Abstract
Objectives
Prior studies have suggested a potential risk of cervical cancer in patients with systemic inflammatory diseases (SID) such as inflammatory bowel disease (IBD) and systemic lupus erythematosus (SLE). The objective of this study was to assess the risk of high-grade cervical dysplasia, a surrogate endpoint for cervical cancer, and cervical cancer in women with SID including IBD, psoriasis, rheumatoid arthritis (RA), or SLE versus non-SID.
Methods
Using U.S. insurance data (2001-2012), we conducted a cohort study that included 133,333 women with SID based on ≥2 diagnoses and ≥1 dispensing for disease-specific treatment and 533,332 women without SID. High-grade cervical dysplasia and cervical cancer was defined by a validated algorithm with a positive predictive value of ≥81%.
Results
Over the mean follow-up of 2.1 years, the crude incidence rate of high-grade cervical dysplasia and cervical cancer per 100,000 person-years was the highest at 141.1 in SLE and the lowest at 82.2 in psoriasis among women with SID, and 73.4 in non-SID women. The multivariable hazard ratio adjusted for potential confounders was 1.07 (95%CI 0.79-1.45) in IBD, 0.96 (95%CI 0.73-1.27) in psoriasis, 1.49 (95%CI 1.11-2.01) in RA, and 1.53 (95%CI 1.07-2.19) in SLE. Multivariable hazard ratios were increased, not statistically significant, in IBD, RA and SLE with baseline use of systemic immunosuppressive drugs or steroids.
Conclusions
The risk of high-grade cervical dysplasia and cervical cancer was 1.5 times higher in RA and SLE compared to non-SID. The risk may be increased in IBD with of systemic immunosuppressive drugs or steroids.
Keywords: cervical dysplasia, systemic inflammatory disease, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease
INTRODUCTION
Human papillomavirus (HPV) infection is the most common sexually transmitted disease (STD) in the U.S. [1] Although most low-grade cervical intraepithelial neoplasia (CIN) lesions regress spontaneously, but the majority of high-grade cervical dysplasia, CIN 2 or 3 do not.[1] Persistent HPV infection, the major risk factor for cervical cancer, is related to other factors such as older age, HPV genotype, coexisting infections, immunosuppression, and inflammation.[1, 2]
Some studies suggested an increased risk of cervical dysplasia and HPV infection in immunocompromised patients including those with systemic inflammatory disease (SID), such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE).[3-8] Although the progression of HPV infection to cervical cancer in immunocompromised women is not yet fully understood, viral reactivation from a latent state in immunocompromised patients has been noted.[9, 10] Impaired innate and cellular immune responses in patients with SID, particularly those taking immunosuppressive drugs, may decrease clearance of HPV infection or regression of CIN and may result in persistent HPV infection and an increased risk of high-grade cervical dysplasia and cervical cancer.[11, 12]
The U.S. Preventive Services Task Force (USPSTF) recommends screening for cervical cancer in women ages 21 to 65 years with Papanicolaou (Pap) smear every 3 years or, for women ages 30 to 65 years who want to lengthen the screening interval, screening with a combination of Pap smear and HPV testing every 5 years.[13] With such routine Pap test, cervical cancer is rare with an estimated annual incidence rate of 7.9/100,000 persons in the U.S., as most women get diagnosed with cervical dysplasia and treated before they develop invasive cervical cancer.[14] High-grade cervical dysplasia, including CIN 2, CIN 3 and carcinoma in situ, has been used as a surrogate endpoint in cervical cancer prevention trials in order to decrease sample size and follow-up duration.[15, 16]
The objectives of this study were 1) to assess the risk of high-grade cervical dysplasia and cervical cancer in women with SID including IBD, psoriasis, RA, and SLE, compared to those without SID and 2) to examine the risk of high-grade cervical dysplasia and cervical cancer specific to each SID compared to those without SID.
METHODS
Data Source
We conducted a cohort study using the claims data from two commercial U.S. health plans, the Wellpoint (2001-2008) and the United Healthcare (2003-2012), which insure primarily working adults and their family members. These databases provide a very large, population-based cohort and contain longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensing on subscribers with medical and pharmacy coverage across the U. S. The quality of data on inpatient diagnoses, procedures, health care utilization and drug dispensing as well as some outpatient diagnoses is known to be high.[17] Both databases have been successfully used in a number of high-quality published studies.[18-22] Because the study databases were de-identified, patient informed consent was not required. The study protocol was approved by the Institutional Review Board of Brigham and Women’s Hospital.
Study Cohort
Among female patients aged 18 years or older, we selected women with SID including RA, SLE, psoriasis, and IBD based on a combination of ≥2 International Classification of Diseases, Ninth Revision (ICD-9) codes on two separate visits that are ≥7 days apart. The start of follow-up period (i.e., index date) was defined as the date of first disease-specific drug dispensing (Supplementary Table 1) after ≥12 months of continuous health plan eligibility; thus, all persons in the ‘SID cohort’ were required to have had two diagnoses of RA, SLE, psoriasis (including psoriatic arthritis), or IBD, and ≥1 filled prescription for disease-specific treatment at the start of follow-up. Nursing home residents, women with hysterectomy, organ transplantation, HIV infection, and malignancy in the 12-month period prior to the index date were excluded. In addition, women with more than one SID diagnosis were excluded; the SID subcohorts, RA, SLE, psoriasis, and IBD, were thus mutually exclusive.
For comparison with the SID cohort, we identified the non-SID cohort among female patients aged 18 years and over who did not have a RA, SLE, psoriasis, or IBD diagnosis during the study period. To compare the risk of high-grade cervical dysplasia and cervical cancer in women with SID with persons who have another chronic medical condition that requires regular visits to a physician, we identified patients who had ≥2 ICD-9 codes for hypertension on 2 separate visits that are ≥7 days apart described herein as the ‘non-SID cohort’. The index date for the non-SID cohort was defined as the date of first anti-hypertensive drug dispensing after ≥12 months of continuous health plan eligibility. Requirement of the same number of visits and the use of prescription as in the SID cohort was chosen to minimize surveillance bias. The aforementioned exclusion criteria were then applied to the non-SID cohort. Patients in the non-SID cohort were then matched to SID patients on age and index date (+/− 30 days) with a 4:1 ratio.
Patients were then followed until the first of any of the following censoring events: development of the outcome, disenrollment, end of study database (i.e. 2008 for the Wellpoint and 2012 for the United databases), or death.
Outcome Definition
The primary outcome of interest was high-grade cervical dysplasia or cervical cancer. To identify the primary outcome, we developed a claims-based algorithm that combined two ICD-9 and a Current Procedural Terminology (CPT) codes for relevant gynecologic procedures or treatment within 30 days after the diagnosis date (Supplementary Table 2) using the billing data in an electronic medical records database.[23] The positive predictive value of the algorithm was 91% in the electronic medical records database and 81% in an independent insurance claims database using cytologic or pathologic diagnosis of CIN 2 or worse as the gold standard.[23] In addition, we assessed number of visits to gynecology (GYN) doctors and number of gynecologic procedures during the follow-up time.
Covariates
A number of predefined variables potentially associated with risk of HPV infection or cervical cancer were assessed using data from the 12-month baseline period prior to the index date. These variables included age, risk factors for HPV infection, comorbidities, medications such as systemic immunosuppressive drugs and steroids, and health care utilization factors (see Table 1). Systemic immunosuppressive drugs included azathioprine, cyclophosphamide, cyclosporine, hydroxyurea, leflunomide, methotrexate, 6-mercaptopurine, mycophenolate, pimecrolimus, tacrolimus, abatacept, adalimumab, alefacept, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab, and ustekinumab. In addition, women who were likely to be sexually active were identified based on a previously validated claims-based algorithm.[24] To quantify patients’ comorbidities at baseline, we also calculated a comorbidity score that combined 20 medical conditions included in both the Charlson Index and the Elixhauser system based on ICD-9.[25]
Table 1. Baseline characteristics of the study cohort in 12 months prior to the index date.
| SID* | Non-SID* | |||||
|---|---|---|---|---|---|---|
| Inflammatory bowel disease |
Psoriasis | Rheumatoid arthritis |
Systemic lupus erythematous |
|||
|
| ||||||
| N | 133,333 | 25,176 | 34,665 | 58,979 | 14,513 | 533,332 |
|
| ||||||
| Percentage or mean ± standard deviation | ||||||
| Follow-up period, years | 2.1 ±1.8 | 2.1 ±1.9 | 2.0 ± 1.8 | 2.1 ± 1.8 | 2.0 ± 1.8 | 2.1 ± 1.9 |
| Age, years | 50.3 ± 11.8 | 47.5 ± 12.0 | 49.0 ± 11.8 | 53.0 ± 11.3 | 47.7 ± 10.8 | 50.3 ± 11.8 |
|
| ||||||
| HPV infection-associated factors | ||||||
|
| ||||||
| Sexually active | 63 | 68 | 61 | 60 | 76 | 54 |
| Oral contraceptives | 11 | 16 | 12 | 9 | 9 | 10 |
| Non-oral contraceptives | 1 | 2 | 1 | 1 | 1 | 1 |
| Smoking | 9 | 10 | 10 | 8 | 11 | 10 |
| Sexually transmitted diseases | 3 | 3 | 3 | 3 | 5 | 3 |
| Substance abuse | 0.5 | 1 | 0.4 | 0.4 | 1 | 0.5 |
| Alcoholism | 0.4 | 0.4 | 0.5 | 0.3 | 0.3 | 0.5 |
| Receipt of HPV vaccine | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 | 0.5 |
|
| ||||||
| Comorbidities | ||||||
|
| ||||||
| Comorbidity score a, median (IQR) |
0.3 ± 1.2 0 (0-1) |
0.5 ± 1.2 0 (0-1) |
0.2 ± 0.9 0 (0-0) |
0.3 ± 1.1 0 (0-1) |
0.7 ± 1.5 0 (0-1) |
−0.3 ± 1.2 −1 (−1-0) |
| Diabetes | 11 | 8 | 12 | 12 | 10 | 21 |
| Chronic kidney disease | 3 | 2 | 1 | 2 | 11 | 2 |
| Liver disease | 5 | 5 | 4 | 4 | 6 | 3 |
| Prior abnormal Pap smears | 4 | 5 | 4 | 3 | 5 | 3 |
|
| ||||||
| Medications | ||||||
|
| ||||||
| Systemic immunosuppressive drugs b |
35 | 13 | 20 | 57 | 21 | 1 |
| Systemic steroids | 38 | 27 | 17 | 52 | 46 | 14 |
| Cumulative steroid dose c, Median (IQR) |
387.5 (940.2) 0 (0-300) |
327.0 (928.7) 0 (0-105) |
77.6 (333.4) 0 (0-0) |
527.0 (1,018.3) 105 (0-605) |
665.5 (1,324.1) 0 (0-750) |
52.0 (299.9) 0 (0-0) |
| NSAIDs | 33 | 20 | 26 | 58 | 39 | 22 |
|
| ||||||
| Health care utilization | ||||||
|
| ||||||
| No. of total physician visits, median (IQR) |
9.6 ± 7.0 8 (5-12) |
8.5 ± 6.5 7 (4-11) |
8.0 ± 6.1 7 (4-10) |
10.5 ± 7.1 9 (6-14) |
11.3 ± 8.1 9 (6-15) |
6.7 ± 5.2 5 (3-9) |
| No. of GYN visits, median (IQR) |
0.8 ±1.9 0 (0-1) |
1.1 ± 2.1 0 (0-1) |
0.9 ± 2.0 0 (0-1) |
0.6 ±1.5 0 (0-1) |
1.0 ± 2.6 0 (0-1) |
0.8 ± 2.3 0 (0-1) |
| No. of emergency room visits, median (IQR) |
0.6 ± 2.9 0 (0-0) |
0.7 ± 3.1 0 (0-0) |
0.6 ± 3.0 0 (0-0) |
0.5 ± 2.6 0 (0-0) |
0.7 ± 3.0 0 (0-0) |
0.5 ± 2.6 0 (0-0) |
| No. of prescription drugs, median (IQR) |
9.8 ± 6.6 9 (5-13) |
9.3 ± 6.4 8 (5-12) |
8.5 ± 6.0 7 (4-11) |
10.6 ± 6.6 9 (6-14) |
11.2 ± 7.3 10 (6-15) |
7.8 ± 5.6 7 (4-11) |
| Acute hospitalizations | 16 | 24 | 10 | 14 | 23 | 15 |
|
| ||||||
| Preventive medical services | ||||||
|
| ||||||
| Pap smears | 44 | 50 | 45 | 40 | 44 | 41 |
| HPV DNA tests | 7 | 8 | 8 | 5 | 8 | 6 |
| Mammograms | 43 | 42 | 42 | 44 | 40 | 41 |
| Fecal occult blood tests | 12 | 13 | 12 | 12 | 11 | 11 |
| Serum cholesterol tests | 41 | 39 | 43 | 41 | 42 | 59 |
| Sigmoidoscopy/colonoscopy | 16 | 51 | 7 | 8 | 9 | 7 |
SID: systemic inflammatory disease, HPV: human papillomavirus, Pap: Papanicolaou, IQR: interquartile range
SID and non-SID cohorts are age- and index date-matched.
The range of combined comorbidity score is −2 to 26.
Systemic immunosuppressive drugs include azathioprine, cyclophosphamide, cyclosporine, hydroxyurea, leflunomide, methotrexate, 6- mercaptopurine, mycophenolate, pimecrolimus, tacrolimus, abatacept, adalimumab, alefacept, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab, and ustekinumab.
cumulative steroid dose equivalent to prednisone was calculated over the 365-day baseline period.
Statistical Analyses
We compared the baseline characteristics of the SID and non-SID cohorts as well as the SID subcohorts. Incidence rates (IRs) of high-grade cervical dysplasia and cervical cancer with 95% confidence interval (CI) were calculated for each cohort and SID subcohort. Unadjusted and multivariable Cox proportional hazard models adjusting for various potential confounders listed in Table 1 were used to compare the risk of high-grade cervical dysplasia and cervical cancer in the SID cohort to those in the non-SID cohort.[26] In separate unadjusted and multivariable Cox models, the risk of high-grade cervical dysplasia and cervical cancer in each SID subcohort was assessed compared to the non-SID cohort. The likelihood ratio test was performed to examine heterogeneity between the SID subcohorts. Kaplan-Meier curves were plotted for the cumulative incidence of high-grade cervical dysplasia and cervical cancer in the SID subcohorts and non-SID cohort.
Multivariable Cox regression models stratified by receipt of baseline Pap smear or HPV testing, and prior abnormal Pap smears were performed for the comparison between the SID and non-SID cohorts. We estimated the proportion of women who had ≥1 visit to GYN or one gynecologic testing done during the follow-up period in each cohort to address surveillance bias as high-grade cervical dysplasia or early cervical cancer would be likely asymptomatic and thus diagnosed mainly with a screening test. Among a subgroup of women with ≥1 visit to GYN or one gynecologic testing (i.e., Pap smear, colposcopy or HPV-DNA test) done during the follow-up period, the risk of high-grade cervical dysplasia and cervical cancer was compared between the SID and non-SID cohorts. Furthermore, we conducted another subgroup analysis in SID patients with and without systemic immunosuppressive drugs or steroids at baseline compared to non-SID patients. All these subgroup analyses were repeated for the comparison between the SID subcohorts and the non-SID cohort
We determined the potential impact of unmeasured confounders on the association between SID and high-grade cervical dysplasia and cervical cancer in a sensitivity analysis.[27] Proportional hazard assumptions for the SID cohort as well as each disease-specific subcohort were not violated, assessed by the Kolmogorov supremum test.[28] All analyses were done using SAS 9.2 Statistical Software (SAS Institute Inc., Cary, NC).
RESULTS
Study Cohort
There were 133,333 SID patients including 25,176 IBD, 34,665 psoriasis, 58,979 RA, and 14,513 SLE patients, and 533,332 non-SID patients. The mean (SD) follow-up time was 2.1 (1.8) years for both SID and non-SID patients. Table 1 shows baseline characteristics of the age- and index date-matched cohorts and SID subcohorts. Little differences across most HPV-infection associated factors and preventive medical services existed between the cohorts. The mean (SD) number of total physician visits was 9.6 (7.0) in the SID cohort and 6.7 (5.2) in the non-SID cohort. However, the mean (SD) number of visits to gynecologists was similar −0.8 (1.9) in the SID cohort and 0.8 (2.3) in the non-SID cohort. Women who were identified as being sexually active or with use of prescription drugs including systemic immunosuppressive drugs and steroids, and comorbidities except diabetes were more common in the SID than the non-SID cohort. The proportion of patients with a sigmoidoscopy or colonoscopy at baseline was higher in the SID cohort due to the IBD subcohort.
Risk of high-grade cervical dysplasia and cervical cancer
The IR of high-grade cervical dysplasia and cervical cancer was 94.2 per 100,000 person-years in the SID and 73.4 per 100,000 person-years in the non-SID cohort (Table 2). Table 3 summarizes the results from various multivariable Cox regression analyses comparing the SID to the non-SID cohort. The HR adjusted for age, HPV infection-associated factors, comorbidities, and the number of prescription drugs was 1.23 (95% CI 1.04-1.46) in the SID cohort. In the fully adjusted model, the HR in the SID cohort became 1.16 (95% CI 0.97-1.39). The likelihood ratio test of the global null hypothesis showed no significant heterogeneity between the SID subcohorts (χ2= 6.04, df=3, p=0.11).
Table 2. Incidence rates (IR) per 100,000 person-years and incidence rate ratios (IRR) of high-grade cervical dysplasia and cervical cancer*.
| SID (N=133,333) |
Non-SID (N=533,332) |
|||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cases (n) |
Person-years, mean (SD) |
IR (95% CI) | Cases (n) |
Person-years, mean (SD) |
IR (95% CI) | |
|
| ||||||
| All patients | 259 | 2.1 (1.8) | 94.2 (83.4-106.4) |
818 | 2.1 (1.9) | 73.4 (68.5-78.6) |
| SID subcohorts | ||||||
|
Inflammatory bowel
disease (n=25,176) |
59 | 2.1 (1.9) | 110.3 (85.4-412.3) |
|||
|
Psoriasis
(n=34,665) |
58 | 2.0 (1.8) | 82.2 (63.5-106.3) |
|||
|
Rheumatoid
arthritis (n=58,979) |
102 | 2.1 (1.8) | 83.3 (68.6-101.1) |
|||
|
Systemic lupus
erythematosus (n=14,513) |
40 | 2.0 (1.8) | 141.1 (103.5-192.4) |
|||
SID and non-SID cohorts are age- and index date-matched.
SID: systemic inflammatory disease, yr: year, SD: standard deviation
Table 3. Hazard ratios (95% confidence intervals) for high-grade cervical dysplasia and cervical cancer.
| SID* (n=133,333) |
IBD (n=25,176) |
Psoriasis (n=34,665) |
RA (n=58,979) |
SLE (n=14,513) |
Non-SID* (n=533,332) |
|
|---|---|---|---|---|---|---|
| Adjustment | ||||||
| Unadjusted | 1.28 (1.11-1.47) |
1.50 (1.16-1.96) |
1.11 (0.85-1.45) |
1.13 (0.92-1.38) |
1.90 (1.38-2.61) |
Referent |
| Age only | 1.27 (1.11-1.47) |
1.22 (0.94-1.59) |
1.00 (0.77-1.31) |
1.40 (1.14-1.72) |
1.66 (1.21-2.28) |
|
| Model 1 | 1.24 (1.07-1.44) |
1.18 (0.90-1.55) |
0.98 (0.77-1.31) |
1.38 (1.12-1.71) |
1.61 (1.17-2.23) |
|
| Model 2 | 1.27 (1.10-1.48) |
1.22 (0.93-1.60) |
0.99 (0.76-1.30) |
1.45 (1.17-1.79) |
1.57 (1.13-2.18) |
|
| Model 3 | 1.23 (1.04-1.46) |
1.20 (0.91-1.58) |
0.99 (0.75-1.30) |
1.44 (1.11-1.88) |
1.56 (1.11-2.19) |
|
| Model 4 | 1.18 (1.00-1.40) |
1.08 (0.80-1.46) |
0.97 (0.74-1.27) |
1.49 (1.14-1.95) |
1.54 (1.08-2.19) |
|
| Model 5 (full model) |
1.16 (0.97-1.39) |
1.07 (0.79-1.45) |
0.96 (0.73-1.27) |
1.49 (1.11-2.01) |
1.53 (1.07-2.19) |
|
SID and non-SID cohorts are age- and index date-matched.
SID: systemic inflammatory disease, IBD: inflammatory bowel disease, RA: rheumatoid arthritis, SLE: systemic luous erythematosus
Model 1 is adjusted for age, comorbidity score, and no. of prescription drugs.
Model 2 is adjusted for alcoholism, smoking, substance abuse, being sexually active, diagnosis of sexually transmitted disease, oral and non-oral contraceptive use, receipt of HPV vaccine, diabetes, chronic kidney disease, and liver disease, in addition to the variables in the model 1.
Model 3 is adjusted for use of systemic immunosuppressive drugs, steroids, and non-steroidal anti-inflammatory drugs, in addition to the variables in the model 2.
Model 4 is adjusted for health care utilization factors and preventive medical services, in addition to the variables in the model 2.
Model 5 is adjusted for health care utilization factors and preventive medical services, in addition to the variables in the model 3.
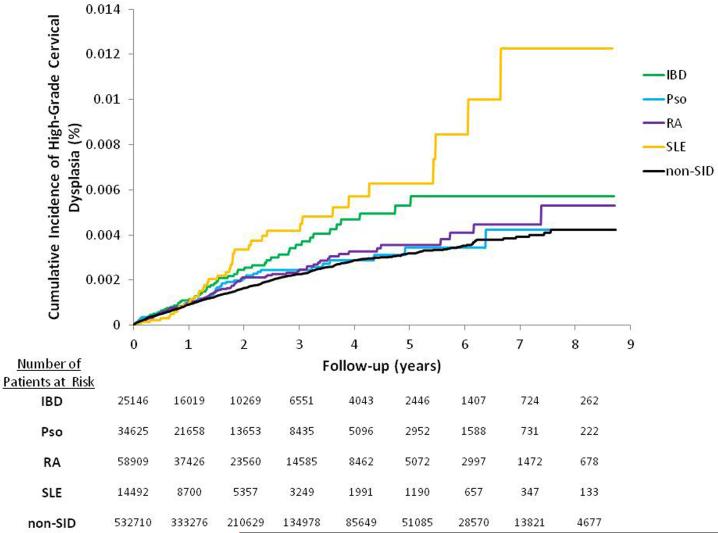
Among the four SID subcohorts, the IR of high-grade cervical dysplasia and cervical cancer was the highest in women with SLE and the lowest in women with psoriasis (Table 2). Kaplan-Meier curves comparing the cumulative incidence of high-grade cervical dysplasia and cervical cancer in the SID subcohorts and non-SID cohort showed an increased risk in the IBD, RA and SLE subcohorts (Figure 1). Unadjusted HRs for high-grade cervical dysplasia and cervical cancer were elevated across all four subcohorts, with the highest HR of 1.90 in SLE and the lowest HR of 1.11 in psoriasis (Table 3). With full adjustment, the HR was not increased in the IBD (1.07, 95% CI 0.79-1.45) and psoriasis (0.96, 95% CI 0.73-1.27) subcohorts. For the SLE subcohort, the HR was attenuated but remained elevated at 1.53 (95% CI 1.07-2.19) in the fully adjusted model. In the RA subcohort, further adjustment for potential confounders moved the HR away from the null with the fully adjusted HR of 1.49 (95% CI 1.11-2.00).
Figure 1. Kaplan-Meier curves for the cumulative incidence of high-grade cervical dysplasia and cervical cancer.
IBD: inflammatory bowel disease, Pso: psoriasis, RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, SID: systemic inflammatory disease
Stratified and Subgroup Analyses (Table 4)
Table 4. Stratified and subgroup analyses: fully adjusted a hazard ratios for high-grade cervical dysplasia and cervical cancer in patients with systemic inflammatory disease (SlD) compared to those without SID.
| SID | IBD | Psoriasis | RA | SLE | Non-SID | ||
|---|---|---|---|---|---|---|---|
| Pap smears or HPV-DNA testing done at baseline | |||||||
| Yes | |||||||
| N Outcomes | 58,110 163 |
12,623 42 |
15,795 38 |
23,362 61 |
6,330 22 |
220,766 468 |
|
| HR (95% CI) | 1.27 (1.01-1.60) |
1.23 (0.85-1.77) |
1.03 (0.73-1.46) |
1.73 (1.18-2.59) |
1.60 (0.98-2.59) |
Ref | |
| No | |||||||
| N Outcomes | 75,223 96 |
12,553 17 |
18,870 20 |
35,617 41 |
8,183 18 |
312,566 350 |
|
| HR (95% CI) | 1.01 (0.75-1.35) |
0.82 (0.48-1.41) |
0.85 (0.53-1.35) |
1.21 (0.77-1.92) |
1.46 (0.86-2.50) |
Ref | |
|
| |||||||
| Prior abnormal Pap smears | |||||||
| Yes | |||||||
| N Outcomes | 5,339 81 |
1,267 19 |
1,456 21 |
1,929 32 |
687 9 |
17,589 220 |
|
| HR (95% CI) | 1.23 (0.88-1.70) |
0.90 (0.52-1.55) |
1.17 (0.73-1.86) |
2.19 (1.29-3.72) |
1.18 (0.56-2.51) |
Ref | |
| No | |||||||
| N Outcomes | 127,994 178 |
23,909 40 |
33,209 37 |
57,050 70 |
13,826 31 |
515,743 598 |
|
| HR (95% CI) | 1.13 (0.92-1.41) |
1.13 (0.78-1.63) |
0.87 (0.62-1.23) |
1.31 (0.92-1.87) |
1.71 (1.14-2.56) |
Ref | |
|
| |||||||
| At least 1 gynecologic visit or procedure during the follow-up time | |||||||
| Yes | |||||||
| N Outcomes | 70,753 259 |
14,969 59 |
19,072 58 |
28,929 102 |
7,783 40 |
267,823 817 |
|
| HR (95% CI) | 1.14 (0.95-1.36) |
1.03 (0.76-1.39) |
0.94 (0.71-1.24) |
1.53 (1.13-2.06) |
1.52 (1.06-2.18) |
Ref | |
|
| |||||||
| Use of systemic immunosuppressive drugs at baseline b | |||||||
| Yes | |||||||
| N Outcomes | 46,626 85 |
3,150 10 |
6,862 11 |
33,568 52 |
3,046 12 |
533,332 c 818 |
|
| HR (95% CI) | 1.31 (0.97-1.77) |
1.72 (0.66-4.45) |
1.04 (0.43-2.51) |
1.40 (0.65-3.03) |
1.52 (0.64-3.61) |
Ref | |
| No | |||||||
| N Outcomes | 86,707 174 |
22,026 49 |
27,803 47 |
25,411 50 |
11,467 28 |
533,332 c 818 |
|
| HR (95% CI) | 1.15 (0.95-1.39) |
1.02 (0.73-1.42) |
0.96 (0.71-1.30) |
1.73 (1.21-2.47) |
1.53 (1.00-2.35) |
Ref | |
|
| |||||||
| Use of systemic steroids at baseline | |||||||
| Yes | |||||||
| N Outcomes | 50,208 102 |
6,814 19 |
5,820 5 |
30,831 55 |
6,743 23 |
533,332 c 818 |
|
| HR (95% CI) | 1.33 (1.00-1.76) |
1.30 (0.79-2.13) |
0.62 (0.25-1.50) |
1.49 (1.00-2.21) |
1.69 (1.05-2.72) |
Ref | |
| No | |||||||
| N Outcomes | 83,125 157 |
18,362 40 |
28,845 53 |
28,148 47 |
7,770 17 |
533,332 c 818 |
|
| HR (95% CI) | 1.14 (0.93-1.41) |
1.04 (0.73-1.48) |
1.04 (0.78-1.39) |
1.59 (1.06-2.38) |
1.45 (0.87-2.42) |
Ref | |
SID: systemic inflammatory disease, IBD: inflammatory bowel disease, RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, HPV: human papillomavirus, HR: hazard ratio, CI: confidence interval
Adjusted for age, comorbidity score, no. of prescription drugs, alcoholism, smoking, substance abuse, being sexually active, diagnosis of sexually transmitted disease, oral and non-oral contraceptive use, receipt of HPV vaccine, diabetes, chronic kidney disease, liver disease, use of systemic immunosuppressive drugs, systemic steroids, and non-steroidal anti-inflammatory drugs, health care utilization factors, and preventive medical services.
Systemic immunosuppressive drugs include azathioprine, cyclophosphamide, cyclosporine, hydroxyurea, leflunomide, methotrexate, 6- mercaptopurine, mycophenolate, pimecrolimus, tacrolimus, abatacept, adalimumab, alefacept, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab, and ustekinumab.
The entire non-SID cohort was used as a comparison.
In women with ≥1 baseline Pap smear or HPV-DNA testing done, the fully adjusted HR was 1.73 (95% CI 1.18-2.59) in RA and 1.60 (95% CI 0.98-2.59) in SLE compared to women without SID. Among the women without prior history of abnormal Pap smear, the fully adjusted HR was 1.13 (95% CI 0.78-1.63) for IBD, 0.87 (95% CI 0.62-1.23) for psoriasis, 1.31 (95% CI 0.92-1.87) for RA, and 1.71 (95% CI 1.14-2.56) for SLE compared to women without SID.
Among the women with baseline use of systemic immunosuppressive drugs, the fully adjusted HR was increased, but not statistically significantly, in IBD (1.72, 95% CI 0.66-4.45), RA (1.40, 95% CI 0.65-3.03) and SLE (1.52, 95% CI 0.64-3.61) compared to non-SID women. Similarly, the fully adjusted HR was increased, albeit not statistically significant, in women with IBD, RA and SLE who used systemic steroids at baseline compared to non-SID women
During the mean 2.1 years of follow-up, 53% of women with SID and 50% of women without SID had ≥1 visit to GYN or ≥1 gynecologic testing done. Among the SID subcohorts, 60% of women with IBD, 55% with psoriasis, 49% with RA, and 54% with SLE had ≥1 visit to GYN or ≥1 gynecologic testing. The mean (SD) number of GYN visits during the follow-up was 3.1 (4.8) in the SID cohort and 3.0 (4.8) in the non-SID. The mean (SD) number of gynecologic tests was also similar between the cohorts, 2.1 (2.1) in the SID and 2.1 (2.0) in the non-SID. Among those women with ≥1 visit to GYN or 1 gynecologic test, the HRs were consistently elevated in RA (1.53, 95% CI 1.13-2.06) and SLE (1.52, 95% CI 1.06-2.18).
Sensitivity Analysis
Supplementary Figure 1 illustrates the potential impact of residual confounding on our results assessed by the rule-out approach.[27] Unless very strong risk factors of cervical cancer that are imbalanced between the two groups are unmeasured and uncontrolled in our fully adjusted models, the increased HR associated with RA and SLE cannot be explained by residual confounding.
DISCUSSION
This study found that the incidence of high-grade cervical dysplasia and cervical cancer was very low. It is still important to note that the diagnosis of high-grade cervical dysplasia and cervical cancer is associated with substantial psychosocial burden and high health care utilization for diagnostic and therapeutic procedures.[29, 30] Women with RA and SLE appeared to have a 1.5 times greater risk of high-grade cervical dysplasia and cervical cancer compared to the non-SID after adjusting for many potential confounders. No significantly increased risks were noted in women with psoriasis or IBD. These findings might be related to the difference in the severity of systemic inflammation or in the use of systemic immunosuppressive drugs or steroids across the 4 subcohorts as the majority of RA and SLE patients, but only 20% of psoriasis and 13% of IBD patients were on systemic immunosuppressive drugs at baseline. Although a few studies reported an increased risk of abnormal Pap smears in IBD patients,[3, 4] others did not find an increased risk of CIN or cervical cancer in IBD.[31-33] Our study also found that, among women with baseline use of systemic immunosuppressive drugs or steroids, IBD, RA and SLE may be associated with an increased risk.
In our multivariable analyses, the HRs were attenuated in IBD, psoriasis and SLE, while the HRs for RA increased with more adjustment. Age appeared to be a major confounder on the association between RA and high-grade cervical dysplasia and cervical cancer; adjusting only for age moved the HR from 1.13 to 1.40. Among all the subcohorts, the mean age was the highest in RA. As the high-grade cervical dysplasia and cervical cancer is more common in younger women, the absolute risk might not be high in women with RA, but the adjusted risk relative to women without SID was increased.
Several strengths of this study are worth noting. First, we examined a large cohort of SID including IBD, psoriasis, RA and SLE, and non-SID patients in a population that is representative of the U.S. commercially-insured population. Studying relatively uncommon exposures and outcomes is methodologically challenging. With the large size of our study cohort, we were able to observe a total of 1,077 women with high-grade cervical dysplasia and cervical cancer. Second, we provided the overall and disease-specific relative risk of high-grade cervical dysplasia and cervical cancer in women with SID, adjusted for age, known HPV infection-associated factors, comorbidities, medications, and health care utilization including preventive medical services. Third, to minimize surveillance bias, we selected the non-SID cohorts as a group of patients with hypertension, a chronic medical condition requiring a regular medical care. Both women with and without SID had overall similar general health care utilization and preventive medical services at baseline as well as during the follow-up. Fourth, we mainly relied on diagnosis and procedure codes for exposure and outcome ascertainment which could potentially lead to exposure and outcome misclassification. To maximize the specificity, we used a combination of diagnosis codes and a dispensing for a disease-specific treatment to select women with SID and identified high-grade cervical dysplasia and cervical cancer with a previously validated claims-based algorithm combining two diagnoses and a procedure. [23, 34-37]
There are limitations to this study. First, this cohort study may still be subject to residual confounding by race, ethnicity, socioeconomic status, behavioral characteristics, and gynecologic history of the patients. As sexual activity is a known risk factor for HPV infection and cervical dysplasia, we used a previously validated claims-based algorithm to identify women who were more likely sexually active [24] and found women with SID were more likely to be sexually active than those without SID. The validity of this algorithm in the SID population may require a further testing since previous studies showed a higher proportion of sexual dysfunction in women with SID.[38-41] However, as illustrated in the sensitivity analysis (Supplementary Figure 1), the positive association between RA or SLE and high-grade cervical dysplasia or cervical cancer is unlikely to be fully explained by an unmeasured or incompletely measured confounder. Second, this study cannot differentiate whether the increased risk noted in RA and SLE is due to the disease itself or treatment. Future studies, however, should address this question. Third, this study was not designed to determine the comparative safety of different immunosuppressive drugs on the risk of high-grade cervical dysplasia or cervical cancer. Fourth, our results may not be generalizable to women with lower socioeconomic status, as the study databases primarily include working adults and their family members. Fifth, it is possible that the 12-month baseline period was not long enough to capture all the information on potential confounders.
To date, there are no specific guidelines addressing HPV vaccination or cervical dysplasia management in the SID population. Currently, two different HPV vaccines are available - a bivalent vaccine for types 16 and 18 and a quadrivalent one for types 6, 11, 16, and 18. Although the efficacy of HPV vaccine in the SID population has not been studied and there are other HPV genotypes that are not covered by the current HPV vaccines, young women with SID, particularly those with RA or SLE, should be considered as a target population for the HPV vaccine, given the high efficacy of HPV vaccine for preventing high-grade cervical dysplasia and cervical cancer in the general population[15, 42] and the safety data in SLE patients.[43, 44]
In conclusion, the risk of high-grade cervical dysplasia and cervical cancer was increased in RA and SLE patients with and without use of systemic immunosuppressive drugs or steroids at baseline. The risk may also be increased among women with IBD who had baseline use of systemic immunosuppressive drugs or steroids. Future research should examine whether a different implementation strategy for HPV vaccination or cervical dysplasia management is needed in patients with RA or SLE.
Supplementary Material
Acknowledgements/Disclosures
This study is funded by the NIH grant K23 AR059677.
Kim is supported by the NIH grant K23 AR059677. She received research support from Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health partially funded by the Pharmaceutical Research and Manufacturers of America (PhRMA) foundation.
Glynn has received research support from AstraZeneca and Novartis for design, monitoring, and analysis of randomized clinical trials.
Hernandez-Diaz is supported by the AHRQ grant R01HS018533 and has consulted for GSK Biologics and Novartis for unrelated projects.
Giovannucci, Liu and Feldman have nothing to disclose.
Karlson is supported by NIH AR052403, AR047782, and AR049880. Schneeweiss is Principal Investigator of the Harvard-Brigham Drug Safety and Risk Management Research Center funded by FDA. His work is partially funded by grants/contracts from PCORI, FDA, and NHLBI. Schneeweiss is consultant to WHISCON, LLC and to Aetion, Inc. of which he also owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, and Boehringer-Ingelheim unrelated to the topic of this study.
Solomon is supported by the NIH grants K24 AR055989, P60 AR047782 and R01 AR056215. Solomon has received research support from Amgen and Lilly. He serves in unpaid roles on studies sponsored by Pfizer, Novartis, Lilly, and Bristol Myers Squibb.
Footnotes
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Kim receives research support from Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health partially funded by the Pharmaceutical Research and Manufacturers of America (PhRMA) foundation. Glynn receives research support from AstraZeneca and Novartis. Hernandez-Diaz has consulted for GSK Biologics and Novartis for unrelated projects. Schneeweiss is consultant to WHISCON, LLC and to Aetion, Inc. of which he also owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, and Boehringer-Ingelheim unrelated to the topic of this study. Giovannucci, Liu, Karlson, and Feldman have nothing to disclose for financial support or conflict of interest. Solomon receives research support from Amgen, Lilly, Pfizer, and CORRONA, and serves in unpaid roles on studies sponsored by Pfizer, Novartis, Lilly, and Bristol Myers Squibb.
REFERENCES
- 1.Wheeler CM. Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin North Am. 2008;35:519–36. doi: 10.1016/j.ogc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Dalstein V, Riethmuller D, Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. International journal of cancer Journal international du cancer. 2003 Sep 1;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia J, Bratcher J, Korelitz B, et al. Abnormalities of uterine cervix in women with inflammatory bowel disease. World journal of gastroenterology: WJG. 2006 Oct 14;12:6167–71. doi: 10.3748/wjg.v12.i38.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. The American journal of gastroenterology. 2008 Mar;103:631–6. doi: 10.1111/j.1572-0241.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 5.Rojo Contreras W, Montoya Fuentes H, Gamez Nava JI, et al. Prevalence and cervical human papilloma virus associated factors in patients with rheumatoid arthritis. Ginecologia y obstetricia de Mexico. 2008 Jan;76:9–17. [PubMed] [Google Scholar]
- 6.Santana IU, Gomes Ado N, Lyrio LD, et al. Systemic lupus erythematosus, human papillomavirus infection, cervical pre-malignant and malignant lesions: a systematic review. Clinical rheumatology. 2011 May;30:665–72. doi: 10.1007/s10067-010-1606-0. [DOI] [PubMed] [Google Scholar]
- 7.Tam LS, Chan PK, Ho SC, et al. Risk factors for squamous intraepithelial lesions in systemic lupus erythematosus: a prospective cohort study. Arthritis care & research. 2011 Feb;63:269–76. doi: 10.1002/acr.20367. [DOI] [PubMed] [Google Scholar]
- 8.Rojo-Contreras W, Olivas-Flores EM, Gamez-Nava JI, et al. Cervical human papillomavirus infection in Mexican women with systemic lupus erythematosus or rheumatoid arthritis. Lupus. 2012 Apr;21:365–72. doi: 10.1177/0961203311425517. [DOI] [PubMed] [Google Scholar]
- 9.Strickler H, Burk R, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Pineres A, Hildesheim A, Herrero R, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070–6. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 11.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2012 Dec;18:807–15. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 12.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clinical microbiology reviews. 2012 Apr;25:215–22. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Force USPST Screening for cervical cancer 2012 March [Google Scholar]
- 14.Cancer US, Statistics Working Group United States Cancer Statistics: 1999-2009 Incidence and Mortality Web-based Report. 2013 cited 2013 May 29. Available from: www.cdc.gov/uscs. [Google Scholar]
- 15.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. The New England journal of medicine. 2007 May 10;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 16.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstetrics and gynecology. 2006 Jan;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 17.Strom BL. Overview of Automated Databases in Pharmacoepidemiology. In: Strom BL, Kimmel SE, editors. Textbook of Pharmacoepidemiology. John Wiley & Sons, Ltd; Philadelphia: 2006. pp. 167–72. [Google Scholar]
- 18.Patorno E, Bohn RL, Wahl PM, et al. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. JAMA: the journal of the American Medical Association. 2010 Apr 14;303:1401–9. doi: 10.1001/jama.2010.410. [DOI] [PubMed] [Google Scholar]
- 19.Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA: the journal of the American Medical Association. 2011 Jun 22;305:2525–31. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss S, Doherty M, Zhu S, et al. Dermatology. Vol. 219. Basel, Switzerland: 2009. Topical treatments with pimecrolimus, tacrolimus and medium-to high-potency corticosteroids, and risk of lymphoma; pp. 7–21. [DOI] [PubMed] [Google Scholar]
- 21.Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinylestradiol/drospirenone and other oral contraceptives. Obstetrics and gynecology. 2007 Sep;110:587–93. doi: 10.1097/01.AOG.0000279448.62221.a8. [DOI] [PubMed] [Google Scholar]
- 22.Wang FT, Mast TC, Glass RJ, et al. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010 Feb;125:e208–13. doi: 10.1542/peds.2009-1246. [DOI] [PubMed] [Google Scholar]
- 23.Kim SC, Gillet VG, Feldman S, et al. Validation of claims-based algorithms for identification of high-grade cervical dysplasia and cervical cancer. Pharmacoepidemiology and drug safety. 2013 Nov;22:1239–44. doi: 10.1002/pds.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangione-Smith R, McGlynn EA, Hiatt L. Screening for chlamydia in adolescents and young women. Archives of pediatrics & adolescent medicine. 2000 Nov;154:1108–13. doi: 10.1001/archpedi.154.11.1108. [DOI] [PubMed] [Google Scholar]
- 25.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 27.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 28.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 29.Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sexually transmitted infections. 2009 Dec;85:508–13. doi: 10.1136/sti.2009.037028. [DOI] [PubMed] [Google Scholar]
- 30.Kruzikas D, Smith JS, Harley C, et al. Costs associated with management of cervical human papillomavirus-related conditions. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012 Sep;21:1469–78. doi: 10.1158/1055-9965.EPI-11-1019. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001 Feb 15;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Hutfless S, Fireman B, Kane S, et al. Screening differences and risk of cervical cancer in inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2008 Sep 1;28:598–605. doi: 10.1111/j.1365-2036.2008.03766.x. [DOI] [PubMed] [Google Scholar]
- 33.Lees CW, Critchley J, Chee N, et al. Lack of association between cervical dysplasia and IBD: a large case-control study. Inflammatory bowel diseases. 2009 Nov;15:1621–9. doi: 10.1002/ibd.20959. [DOI] [PubMed] [Google Scholar]
- 34.Abuabara K, Lee H, Kimball AB. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br J Dermatol. 2011 Nov;165:1066–73. doi: 10.1111/j.1365-2133.2011.10525.x. [DOI] [PubMed] [Google Scholar]
- 35.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010 May;19:741–3. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis research & therapy. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013 Mar;65:753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marin L, Manosa M, Garcia-Planella E, et al. Sexual function and patients’ perceptions in inflammatory bowel disease: a case-control survey. Journal of gastroenterology. 2013 Jun;48:713–20. doi: 10.1007/s00535-012-0700-2. [DOI] [PubMed] [Google Scholar]
- 39.Kobelt G, Texier-Richard B, Mimoun S, et al. Rheumatoid arthritis and sexuality: a patient survey in France. BMC musculoskeletal disorders. 2012;13:170. doi: 10.1186/1471-2474-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia Morales M, Callejas Rubio JI, Peralta-Ramirez MI, et al. Impaired sexual function in women with systemic lupus erythematosus: a cross-sectional study. Lupus. 2013 Sep;22:987–95. doi: 10.1177/0961203313500370. [DOI] [PubMed] [Google Scholar]
- 41.Maaty AS, Gomaa AH, Mohammed GF, et al. Assessment of female sexual function in patients with psoriasis. The journal of sexual medicine. 2013 Jun;10:1545–8. doi: 10.1111/jsm.12119. [DOI] [PubMed] [Google Scholar]
- 42.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012 Nov 20;30(Suppl 5):F123–38. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mok CC, Ho LY, Fong LS, et al. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Annals of the rheumatic diseases. 2013 May;72:659–64. doi: 10.1136/annrheumdis-2012-201393. [DOI] [PubMed] [Google Scholar]
- 44.Soybilgic A, Onel KB, Utset T, et al. Safety and immunogenicity of the quadrivalent HPV vaccine in female Systemic Lupus Erythematosus patients aged 12 to 26 years. Pediatric rheumatology online journal. 2013 Aug 7;11:29. doi: 10.1186/1546-0096-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.