Abstract
Rationale
Several laboratories have conducted placebo-controlled drug challenge studies with MDMA, providing a unique source of data to examine the reliability of the acute effects of the drug across subject samples and settings. We examined the subjective and physiological responses to the drug across three different laboratories, and investigated the influence of prior MDMA use.
Methods
Overall, 220 healthy volunteers with varying levels of previous MDMA experience participated in laboratory-based studies in which they received placebo or oral MDMA (1.5 mg/kg or 125 mg fixed dose) under double blind conditions. Cardiovascular and subjective effects were assessed before and repeatedly after drug administration. The studies were conducted independently by investigators in Basel, San Francisco and Chicago.
Results
Despite methodological differences between the studies and differences in the subjects' drug use histories, MDMA produced very similar cardiovascular and subjective effects across the sites. The participants' prior use of MDMA was inversely related to feeling `Any Drug Effect' only at sites testing more experienced users.
Conclusions
These data indicate that the pharmacological effects of MDMA are robust and highly reproducible across settings. There was also modest evidence for tolerance to the effects of MDMA in regular users.
Keywords: MDMA, tolerance, mood, humans
Introduction
Several laboratories across the world have conducted controlled studies administering 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) to healthy volunteers (de la Torre 2000; Liechti et al. 2001; Tancer and Johanson 2003; Dumont et al. 2009; Bedi et al. 2010; Kirkpatrick et al. 2012). These studies provide a unique source of data regarding possible sources of variability in drug responses, such as drug use history or environmental setting. However, the extent to which responses to the drug are consistent across different laboratory sites is not known. The literature is replete with failures to replicate results, especially in behavioral studies (Pashler et al. 2012; Yong 2012; Smith et al. 2013), including recent investigations of acute amphetamine effects (Hart et al. 2013). Therefore, reports of consistencies across studies and across laboratories are important, and greatly strengthen the conclusions. In this study, we examined the quality and magnitude of responses to MDMA in healthy volunteers obtained in three different laboratories.
One factor known to influence an individual's response to drugs is past drug use. With MDMA there is some evidence that chronic tolerance develops to its “positive” subjective effects (Solowij et al. 1992; Verheyden et al. 2003; Parrott 2005; Sumnall et al. 2006). However, apparent decreases in effects may result from changes in the purity of MDMA tablets over time or geographical location (Sherlock et al. 1999; Baggott et al. 2000; Spruit 2001; Parrott 2004; Morefield et al. 2011). Nevertheless, there is evidence of chronic tolerance to the behavioral effects of MDMA in studies with rats and rhesus monkeys (Zacny et al. 1990; Frederick et al. 1998; Fantegrossi et al. 2004; Baumann et al. 2008), suggesting that tolerance may also occur in humans.
In this report we examine responses to a single dose of MDMA in participants whose self-reported lifetime use of ecstasy/MDMA ranged from 0 to 200 separate occasions of use. We examined the drug's effects on heart rate, blood pressure, and self-reported subjective effects using data conducted at the California Pacific Medical Center Research Institute in San Francisco (SF), the University of Chicago (Chicago), and the University Hospital Basel (Basel). Some of the data were previously published (Harris et al. 2002; Bedi et al. 2010; Hysek et al. 2012); some are as yet unpublished (SF, Chicago). The studies at the three sites had both methodological commonalities and differences (Table 1). At all laboratories, healthy adults received comparable, moderate doses of MDMA (1.5 mg/kg or 125 mg fixed dose) and placebo under double-blind conditions, and the studies used similar outcome measures. However, participants at the sites differed in nationality (Swiss, U.S.), native language (German, English) and drug use history (no prior use to high levels of use), and there were minor procedural differences including instructions, drug expectancies and environmental conditions during sessions (e.g., presence or absence of researchers, participants in bed or ambulatory). Also, some of the studies included additional test sessions with other doses of MDMA, an active control drug or a pretreatment drug.
Table 1.
Demographics and methods by laboratory
| Sample size | Age (years) | Weight (kg) | MDMA use (lifetime) | MDMA dose (mg) | Blinding | Testing context | Time of Dosing | |
|---|---|---|---|---|---|---|---|---|
| SF | N=45 (20 F, 25 M) | 28.0±6.7*† | 68.1±12.9 | 32.8±37.0*† | 1.5mg/kg | MDMA or placebo | researchers present | 11:00 am |
| Chicago | N=95 (37 F, 58 M) | 24.3±4.5 | 69.9±11.1 | 13.1±13.5† | 1.5mg/kg | Stimulant, sedative, cannabinoid, placebo | participants alone | 9:30 am |
| Basel | N=80 (40 F, 40 M) | 25.4±5.2 | 68.1±11.4 | 0.6±0.6 | 1.89 ± 0.33 mg/kg | MDMA or placebo | researchers present | 8–9:00 am |
Note: Blinding refers to the information the participant is given about the range of possible drugs administered in each study
denotes significantly different than Chicago lab (p < 0.05)
denotes significantly different than Basel lab (p < 0.05)
Overall, these methodological differences across sites provide a rigorous test of the reliability of the subjective and behavioral effects of MDMA. Moreover, most previously published studies with MDMA present aggregated data, whereas the current report provides individual-level analysis allowing us to examine the role of MDMA use history in acute response to the drug.
Methods and Materials
Participants
At all three sites healthy young adults were screened for psychiatric and medical problems. Inclusion criteria were similar across laboratories, with some differences in current drug use. Participants at all three sites included current alcohol and nicotine users, but current smoking was limited in Basel to less than 7 cigarettes/day and in Chicago to less than 10 cigarettes/day. Current marijuana users were excluded in the Basel laboratory but not in Chicago or SF. Participants in Chicago and SF, but not in Basel, had to report some previous use of MDMA. Participants at all sites were required to test negative for alcohol and other drugs of abuse before each experimental session. Participants were excluded if they had a current Axis I disorder, including substance dependence (DSM-IV), or any cardiovascular or neurological disorder that would increase risk for study participation.
The studies were conducted according to the principles expressed in the Declaration of Helsinki. All participants provided written informed consent. Although ethical considerations vary from site to site, depending on the local cultural milieu and on aspects of the specific laboratories, the protocols were fully approved by the Ethics Committee of the Canton of Basel, Switzerland and by the Institutional Review Boards at the University of California, San Francisco, the California Pacific Medical Center Research Institute, or the University of Chicago Medical Center.
Procedure, Dependent Measures, and Drug
Before each session, participants provided urine (SF, Chicago, Basel) and breath samples (SF, Chicago) to confirm abstinence from drugs and alcohol, and women were tested for pregnancy. Placebo or MDMA (dispensed by weight: 1.5 mg/kg [SF and Chicago] or fixed-dose: 125 mg [Basel]) was administered orally, under double blind conditions, between 8 and 11 am. Dependent measures, including heart rate and blood pressure and mood states were obtained at baseline and at repeated intervals for several hours after capsule administration. Mood states were measured using visual analog scales (VAS), with descriptors `Do you feel any drug effect?' `Do you like the drug effects?' `Do you feel Stimulated?' `Anxious?' `Closeness to others?'. Each adjective was presented with a 100-mm line labeled `not at all' at one end and `extremely' at the other end.
Data Analysis
To evaluate whether the effects of MDMA differed between laboratories, peak cardiovascular measures and subjective effects were analyzed using multilevel linear models (MLMs). Independent (fixed) effects were Dose (placebo, MDMA), Lab (SF, Chicago, Basel), and Sex. Past MDMA use and Participant were used as random effects. MLMs provided the error terms needed to calculate planned comparisons between the three laboratories following both placebo and active MDMA administration. To evaluate the potential relationship between MDMA use history and acute drug response, we made linear models predicting individual effects with past MDMA use, lab, and their interaction term. Due to significant Shapiro-Wilks tests of residuals, appearance of qqplots, and significant linear relationships between absolute values of residuals and fitted values in models, past MDMA use was transformed by taking the log of 1 + the original value (hereafter log + 1) to reduce non-normality of residuals. For all analyses, p values were considered statistically significant at less than 0.05. For all statistical models, participant Sex did not alter any results and thus is not discussed further in this report.
Results
Participants
In all, 220 individuals (44% Female) completed study procedures. As a group, they were 25.4 ± 5.2 (mean ± SD) years old and weighed 68.8 ± 11.6 kg. Self-reported past MDMA use (i.e., number of occasions of use) ranged from 0 to 200 lifetime uses. SF participants were significantly older and reported greater lifetime MDMA use compared to Chicago and Basel participants, and Chicago participants reported greater lifetime MDMA use than Basel participants (p < 0.05 for all comparisons). A total of 35 participants (all in Basel) had never experienced MDMA outside of the laboratory.
Due to reporting differences between the labs, it is difficult to directly compare the participants' current use of other drugs. Detailed use histories for drugs other than MDMA were unavailable from SF participants. In Basel, 70 out of 80 participants currently used caffeine (2.2 ± 1.3 cups/day), 79 drank alcohol (5.6 ± 3.2 drinks/week), and 21 smoked tobacco (1.1 ± 2.3 cigarettes/day). Additionally, 69 participants had used marijuana at least once in their lifetime (range 1–200). In Chicago, 80 out of 95 participants currently used caffeine (1.8 ± 1.5 cups/day), 89 drank alcohol (9.7 ± 6.8 drinks/week), 36 smoked tobacco (5.1 ± 4.3 cigarettes/day), and 75 currently smoked marijuana (10.1 ± 9.3 days/month).
Acute Effects of MDMA across Sites
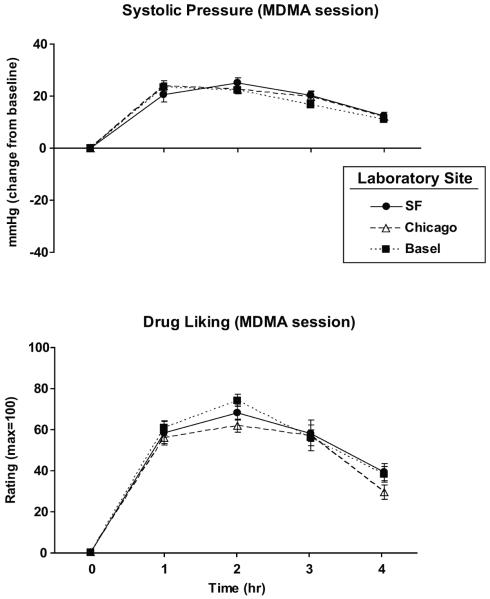
MDMA produced very similar effects at all three sites. Figure 1 shows the time course of the effects of MDMA at the three sites, on a representative subjective measure (`Drug Liking') and a cardiovascular measure (systolic blood pressure). Across sites, the drug produced similar increases in heart rate and blood pressure (Figure 2 top panels; p = 0.15–1.00) and it produced similar reports on most subjective responses. Participants in the three sites did not differ in their peak ratings of `Any Drug Effect,' `Drug Liking,' and `Stimulated' (Figure 2 bottom panels; some data not shown; p = 0.07–1.00) after MDMA administration. Some minor site differences were noted. Chicago participants reported greater peak ratings of `Anxious' after MDMA compared to SF and Basel participants, and SF participants reported greater peak ratings of “Closeness to others” compared to Basel participants (Figure 2; p < 0.05 for all comparisons). Interestingly, following placebo Chicago participants reported significantly greater peak ratings of `Any Drug Effect' and `Drug Liking' compared to SF and Basel participants (Figure 2; p < 0.01 for all comparisons). Additionally, Basel participants exhibited lower peak systolic and diastolic pressure on placebo sessions, compared to the other laboratories (Figure 2; p < 0.05 for all comparisons).
Figure 1.
Systolic pressure (top) and subjective ratings of `Drug Liking' (bottom) during the MDMA session as a function of time and laboratory site. Error bars represent SEM.
Figure 2.
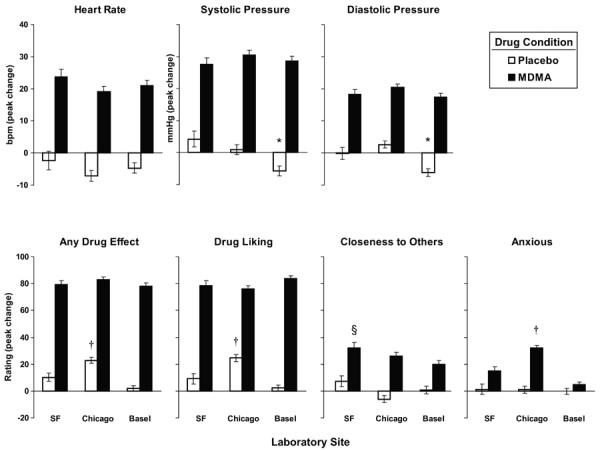
Mean peak (change from baseline) cardiovascular measures and subjective-effect ratings as a function of laboratory site and drug (placebo, MDMA). Error bars represent one SEM. An * indicates significant difference between Basel and the other two sites (p<0.05). An † indicates significant difference between Chicago and the other two sites (p<0.05). An § indicates significant difference between SF and Basel (p<0.05).
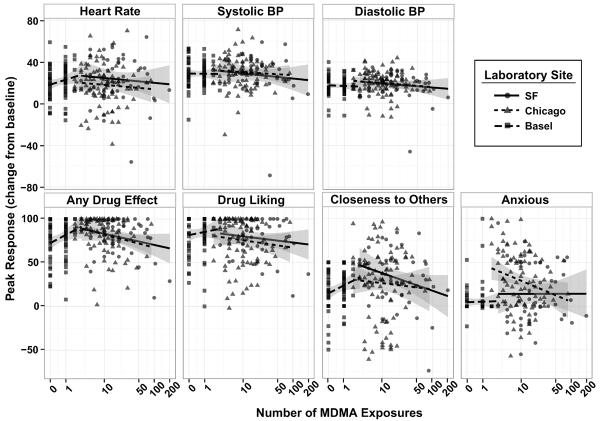
Relationship between acute drug-related effects and MDMA use history
Figure 3 depicts individual MDMA cardiovascular and subjective responses as a function of previous MDMA use. Ratings of feeling `Any Drug Effect' after MDMA were related to prior lifetime use in ways that varied across sites (significant Use × Site interaction; F2, 218 = 4.74, p = 0.010). For Chicago participants, ratings of `Any Drug Effect' decreased by 6.8 (95% CI: 0.913 to 12.7) points for each prior log+1 exposure, and for SF participants a similar decreased response was observed at a trend level (estimated 5.8 points; 95% CI: −1.00 to 12.53; p = 0.094; Chicago and SF sites 6.1 points (95% CI: 2.18 to 10.0 decrease) for each previous log+1 exposure). However, the Basel participants, who had used MDMA less than three times or not at all, reported increased ratings of feeling a drug effect across their uses, by 13.5 (95% CI: 1.63 to 25.3) points for each prior log+1 exposure. No other relationships between past use and acute effects reached statistical significance.
Figure 3.
Scatter plots of peak (change from baseline) cardiovascular measures and subjective-effect ratings during the MDMA session as a function of number of MDMA exposures. Linear fit lines are shown for each laboratory site.
Discussion
We showed that acute responses to MDMA are highly stable across different testing sites, and in different subject samples. The drug produced very similar increases in heart rate, blood pressure and subjective effects across the three laboratories, despite a number of methodological differences. Additionally, the data provide modest evidence for the development of tolerance to some effects of the drug among heavier users if the drug.
The fluctuations that can be expected across different testing sites failed to influence the subjective and cardiovascular responses to the drug. Across the three sites, participants differed in language, culture, age and past MDMA use and probably other drug use and other unmeasured demographic characteristics, as well. Different experimenters monitored the subjects under varying conditions (in a hospital bed, in a recreational environment), and the instructions to subjects and subjects' expectancies varied across settings. Some subjects were tested in the context of larger studies involving other drug administration. The pharmacological effects of the drug were consistent and robust. These findings may be compared to data with other drugs: the effects of amphetamines appear to be stable across contexts (Zacny et al. 1992; de Wit et al. 1997), whereas the effects of hallucinogens depend significantly on the context in which they are used (Studerus et al. 2012).
Despite the marked similarities in responses to MDMA across sites, there were some differences in subjective drug response across sites. That is, SF participants reported relatively greater ratings of `Closeness to others' and Chicago participants reported greater ratings of `Anxious' following MDMA administration. These differences may reflect either procedural differences, or subtle differences in the subject samples. SF and Basel participants received MDMA in a social setting (i.e., researchers were always present in the room) whereas Chicago participants were tested alone. Given that the psychosocial context can influence and enhance subjective responses to drugs (Carlin et al. 1972; Pliner and Cappell 1974; Doty and de Wit 1995; de Wit et al. 1997; Kirkpatrick and de Wit 2013), it is possible that this social setting enhanced the “prosocial” – and reduced the “negative” – effects of MDMA. Additionally, MDMA-induced feelings of interpersonal closeness may result from a learned association between the drug and the social setting in which is has been used. Thus, the SF participants, who had more MDMA experience, may have felt more prosocial effects than the Basel participants because of their conditioning history. Interestingly, however, ratings of `Closeness to Others' were negatively related to prior MDMA use in the SF group (at trend level: p = 0.07), suggesting that tolerance may develop to this effect.
There was also a minor difference between the sites in placebo response: Basel participants exhibited lower blood pressure after placebo compared to the other laboratories, possibly because they remained at rest in hospital beds throughout the session. Additionally, Chicago participants reported greater drug liking after placebo compared to the other laboratories, perhaps because of subtle aspects of instructions and blinding procedures (e.g., SF and Basel participants were explicitly told that they would receive either MDMA or placebo whereas in Chicago participants were informed that they could receive a stimulant, sedative, opioid, cannabinoid or placebo). Thus, it is possible that the expectancy of receiving any drug affected placebo responses in the Chicago population.
We found some evidence that greater prior use of MDMA was associated with lesser ratings of feeling `Any Drug Effect.' This finding appears consistent with epidemiological evidence suggesting that more experienced users of MDMA feel lesser effects of the drug (Solowij et al. 1992; Verheyden et al. 2003; Parrott 2005; Sumnall et al. 2006). To our knowledge, the current study is the first to report a relationship between MDMA use history and acute response to a known, standardized MDMA dose. Thus, these data provide the strongest support for the idea that repeated use of MDMA leads to an attenuation of the subjective drug experience. Surprisingly, participants in Basel, who had little or no prior experience, appeared to show the opposite pattern. The subjects in Basel typically had no experience with MDMA before participating in the studies, and so their responses reflected only their 1st, 2nd or 3rd experience with the drug, ever. It is possible that subjects come to identify the drug effect more accurately across these initial experiences. In any case, however, the present findings provide little support for the idea that the first response to this drug is dramatically greater than subsequent uses.
The negative relation between prior drug use and ratings of feeling `Any Drug Effect' in the SF and Chicago sites is consistent with chronic pharmacological tolerance, as has been reported in animal studies (Zacny et al. 1990; Frederick et al. 1998; Fantegrossi et al. 2004; Baumann et al. 2008). Surprisingly this relationship only reached significance with this single measure, suggesting that it may have resulted from factors other than pharmacological tolerance. For example, heavier users of one drug are likely also to use relatively high doses of other potent recreational drugs, which may lessen their assessments of this moderate dose of MDMA, administered in a laboratory environment. It is also possible that tolerance develops in the natural ecology but is less detectable in the novel laboratory setting because of differences in the doses used, the time of day of use, or the context of use (Winstock et al. 2001; Degenhardt et al. 2004). That is, tolerance may be environment-specific. Another possibility is that the relationship between prior use and acute drug effects was limited to the measure of `Any Drug Effect' because this is a global rating that encompasses the full range of perceived drug effects; high variability on other measures may reduce the sensitivity to detect relationships. Nevertheless, the current results suggest that chronic tolerance may develop to some MDMA-related subjective effects, and this would be an interesting topic for future research.
Unlike some previous reports, we did not observe any gender differences in MDMA-related subjective or cardiovascular effects (e.g., Verheyden et al. 2002; Liechti et al. 2001). For example, Liechti and colleagues (2001) observed that women reported greater subjective responses to the drug, but lesser cardiovascular responses. However, other studies have not detected these differences (Dumont et al. 2008). The differences between these studies could be related to differences in subjective-effects measures (e.g., perceptual effects and thought disturbances versus mood), subject samples, or differences in hormonal states in women at the time of testing (White et al. 2002). Future studies might systematically investigate the influence of menstrual cycle phase on the acute subjective, physiological, and behavioral effects of MDMA.
In conclusion, we found that an acute dose of MDMA, administered under controlled conditions, produced very similar effects at three quite different laboratory sites. This indicates that the drug's effects are robust and reproducible. We also found that previous exposure to MDMA or ecstasy was related to a measure of subjective drug response, providing some evidence of tolerance to the drug's effects. Thus, overall the current data provide modest evidence for chronic tolerance to the effects of MDMA and provide little evidence that the initial experiences are dramatically greater than subsequent experiences. These findings extend our understanding of the repeatability and pharmacological profile of MDMA. Similar studies with other drugs might reveal the specific and common effects of drugs studied across sites and study populations.
Acknowledgements
This research was supported by DA02812 and DA026570 (Chicago: Harriet de Wit PI), DA017716 and DA016776 (SF: John E. Mendelson PI) and SNSF320030_138481 and SNFF32323B_144996 (Basel: Matthias E. Liechti PI).
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
REFERENCES
- Baggott M, Heifets B, Jones RT, Mendelson J, Sferios E, Zehnder J. Chemical analysis of ecstasy pills. JAMA. 2000;284:2190. doi: 10.1001/jama.284.17.2190. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is Ecstasy an “Empathogen”? Effects of ±3,4-Methylenedioxymethamphetamine on Prosocial Feelings and Identification of Emotional States in Others. Biological Psychiatry. 2010;68:1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin AS, Bakker CB, Halpern L, Post RD. Social facilitation of marijuana intoxication: impact of social set and pharmacological activity. J Abnorm Psychol. 1972;80:132–140. doi: 10.1037/h0033317. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Barker B, Topp L. Patterns of ecstasy use in Australia: findings from a national household survey. Addiction. 2004;99:187–195. doi: 10.1111/j.1360-0443.2003.00622.x. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Ortuno J, Mas M, Brenneisen R, Roset PN, Segura J, Cami J. Non-linear pharmacokinetics of MDMA (`ecstasy') in humans. Br J Clin Pharmacol. 2000;49:104–9. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Clark M, Brauer LH. Effects of d-amphetamine in grouped versus isolated humans. Pharmacol Biochem Behav. 1997;57:333–340. doi: 10.1016/s0091-3057(96)00316-4. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl) 1995;118:19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Sweep FC, van der Steen R, Hermsen R, Donders AR, Touw DJ, van Gerven JM, Buitelaar JK, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. 2009;4:359–66. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, et al. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Frederick DL, Ali SF, Gillam MP, Gossett J, Slikker W, Paule MG. Acute effects of dexfenfluramine (d-FEN) and methylenedioxymethamphetamine (MDMA) before and after short-course, high-dose treatment. Ann N Y Acad Sci. 1998;844:183–190. [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 2013;38:802–16. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME. Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 2012;224:363–376. doi: 10.1007/s00213-012-2761-6. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2012;219:109–22. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H. In the company of others: social factors alter acute alcohol effects. Psychopharmacology (Berl) 2013;230:215–26. doi: 10.1007/s00213-013-3147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Geyer MA, Hell D, Vollenweider FX. Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001;24:240–52. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Morefield KM, Keane M, Felgate P, White JM, Irvine RJ. Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational `ecstasy' users. Addiction. 2011;106:1293–1300. doi: 10.1111/j.1360-0443.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology (Berl) 2004;173:234–241. doi: 10.1007/s00213-003-1712-7. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J Psychopharmacol. 2005;19:71–83. doi: 10.1177/0269881105048900. [DOI] [PubMed] [Google Scholar]
- Pashler H, Coburn N, Harris CR. Priming of social distance? Failure to replicate effects on social and food judgments. PLoS One. 2012;7:e42510. doi: 10.1371/journal.pone.0042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner P, Cappell H. Modification of affective consequences of alcohol: a comparison of social and solitary drinking. J Abnorm Psychol. 1974;83:418–425. doi: 10.1037/h0036884. [DOI] [PubMed] [Google Scholar]
- Sherlock K, Wolff K, Hay AW, Conner M. Analysis of illicit ecstasy tablets: implications for clinical management in the accident and emergency department. J Accid Emerg Med. 1999;16:194–197. doi: 10.1136/emj.16.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Barry RJ, Steiner GZ. CNV resolution does not cause NoGo anteriorisation of the P3: A failure to replicate Simson et al. Int J Psychophysiol. 2013 doi: 10.1016/j.ijpsycho.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Solowij N, Hall W, Lee N. Recreational MDMA use in Sydney: a profile of `Ecstacy' users and their experiences with the drug. Br J Addict. 1992;87:1161–1172. doi: 10.1111/j.1360-0443.1992.tb02003.x. [DOI] [PubMed] [Google Scholar]
- Spruit IP. Monitoring synthetic drug markets, trends, and public health. Subst Use Misuse. 2001;36:23–47. doi: 10.1081/ja-100000227. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PLoS One. 2012;7:e30800. doi: 10.1371/journal.pone.0030800. doi: 10.1371/journal.pone.0030800. Epub 2012 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Henry JA, Curran HV. Acute, sub-acute and long-term subjective consequences of `ecstasy' (MDMA) consumption in 430 regular users. Hum Psychopharmacol. 2003;18:507–517. doi: 10.1002/hup.529. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Griffiths P, Stewart D. Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug Alcohol Depend. 2001;64:9–17. doi: 10.1016/s0376-8716(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Yong E. Replication studies: Bad copy. Nature. 2012;485:298–300. doi: 10.1038/485298a. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Virus RM, Woolverton WL. Tolerance and cross-tolerance to 3,4-methylenedioxymethamphetamine (MDMA), methamphetamine and methylenedioxyamphetamine. Pharmacol Biochem Behav. 1990;35:637–642. doi: 10.1016/0091-3057(90)90301-w. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Bodker BK, de Wit H. Effects of setting on the subjective and behavioral effects of d-amphetamine in humans. Addict Behav. 1992;17:27–33. doi: 10.1016/0306-4603(92)90050-6. [DOI] [PubMed] [Google Scholar]