by August, 2013 that assessed the neural response to drug-related cues using fMRI. Keywords for the imaging component were “imaging”, “MRI”, and “BOLD”. For the addiction component we searched “addict*”, “drug”, “abuse”, as well as individual classes/types of drugs including “nicotine”, “smok*”, “cocaine”, “stimulant”, “methamphetamine”, “alcohol*”, “opiate”, “heroin”, “marijuana” and “cannabis”. Lastly, we searched all “cue”, “cue-reactivity”, and “craving” studies. The goal was to identify (1) f MRI experiments of drug cue-reactivity that (2) presented visual cues to a substance-abusing population, (3) analyzed the data in a whole-brain approach (that included the occipital lobe), and (4) reported the results of drug versus non-drug cues within the substance-using population. Papers were selected after examination of the methods, and chosen if criteria of an fMRI drug cue-reactivity primary investigation were met. The reference sections of those selected were also searched for additional pertinent investigations.
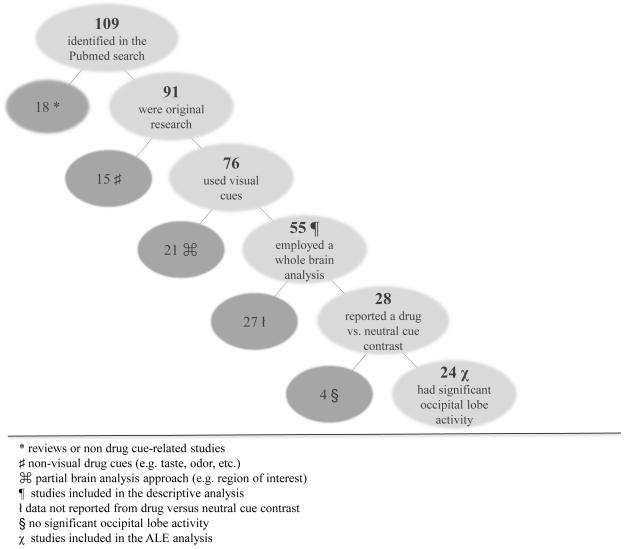
This literature search resulted in 109 manuscripts of which 5 meta-analyses and 13 noncue-related or inaccessible papers were excluded, leaving 91 original fMRI drug cue-reactivity articles. Of the 91 studies, 76 used visual cues [static images (n=61), video (n=11), viewing a self-holding cue (n=3), and virtual cues (i.e., 2D/3D smoking-related images and environments; n=1)]. Studies using non-visual cues [script guided imagery (n=5), taste cues (n= 3), presentation of words (n=5), odors (n=1), and infusion (n=1)] were not included in the subsequent analyses. Fifty-five of these 76 studies used a whole-brain approach.
To determine the frequency with which cue-induced craving studies observed visual cortex activity to drug cues, these 55 articles were thoroughly examined, noting information from all tables and figures, sample size, characteristics of the subject population, cue-exposure paradigm, analytic approach, statistical thresholding, the nature of the control condition/group, the cluster size, and the spatial location of visual cortex activity if it was observed. Of these 55 studies that performed a whole-brain voxel-based analysis of the BOLD response to visual cues of a drug in a substance-using group, 28 reported a detailed table of results describing regional brain activity elicited from a drug-cue versus neutral contrast in the substance-using group (Figure 1, Table 1, top).
Figure 1.
Flowchart of studies included in the descriptive and Activation Likelihood Estimation (ALE) analyses. Each step represents an additional inclusion criterion and subsequent exclusion of studies not meeting that criterion.
Table 1.
Number of studies by substance.
| Search Criteria | Total | Nicotine | Alcohol | Cocaine | Marijuana | Opiates | Poly/Other |
|---|---|---|---|---|---|---|---|
| Original data (n=91) |
91 | 37(41%) | 27(30%) | 13(14%) | 3(3%) | 9(10%) | 2(2%) |
| fMRI visual cues whole-brain |
55 | 25(45%) | 15(27%) | 4(7%) | 2(4%) | 8(15%) | 1(2%) |
| fMRI visual cues, drug vs neutral contrast |
28 * | 14(50%) | 7(25%) | 1(4%) | 1(4%) | 5(18%) | 0(0%) |
| Results: Occipital Activity | |||||||
| …to drug cues, any contrast (n=55) |
50 (91%) | 27(54%) | 12(24%) | 5(10%) | 2(4%) | 7(14%) | 1(2%) |
| …to drug vs neutral cues (n=28) |
24 (86%) | 14(58%) | 7(29%) | 1(4%) | 1(4%) | 5(21%) | 0(0%) |
=these studies were entered into the ALE analysis given the similarity of their contrast [substance dependent group, BOLD response to drug-cues versus neutral cues (see Table 2 for list)].
2.2. Activation Likelihood Estimation (ALE)
To determine and localize the regions within the visual cortex (e.g. primary versus secondary visual cortex) that were most consistently activated, an activation likelihood analysis (ALE) was performed on the subset of the 28 studies, namely the ones that detected significant occipital lobe activity to drug versus neutral cues (n=24). The coordinates of the peak activity within the occipital cortex for these 24 studies were entered into an ALE analysis. Only the clusters in the visual cortex were entered into the analysis, as this investigation was not designed as a comprehensive analysis of all brain regions implicated in drug cue-reactivity. ALE was performed with the GingerALE software (Turkeltaub et al., 2002), which uses an updated ALE algorithm for minimizing within-experiment and within-group effects (Eickhoff et al., 2012). Likelihood estimation values were computed for all focal locations from contributing contrasts. The null distribution statistic from the likelihood estimation was calculated with full-width at half maximum values empirically determined by the sample size of each contributing study. Values were then subjected to a false discovery rate (FDR) of p < .01 with a minimum extent threshold cluster threshold of 100 mm3.
3. RESULTS
3.1. Visual cortex activity during cue-induced craving: descriptive analyses
Of the 55 studies that used fMRI and whole-brain analyses to examine drug-related visual cues, 50 (91%) reported significant activity in the occipital lobe (primary and secondary visual cortices) during cue exposure. This drug-cued activity in occipital cortex was observed across multiple drug classes (alcohol, cocaine, marijuana, tobacco; Table 1) and there was no significant difference in frequency between the drug classes. Among the 28 studies that reported detailed results from a whole brain analysis of drug cues versus neutral cues in a substance abusing population, 24 (86%) reported significant drug-cued activity in the occipital cortex (Table 1, bottom, Table 2).
Table 2.
Studies included in the ALE analysis.
| Article | Cue Type | Substance | N (users) | Occipital Found |
|---|---|---|---|---|
| Braus et al 2001 | images | alcohol | 4 | Y |
| Brody et al 2007 | video | nicotine | 42 | Y |
| Dagher et al 2009 | video | nicotine | 15 | Y |
| David et al 2007 | images | nicotine | 8 | Y |
| Filbey et al 2009 | hold/view | marijuana | 38 | Y |
| Janes et al 2009 | images | nicotine | 13 | Y |
| Janes et al 2012 | images | nicotine | 24 | Y |
| Kang et al 2012 | images | nicotine | 25 | Y |
| Kushnir et al 2013 | images | nicotine | 20 | Y |
| Lee et al 2005 | virtual | nicotine | 8 | Y |
| Li et al 2012 | images | opiate | 24 | Y |
| Li et al 2013 | images | opiate | 18LT | Y |
| Lou et al 2012 | images | opiate | 17ST 17 LT | Y |
| McClernon et al 2009 | images | nicotine | 18 | Y |
| McClernon et al 2008 | images | nicotine | 30 | Y |
| Mei et al 2010 | images | opiate | 15 | Y |
| Prisciandaro et al 2014 | images | cocaine | 31 | Y |
| Rubenstein et al 2011 | images | nicotine | 12 | Y |
| Vollstadt-Klein et al 2011a | images | nicotine | 21 | Y |
| Vollstadt-Klein et al 2011b | images | alcohol | 30 | Y |
| Vollstadt-Klein et al 2010 | images | alcohol | 31 | Y |
| Wrase et al 2002 | images | alcohol | 6 | Y |
| Wrase et al 2007 | images | alcohol | 16 | Y |
| Zijlstra et al 2009 | images | opiate | 12 | Y |
Criteria include: visual stimulus, report whole-brain analysis that includes the occipital lobe, report within user group contrast results for cue relative to baseline, no covariates, report coordinates in the occipital lobe for the contrast meeting study criteria. N=number of users included in the contrast of interest. LT=Long Term Abstinence, ST=Short Term Abstinence.
Although not included in the initial analysis, a posthoc assessment of drug cue-reactivity studies that used stimuli other than visual (n=15) revealed that six (40%) observed visual cortex activity to the non-visual drug cues (2 script, 1 odor, 2 word, 1 taste).
3.2. Distribution of activity within the occipital lobe: Activation Likelihood Estimation analyses
Among the 24 studies that reported results from a whole-brain analysis of drug versus neutral cues and observed activity in the occipital cortex, ALE revealed several independent clusters that were consistently activated. These included discrete clusters in both the primary visual (left BA 17) and secondary (left and right BA 18/19) visual cortices (Table 3 and Figure 2).
Table 3.
Locations of centered Talairach peak coordinates with significant ALE values among addiction studies that observed higher activity in the occipital lobe when individuals were viewing drug-related cues versus neutral cues.
| Brain | Region^ | Volume* | ALE extrema | x | y | z |
|---|---|---|---|---|---|---|
| Right | BA 19 | 7520 | 0.0292 | 36 | −80 | −12 |
| Right | BA 17 | 2952 | 0.0187 | 22 | −82 | 8 |
| Left | BA 17 | 1600 | 0.0189 | −20 | −92 | −10 |
| Left | BA 19 | 1592 | 0.0144 | −10 | −84 | 32 |
| Left | BA 19 | 1336 | 0.0127 | −44 | −78 | −6 |
| Right | BA 18 | 872 | 0.0137 | 6 | −94 | 12 |
| Left | BA 18 | 480 | 0.0125 | −22 | −56 | 4 |
| Left | BA 19 | 232 | 0.0112 | −40 | −68 | 12 |
| Right | BA 19 | 144 | 0.0101 | 18 | −56 | 0 |
mm3,
BA = Brodmann Area
Figure 2.
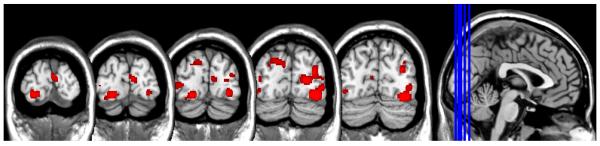
Areas of consistent overlap in occipital lobe activity as revelaed by Activation Likelihood Estimation (ALE). These data are intended to refine the spatial localization of occipital lobe activity (primary versus secondary visual corticies) among functional MRI studies that have observed significantly more occipital lobe activity in repsonse to drug-related versus neutral visual cues. FDR corrected, p<0.01 for multiple comparisons, cluster size > 100.
4. DISCUSSION
Drug-cue reactivity is one of the most common paradigms employed in human substance abuse literature, largely because drug cue-induced craving is one of the most robust factors that lead to continued use and relapse across substances. Our discussions on treatable-targets for addiction typically focus on the frontal and striatal areas that are activated by drug-related cues. The results of the present investigation however highlight the importance of another often overlooked brain region that is consistently activated by drug cues relative to non-drug cues, the primary and secondary visual cortices. Although the role of the visual cortex in addiction is seldom discussed at scientific meetings, the vast majority of drug cue exposure studies (86%) found significant occipital cortex activation in substance users who were exposed to visual drug cues compared to neutral cues. Activation likelihood estimation revealed that this activity is present in distinct areas of BA 17 and BA 19. Furthermore this activity was observed across multiple drug classes (alcohol, cocaine, marijuana, tobacco). Thus, it appears that addiction can directly affect primary sensory cortex, suggesting that the effects of drug cue-reactivity are more widespread and fundamental than previously appreciated. It is highly likely that addiction influences basic sensory processing via its effects on cognitive processes that are known to modulate activity in BA 17. Here, we discuss some of the more likely cognitive processes underlying the enhancement of activity in BA 17, and the implications for our basic understanding of addiction.
4.1. Attention and Reward processing in the Visual Cortex
The primary visual cortex (BA 17) encodes elementary features of visual stimuli such as local contrast, spatial location, spatial frequency, orientation, and ocularity (Hubel, 1982). An extensive body of work has shown that the representation of these basic visual features can be significantly modulated by attention as well as the association of stimuli with reward. Both attention and reward are thus potential candidates for mediating the effect of drug cues on visual cortical activity.
Attention can increase the gain, reduce the noise, and reduce noise correlations in response to attended visual stimuli (Pratte et al., 2013; Keitel et al., 2013). Any of these attention-related effects could result in the increased probability of activation to drug cues noted in our meta-analysis. Thus, one way in which addiction might exert it effects upon sensory processing in BA 17 is by establishing an attentional bias toward images, either external or internally generated, that depict drug-related materials. Such an attentional bias could increase the likelihood of relapse by increasing drug craving (Field and Cox, 2008). Thus, an important future direction of this work will be to determine if an attentional bias toward drug-related cues can explain our finding of increased visual cortex activity in response to drug cues. This could be done, for example, by determining if BA 17 in non-users shows enhanced activation when subjects are specifically instructed to attend to drug-related cues. If so, it will then be important to assess whether or not this attentional bias is effectively permanent, or if it can be manipulated or diverted by, for example, a demanding attentional task.
Several recent animal studies have demonstrated that in addition to processing basic features of visual stimuli, BA 17 is also involved in reward processing. For example, Schuler and Bear (2006) demonstrated that in rodents exposed to visual stimuli previously paired with a reward, neurons in the primary visual cortex responded to the timing of the reward rather than the physical attributes of the stimulus itself. The sensitivity of the visual cortex to reward has also been described in non-human primates using electrophysiological (Keliris et al., 2010) and neuroimaging approaches (Arsenault et al., 2013), as well as in rodents using two-photon imaging of visual cortex (Goltstein et al., 2013). Arsenault and colleagues (2013), for example, demonstrated that dopamine signaling can selectively modulate visual cortex activity and that, following a visually paired reward association task, the visual cortex of monkeys was activated by being rewarded in the absence of any image. Taken together, these basic science findings are consistent with the results of the present study, that the rewarding properties of a cue may be reflected by activation in primary visual cortex.
4.2. Implications for Addiction
As discussed, visual cortex activity is modulated by both attention and reward. From the perspective of addiction, it is well established that substance dependent individuals have elevated attentional bias to drug-related cues (reviews see: Field and Cox, 2008; Robbins and Ehrman, 2004). One theory of attentional bias in addiction is that over time, as the individual repetitively uses the substance and finds it rewarding, incentive salience for the substance transfers to the substance-related cue. The cue then elicits a conditioned motivational state (Robinson and Berridge, 1993) which promotes attentional bias towards the drug cue (Goldstein and Volkow, 2002; Robinson and Berridge, 1993), and subsequent craving (Waters et al., 2012). The attentional bias to cues is remarkably frequent and stable across drug classes. This has led to enthusiasm about attentional bias being a potential biomarker for addiction, an indicator of vulnerability, or even a treatment target.
Although attentional bias to drug cues is often quantified by measuring performance on an interference task such as the Stroop task which requires the participant to detect and then respond to a cue, there are a number of other studies which have shown that basic visual metrics of attention such as visual fixation are even more robust markers of attentional bias to drug cues. These visual metrics are affected in multiple classes of drug users including marijuana (Field et al., 2006), alcohol (Miller and Fillmore 2010, 2011), nicotine (Mogg et al., 2003), and cocaine users (Marks et al., 2014). Marks and colleagues (2014), for example, recently demonstrated that cocaine users, compared to non-using controls, visually fixate for a longer amount of time on cocaine-related images, and that this attentional bias was significantly correlated with self-reported lifetime cocaine use. The advantage of visual fixation time as a metric of attentional bias is that it may have higher test-retest reliability than response time in an interference task (Ataya et al., 2012) and that it does not require a motor response (which may be slowed in drug users). While the results from this meta-analysis of functional imaging studies in drug cue exposure are consistent with other data demonstrating longer fixation times to drug related cues, additional studies are needed to determine whether longer fixation times correspond to elevated visual cortex activity in the presence of salient drug cues.
There is a strong interest in using attentional bias to drug cues as a biomarker for addiction. The collection of human functional neuroimaging studies examined in this manuscript demonstrate with remarkable consistency that substance-dependent individuals have significantly higher activity in the primary and secondary visual cortices when exposed to a drug versus non-drug cue. Although visual gaze tracking is routinely performed in human functional neuroimaging studies, none of the studies included in this meta-analysis directly compared visual gaze tracking metrics to occipital cortex activity. Future research in this area will be able to directly test whether human laboratory metrics of attentional bias to drug cues, including fixation time, are directly related to visual cortex activity—which would suggest that visual cortex activity is a strong, consistent biomarker of addiction.
The interpretation that elevated visual cortex activity to drug cues versus neutral cues indicates an attentional bias to these stimuli has implications for predicting relapse following substance-use treatment. Many studies have investigated attentional bias as a bell-weather for treatment success across multiple drug-classes including in cocaine (Kennedy et al., 2014; Kilts et al., 2014), heroin (Marrisen et al., 2006), smoking (Waters et al., 2003, Janes et al., 2010), and alcohol (Cox et al., 2002). Other studies have even used attentional bias as a target of treatment itself including Attentional Retraining (Atwood et al., 2008). While many of these studies have mixed results, they typically utilized interference tasks such as the Stroop, rather than direct visual fixation metrics (or visual cortex activity metrics), as their indicators for success. Given the consistency with which the functional neuroimaging studies in this meta-analysis observed visual cortex activity to drug-related cues, it is possible that drug cue-elicited visual cortex activation might be a more sensitive metric to predict treatment outcome.
It is also possible that these substance users had a higher visual cortex response to these cues even before they initiated and escalated their use. That is, the occipital cortex activity to drug cues may not reflect an attentional bias that has developed over time, but rather a preexisting sensitivity to drug cues. In a society which both aggrandizes and demonizes images of drugs, it is possible that drug-related images are particularly salient even before being paired with the experience of using the drugs. That said, the few studies in this meta-analysis that showed images of drug cues (typically alcohol or smoking) to non-drug users however did not report significant activity in the occipital cortex among the non-drug users. Future longitudinal investigations of adolescents at risk for substance dependence (e.g., those with a positive family history of alcohol dependence or high sensation-seekers) may be able to test directly whether visual cortex activity to drug related images, and visual fixation, predicts drug initiation even before a traditional Stroop-related attentional bias develops. Although fMRI will likely never be a realistic screening tool due to cost concerns, it is possible that visual cortex activity could still be used as a biomarker for vulnerability to addiction or substance dependence itself through portable, lower-cost technologies such as EEG which can sample a more reliable signal from the visual cortical areas rather than deeper structures such as the orbitofrontal cortex or anterior cingulate cortex that are also involved in attention to drug cues.
4.3. Limitations and future directions
While this study was motivated by a desire to explore the frequency and pattern of visual cortex activity to drug-related cues among multiple substance-dependent populations—an area not yet explored in this focused manner—it was not intended to be a comprehensive investigation of brain regions involved in drug craving. Nor was it intended to place larger value on occipital cortex activity relative to other brain regions implicated in drug craving—including the ventral and dorsal striatum, as well as prefrontal regions including medial and orbital prefrontal cortex. Consequently we did not do a comprehensive analysis of all other brain regions that are activated, or potentially co-activated with the occipital lobe, which may be an informative future direction from the perspective of understanding the way in which substance-dependent individuals process drug cues.
The inclusion of multiple classes of drug users emphasizes the consistency of this visual cortex activity in substance use literature, (i.e., nicotine, alcohol, marijuana, cocaine, opiates, etc.). The generalizability of the results, however; is influenced by the disproportionately large number of studies on nicotine and alcohol. While this distribution generally reflects the historic trends in drug use and abuse in the United States (Johnston et al., 2014), there were very few available studies on marijuana or prescription opiate cue-reactivity, which are growing segments of the population.
Additionally, while the presence of occipital activity was high across all classes of substance-dependent populations, it is still unclear whether this activity is indicative of something specific to dependence or vulnerability. Many questions remain to be answered with regard to the role of visual cortex activity in response to visual drug cues. Future investigations range from both basic science inquiries (e.g., salience versus reward, temporal dynamics of visual versus striatal activity to drug cues), to the potential clinical value of detecting vulnerability to drug use or relapse based upon visual cortical response to drug images. Furthermore, if the primary visual cortex is more stimulated by drug cues than neutral cues, there are potential implications for public policy including detection of vulnerable youth, variations between legal and illegal substances, and the role of visual cortex stimulation in maintaining the chronic process of addiction, abstinence, and relapse. The present study is an initial step in recognizing a potentially informative new target of investigation in addiction research. These results support future basic and clinical addiction science inquiry focused on the visual cortex.
Highlights.
The majority of fMRI drug cue-reactivity studies report effects in visual areas.
Drug cue-elicited BOLD activation is found in primary and secondary visual areas.
Attentional bias and/or reward processing may relate to drug-cued visual activation.
Acknowledgments
Role of Funding Source Funding for this study was provided by NIDA Grant K01 DA027756 (CH) and NIMH Grant K01 MH090548 (BC); the NIDA and NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors CH and BC conceived and developed the study. CH, BC, MC, TN, and LD all contributed to the literature searches, data analysis, manuscript composition and illustrations. CH wrote the first draft of the manuscript. All authors have approved the final manuscript.
Conflict of Interest All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arsenault JT, Nelissen K, Jarraya B, Vanduffel W. Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron. 2013;77:1174–1186. doi: 10.1016/j.neuron.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 2012;121:148–151. doi: 10.1016/j.drugalcdep.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafò MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103:1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J. Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, Niaura R, Rogers RD, Matthews PM, Walton RT. Effects of acute nicotine abstinence on cue-elicited ventral striatum/nucleus accumbens activation in female cigarette smokers: a functional magnetic resonance imaging study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol. Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht J, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc. Natl. Acad. Sci. USA. 2009;106:13016–21. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltstein PM, Coffey EB, Roelfsema PR, Pennartz CM. In vivo two-photon Ca2+ imaging reveals selective reward effects on stimulus-specific assemblies in mouse visual cortex. J. Neurosci. 2013;33:11540–11555. doi: 10.1523/JNEUROSCI.1341-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH. Exploration of the primary visual cortex, 1955-78. Nature. 1982;299:515–524. doi: 10.1038/299515a0. [DOI] [PubMed] [Google Scholar]
- Janes AC, Frederick B, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp. Clin. Psychopharmacol. 2009;17:365–373. doi: 10.1037/a0017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Smoller JW, David SP, Frederick BD, Haddad S, Basu A, Fava M, Evins AE, Kaufman MJ. Association between CHRNA5 genetic variation at rs16969968 and brain reactivity to smoking images in nicotine dependent women. Drug Alcohol Depend. 2012;120:7–13. doi: 10.1016/j.drugalcdep.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Institute for Social Research. The University of Michigan; Ann Arbor: 2014. Monitoring The Future National Survey Results On Drug Use: 1975-2013: Overview, Key Findings On Adolescent Drug Use. [Google Scholar]
- Kang OS, Chang DS, Jahng GH, Kim SY, Kim H, Kim JW, Chung SY, Yang SI, Park HJ, Lee H, Chae Y. Individual differences in smoking-related cue reactivity in smokers: an eye-tracking and fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;38:285–293. doi: 10.1016/j.pnpbp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Keitel C, Andersen SK, Quigley C, Müller MM. Independent effects of attentional gain control and competitive interactions on visual stimulus processing. Cereb. Cortex. 2013;23:940–946. doi: 10.1093/cercor/bhs084. [DOI] [PubMed] [Google Scholar]
- Keliris GA, Logothetis NK, Tolias AS. The role of the primary visual cortex in perceptual suppression of salient visual stimuli. J. Neurosci. 2010;30:12353–12365. doi: 10.1523/JNEUROSCI.0677-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Gross RE, Ely T, Drexler KP, Kilts CD. Clinical correlates of attentional bias to drug cues associated with cocaine dependence. Am. J. Addiction. 2014 doi: 10.1111/j.1521-0391.2014.12134.x. doi: 10.1111/j.1521-0391.2014.12134.x. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM, James GA. Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology. 2014;39:1135–1147. doi: 10.1038/npp.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Kushnir V, Menon M, Balducci XL, Selby P, Busto U, Zawertailo L. Enhanced smoking cue salience associated with depression severity in nicotine-dependent individuals: a preliminary fMRI study. Int. J. Neuropsychopharmacol. 2013;16:997–1008. doi: 10.1017/S1461145710000696. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl. Psychophysiol. Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, Wu N, Chang H, Zheng Y, Qin W, Zhao L, Yuan K, Liu J, Wang W, Tian J. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Zhu J, Zheng Y, Chen J, Zhao L, Zhou Z, Liu Y, Wang W, Tian J. Assessing cue-induced brain response as a function of abstinence duration in heroin-dependent individuals: An event-related fmri study. PLoS One. 2013;8:e62911. doi: 10.1371/journal.pone.0062911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M, Wang E, Shen Y, Wang J. Cue-elicited craving in heroin addicts at different abstinent time: an fMRI pilot study. Subst. Use Misuse. 2012;47:631–639. doi: 10.3109/10826084.2011.646381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, Van Den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Marks KR, Roberts W, Stoops WW, Pike E, Fillmore MT, Rush CR. Fixation time is a sensitive measure of cocaine cue attentional bias. Addiction. 2014 doi: 10.1111/add.12635. doi: 10.1111/add.12635. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei W, Zhang JX, Xiao Z. Acute effects of sublingual buprenorphine on brain responses to heroin-related cues in early-abstinent heroin addicts: an uncontrolled trial. Neuroscience. 2010;170:808–15. doi: 10.1016/j.neuroscience.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT. The effect of image complexity on attentional bias towards alcohol-related images in adult drinkers. Addiction. 2010;105:883–890. doi: 10.1111/j.1360-0443.2009.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT. Persistence of attentional bias toward alcohol-related stimuli in intoxicated social drinkers. Drug Alcohol Depend. 2011;117:184–189. doi: 10.1016/j.drugalcdep.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J. Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratte MS, Ling S, Swisher JD, Tong F. How attention extracts objects from noise. J. Neurophysiol. 2013;110:1346–1356. doi: 10.1152/jn.00127.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict. Biol. 2014;19:240–249. doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. The role of attentional bias in substance abuse. Behav. Cogn. Neurosci. Rev. 2004;3:243–260. doi: 10.1177/1534582305275423. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int. J. Addict. 1991;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Moscicki AB, Dryden W, Rait MA, Simpson GV. Smoking-related cue-induced brain activation in adolescent light smokers. J. Adolesc. Health. 2011;48:7–12. doi: 10.1016/j.jadohealth.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Kobiella A, Buhler M, Graf C, Fehr C, Mann K, Smolka MN. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict. Biol. 2011a;16:166–175. doi: 10.1111/j.1369-1600.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol. Psychiatry. 2011b;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Marhe R, Franken IH. Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology (Berl.) 2012;219:909–921. doi: 10.1007/s00213-011-2424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur. Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Beck A, Ströhle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behav. Brain Res. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]