Abstract
We (Kranzler et al. 2014) reported that topiramate 200 mg/day reduced heavy drinking days and increased abstinent days in 138 heavy drinkers whose treatment goal was to reduce drinking to safe levels. In that 12-week, placebo-controlled study, we measured drinking using the Timeline Follow-back method at each treatment visit. In addition to the intent-to-treat effects of topiramate, we found that a single nucleotide polymorphism (rs2832407) in GRIK1, encoding the GluK1 subunit of the kainate receptor, moderated the treatment effect in European Americans (EAs; n=122). Topiramate reduced heavy drinking only in rs2832407*C allele homozygotes. Here, we augment those analyses by using patients’ daily reports obtained using interactive voice response technology (a) to validate the interactive effects of GRIK1 and topiramate as predictors of drinking level and (b) to examine changes in expected positive effects of drinking (i.e., positive outcome expectancies) and desire to drink. We found that rs2832407*C allele homozygotes treated with topiramate drank less overall during treatment than those receiving placebo, validating our earlier findings for heavy drinking days (Kranzler et al. 2014). There was also a study day × medication group × genotype group interaction that predicted both positive alcohol expectancies and desire to drink, with rs2832407*C-allele homozygotes treated with topiramate showing the largest decreases in these outcomes during the study period. Changes in positive alcohol expectancies or desire to drink did not mediate the effects on drinking. These findings validate and extend our previous pharmacogenetic findings with topiramate.
Keywords: Topiramate, Heavy Drinking, GRIK1, Pharmacogenetics, Subjective Effects
INTRODUCTION
Two 14-week, randomized controlled trials of topiramate 300 mg/day, including a single-site study (Johnson et al. 2003) and a multi-center study (Johnson et al. 2007) of alcohol-dependent subjects showed the medication to be superior to placebo in reducing the frequency of drinking and heavy drinking in alcohol-dependent patients. In the multi-center study (Johnson et al. 2008), topiramate also reduced scores on the Obsessive-Compulsive Drinking Scale (OCDS; Anton et al. 1995), a measure of alcohol craving. That study also showed a significantly lower rate of treatment completion in the topiramate group. Recently, topiramate 100-300 mg/day was reported to be no better than placebo in reducing drinking in alcohol-dependent patients (Likhitsathian et al. 2013). However, in that trial, the completion rate was 50% and adherence to the prescribed regimen was not evaluated, making it difficult to interpret the results.
In a three-arm, placebo-controlled study in non-treatment-seeking individuals (Miranda et al., 2008), topiramate was gradually increased to a daily dosage of either 200 mg or 300 mg over a 32-day period and maintained there for one week. During dosage adjustment, the frequency of heavy drinking decreased significantly more in both topiramate groups than in the placebo group (Miranda et al., 2008). In a recent 12-week, parallel-groups, placebo-controlled trial in 138 heavy drinkers who sought to reduce drinking to safe levels, treatment with topiramate 200 mg/day reduced heavy drinking days and increased abstinent days more than placebo (Kranzler et al. 2014). The 200-mg/day dosage was chosen to reduce the risk of dropout due to adverse events, which was seen at a dosage of 300 mg/day in the multi-center trial (Johnson et al. 2007). In the Kranzler et al. (2014) study there were high levels of study completion, and efficacy similar to that seen with the higher dosage of topiramate.
Topiramate's multiple pharmacologic effects include the facilitation of GABAergic function by interacting with a non-benzodiazepine site on the GABAA receptor (White et al. 2000) and antagonism of glutamate activity at AMPA and kainate receptors (Skradski and White 2000, Gibbs et al. 2000). Topiramate's effects on AMPA/kainate receptors are most potent and selective for those containing the GluK1 and GluK2 subunits (encoded by GRIK1 and GRIK2, respectively) (Gryder and Rogawski 2003, Kaminski et al. 2004). Topiramate also inhibits carbonic anhydrase, enhances K+ conductance, and blocks voltage-dependent Na+ and L-type voltage-gated Ca++ channels (McDonald and Rogawski 2006).
Based on the effects of topiramate at GluK1-containing kainate receptors, we (Kranzler et al. 2009) examined the association to alcohol dependence of seven single nucleotide polymorphisms (SNPs) in GRIK1. One SNP, rs2832407, a C-to-A non-coding substitution, was significantly associated with alcohol dependence, with the rs2832407*C allele, the major allele in EAs, being more common in alcohol-dependent subjects. Rs2832407 was not in linkage disequilibrium (LD) with any of the other six SNPs examined by Kranzler et al. (2009).
As hypothesized, based on these findings, we found that in the EA subsample from our topiramate treatment study (Kranzler et al. 2014), rs2832407 moderated the reduction in heavy drinking days. Specifically, topiramate's effect on heavy drinking was greater than placebo only in rs2832407*C-allele homozygotes. Although the mechanism for this moderating effect remains to be determined, rs2832407 is located 800 bp upstream of a GRIK1 antisense transcript. Analysis of data from the 1000 Genomes Project (1000 Genomes Project Consortium 2012) shows that rs2832407 is in near-complete linkage disequilibrium (r2=0.99) with rs363431, which maps to an antisense transcript in GRIK1. Thus, one possible mechanism for the moderating effect of rs2832407 is that the A allele, either by directly or indirectly influencing the expression of GRIK1 antisense RNA, reduces the effect of topiramate at GluK1-containing kainate receptors.
Although the potent AMPA/kainate competitive antagonist CNQX suppressed drinking in rats in a cue-induced reinstatement model (Bäckström and Hyytiä 2004), there are no published studies of the effects of specific antagonists of GluK1-containing kainate receptors on alcohol consumption. Further, there is little known about whether topiramate's impact on the subjective effects of alcohol is associated with the reduced drinking seen in most topiramate trials. Finally, it was of interest to determine whether variation in the gene encoding GluK1 moderated any subjective effects of topiramate.
Based on evidence that topiramate reduced obsessional thoughts and compulsions about using alcohol (Johnson et al. 2008), in the present study, we examined the medication's effect on the self-reported desire to drink. There is also a substantial body of evidence showing that cognitions such as expectancies regarding alcohol's effects predict subsequent drinking behavior (Jones et al. 2001). Much of this literature focuses on college students, in whom expectations of positive effects of alcohol predicted changes in hazardous or problem drinking in both sexes (Werner et al. 1995, Zamboanga et al. 2006). Other studies provide similar evidence of the predictive effects of positive alcohol expectancies on drinking behavior in adults of a variety of ethnic groups (Grotmol et al. 2010, Nicolai et al. 2012, Sawayama et al. 2012). Although topiramate has prominent cognitive adverse effects (Sommer et al. 2013), there is no evidence as to whether its use in alcohol treatment is associated with effects on cognitions such as expectancies.
Consistent with our previous findings (Kranzler et al. 2014), we hypothesized that topiramate-treated rs2832407*C homozygotes would show the greatest reductions in patients’ daily reports of drinking, desire to drink, and positive alcohol expectancies across the 12 weeks of the study. This hypothesis is based on evidence that glutamatergic neurotransmission (Tabakoff and Hoffman 2013), particularly that mediated by kainate receptors (Holmes et al. 2013), is an important neurochemical substrate for alcohol's effects. This analysis uses daily reports of drinking, desire to drink, and alcohol expectancies to test the concurrent validity of our prior findings (Kranzler et al. 2014), which were based on drinking measures obtained retrospectively at each treatment visit, and extends them to include topiramate's effects on cognitions. We also explored whether the observed interactive effects of medication and genotype on drinking were mediated by either expectancies or desire to drink.
METHODS AND MATERIALS
Overview
Kranzler et al. (2014) provides a detailed description of the methods employed in this 12-week, parallel-groups, placebo-controlled trial of topiramate in heavy drinkers. All patients received medical management (MM), a brief psychosocial intervention (Pettinati et al. 2004), at each of nine treatment visits. Patients were randomly assigned to medication group and double-blind conditions were maintained throughout the study.
The study was initiated at the University of Connecticut Health Center (UCHC; N=76) and completed at the University of Pennsylvania Treatment Research Center (Penn; N=62). The institutional review boards at both institutions approved the consent forms and study protocol. Study participants gave written, informed consent. They were paid to complete daily reports and an assessment battery at the end of treatment. Throughout the trial, patients responded to daily interactive voice response (IVR) surveys that were computer administered.
Patients
Following a telephone screen, we interviewed 200 prospective participants in person. One hundred thirty-eight patients (86 men, 62.3%) were randomly assigned to treatment with topiramate (N=67, 48.6%) or placebo (N=71, 51.4%). The study sample was predominantly middle-aged (mean=51.1 yr, SD=8.2), EA (88.4%), married (60.9%), employed (80.4%), and male (62.3%), with an average of more than three years of college. During pretreatment, patients drank alcohol on approximately 6 days/week, drinking heavily on approximately 5 days/week. The only pretreatment demographic or clinical measure on which the groups differed significantly was age: placebo patients were approximately 3.5 yr older than topiramate patients.
Of the 138 patients randomly assigned to treatment with topiramate or placebo, 122 (88.4%) were self-reported EAs. Because of the potential for confounding due to substantial population differences in the frequency of rs2832407 alleles, we limited our analyses to the EA subsample. Although considering a single population group does not completely eliminate the risk of stratification, it is comparatively unlikely in European-ancestry subjects who generally do not show high degrees of admixture with other populations and where allele frequency differences are comparatively small (Halder et al. 2009). These considerations led us to use self-identified race to classify population group.
Procedures
Subjects were recruited through advertisements. Following an initial telephone screening, eligible individuals were seen in person, where they gave written, informed consent to participate and underwent a history, physical examination, routine laboratory testing, a urine drug screen, and pregnancy testing (for women of childbearing potential). Prior to randomization, patients completed questionnaires and were administered research interviews by a trained research evaluator, following which they received their first counseling session and supply of study medication. Patients were seen weekly for medication increases during the first six weeks of treatment, followed by three biweekly visits. At each visit, the patient's breath alcohol concentration, weight, and vital signs were measured; patients completed questionnaires; and the research nurse elicited information on concurrent medications, the occurrence of adverse events, and protocol adherence, and delivered the MM counseling (Pettinati et al. 2004). At the end of treatment, patients again completed questionnaires and were interviewed by the research nurse and the research evaluator.
Study Treatments
Counseling
The MM manual focuses on medication adherence and treatment participation through education and support and was modified for the current study to be consistent with a goal of sensible drinking. Based on guidelines for non-hazardous drinking (Sanchez-Craig et al. 1995), men were advised to consume no more than 3 standard drinks per day and 12 standard drinks per week and women were advised to consume no more than 2 drinks per day and 8 drinks per week.
Medication
Topiramate treatment was initiated at a dosage of 25 mg at bedtime and at weekly intervals was increased gradually to 100 mg twice daily in week 6. Placebo and topiramate were encapsulated and indistinguishable from one another.
Interactive voice response technology uses the telephone to administer survey questions (Kranzler et al. 2004). At the outset, patients were given a wallet-sized, follow-along sheet detailing each question in the IVR phone call, including answer options as a guide, and trained to use the IVR. They called daily between 5 and 8 PM to report on their desire to drink and drinking expectancies and their alcohol consumption by pressing the keys on the keypad, with responses entered automatically in a database. We chose the time of the calls to minimize the potential for patients to have begun drinking heavily prior to making the calls. Patients who failed to call in during the allotted time received a computerized reminder call. A research assistant monitored calls to ensure that they were made daily and that problems and questions were addressed immediately (to enhance accuracy and adherence). Patients that failed to make multiple calls were contacted and reminded of the importance of the daily assessments to the study's success.
Patients were queried daily about their current desire to drink using three items adapted from the Alcohol Urge Questionnaire (AUQ; Bohn et al. 1995) (e.g., “Today I felt like I could really use a drink”). Responses were made on a 4-point scale (0=“not at all” to 4=”extremely likely”). We also used two items that assessed anticipated positive outcomes for drinking later that night. The items, adapted from existing expectancy scales (Rohsenow 1983, Fromme et al. 1993, Leigh and Stacy 1993), correspond to the commonly identified expectancy domains of general pleasure and tension reduction. Patients responded to the questions, “If you were to drink tonight how likely is it that you” (a) “would have a good time?” and (b) “would feel less tense/more relaxed?” Response options here were the same as for desire to drink. For analysis, we averaged the appropriate items to create a daily desire to drink composite (alpha = 0.79) and a positive expectancy composite (alpha = 0.72).
Patients also reported daily on the number of standard drinks of beer, wine, liquor and “other” category that they consumed. To capture all drinking during the preceding 24-hour period, patients were asked to report separately drinking from yesterday (in total), and any drinking during the current day, up until the time of the IVR report. Two percent of drinking values exceeded 20 drinks; we set these to a value of 20.
Genotyping Procedure
DNA was extracted from whole blood using the PureGene kit (GentraSystems, Minneapolis, MN). We genotyped rs2832407 using the TaqMan SNP genotyping assay (Life Technologies, Grand Island, NY). All genotypes were obtained in duplicate with consistent results. As reported in Kranzler et al. (2014), the genotype frequencies in the 122 European Americans were consistent with Hardy-Weinberg equilibrium expectations (χ2=0.61, df=2, p=0.74)
Statistical Analysis
We used linear multilevel regression analysis to test whether changes in desire to drink, expectancies, and drinking level across study day varied by medication and genotype groups. This approach allowed for the unbalanced nature of the data, i.e., no subjects were excluded due to missing daily reports. We modeled the interactions between study day (coded 1-84), medication condition (coded 0=placebo, 1=topiramate) and genotype (coded 0=A-allele carrier, 1=C-allele homozygote). Both intercepts and study day slopes were specified as random effects. Because there was evidence from our clinical trial (Kranzler et al., 2014) that the two-level rs2832407 genotypes were significant moderators of topiramate effects, we combined the AA and AC groups, comparing them with the CC group as a dichotomous genotype. We entered the predictors in 3 steps: study day, medication group and genotype group. We also included sex and, in the model predicting drinking level, the proportion of baseline heavy drinking days as control variables. In block 2, we entered the product terms for the two-way interactions of study day, medication group and genotype group, and in block 3 we entered the 3-way product term. Despite a significant age difference between treatment groups, age did not impact the observed effects and was removed from the models. Further, as described in Kranzler et al. (2014), when we examined the effects of treatment site, we found no association with the drinking outcomes and including treatment site it in the analytic model did not substantially alter the findings.
Finally, we used a multilevel SEM procedure described by Preacher et al. (2010) to examine whether the predicted interaction effect on drinking levels was mediated by effects on desire to drink and expectancies. This approach allows for the specification of indirect effects across levels of analysis (i.e., within persons vs. between persons). Specifically, we modeled the slopes that resulted from the regression of desire to drink and expectancies on study day as intervening variables in a parallel multiple mediator model (Dagne et al 2007).
RESULTS
IVR Adherence
The sample of 122 EAs provided complete data on 7,810 person-days [mean=64.0 daily reports per person (SD=22.0) or 76.2% (SD=26.2) of study days]. Patients reported drinking on 78.5% of those days, consuming a mean of 4.5 drinks (SD = 2.6) per drinking day. Table 1 shows the mean values for the daily variables broken down by medication group and genotype group and the mean number of reporting days for the IVR period, which did not differ as a function of medication group [F(1, 118)=0.38, p=0.54], genotype group [F(1, 118)=1.75, p=0.19], or the medication group × genotype group interaction [F(1, 118)=1.07, p=0.30].
Table 1.
Descriptive Statistics
| Genotype Group | C-allele Homozygotes | A-allele Carriers | ||
|---|---|---|---|---|
| Medication Group | Placebo | Topiramate | Placebo | Topiramate |
| Person N | 30 | 21 | 36 | 35 |
| Mean person-days | 64.03 | 70.76 | 62.86 | 61.14 |
| Mean drinks per day | 4.09 | 2.73 | 3.42 | 3.75 |
| Mean daily expectancies | 1.74 | 1.31 | 1.40 | 1.47 |
| Mean daily desire to drink | 2.04 | 1.83 | 2.03 | 1.91 |
Note. Values for drinking, expectancies, and desire to drink variables are based on aggregate person-level values that are not weighted for the number of reporting days.
Changes in Drinking Level, Positive Alcohol Expectancies, and Desire to Drink Across the Study Period
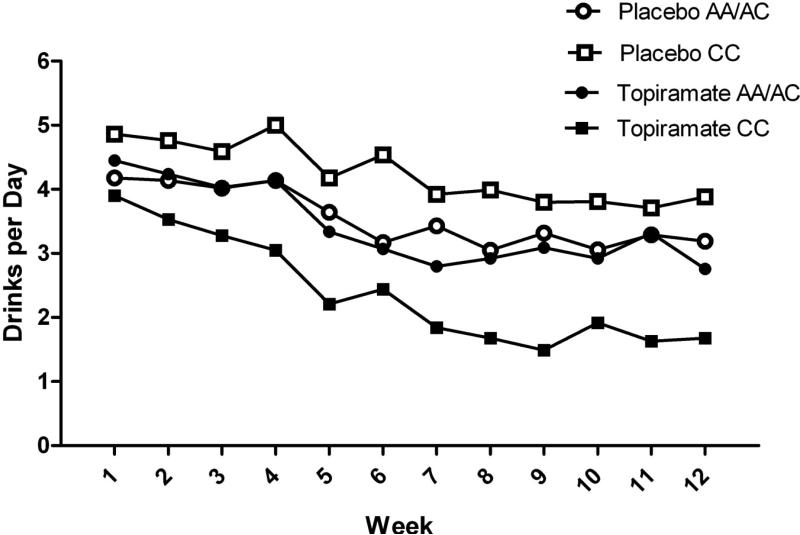
As shown in Table 2, where the coefficients correspond to the step when entered, the study day × medication group × genotype group interaction did not reach significance in the model predicting drinking level. However, similar to the results of Kranzler et al. (2014), the medication group × genotype interaction in block 2 was significant. Figure 1 shows daily drinking level during the 12 treatment weeks broken down by genotype and treatment groups. Probing of this interaction indicated a significant effect of topiramate for CC individuals (b= −1.83, SE=0.53, p=0.001, 95%CI: −2.87, −0.79), but not for A-allele carriers (b= −0.33, SE=0.46, p=0.49, 95%CI: −1.24, 0.59).
Table 2.
Results from Multilevel Linear Regressions
| Drinking Level | ||||||
|---|---|---|---|---|---|---|
| Block | Predictor | B | SE | p | 95% CI | |
| 1 | Diary Day | −0.021 | 0.003 | <0.001 | −0.026 | −0.016 |
| Sex | 0.546 | 0.314 | 0.085 | −0.076 | 1.168 | |
| Baseline Heavy Drinking | 3.067 | 0.574 | <0.001 | 1.931 | 4.203 | |
| Medication Group | −0.547 | 0.306 | 0.076 | −1.153 | 0.059 | |
| Genotype Group | −0.221 | 0.312 | 0.481 | −0.838 | 0.397 | |
| 2 | Diary Day × Genotype Group | −0.005 | 0.005 | 0.330 | −0.015 | 0.005 |
| Diary Day × Medication Group | −0.008 | 0.005 | 0.122 | −0.018 | 0.002 | |
| Medication Group × Genotype Group | −1.506 | 0.612 | 0.015 | −2.719 | −0.293 | |
| 3 | Diary Day × Medication Group × Genotype Group | −0.007 | 0.010 | 0.485 | −0.028 | 0.013 |
| Positive Alcohol Expectancies | ||||||
|---|---|---|---|---|---|---|
| Block | Predictor | B | SE | p | 95% CI | |
| 1 | Diary Day | −0.007 | 0.001 | <0.001 | −0.009 | −0.005 |
| Sex | 0.418 | 0.150 | 0.005 | 0.129 | 0.707 | |
| Medication Group | −0.072 | 0.143 | 0.616 | −0.354 | 0.211 | |
| Genotype Group | 0.181 | 0.143 | 0.209 | −0.103 | 0.467 | |
| 2 | Diary Day × Genotype Group | −0.001 | 0.002 | 0.772 | −0.005 | 0.003 |
| Diary Day × Medication Group | −0.004 | 0.002 | 0.030 | −0.008 | −0.001 | |
| Medication Group × Genotype Group | −0.401 | 0.290 | 0.170 | −0.980 | 0.173 | |
| 3 | Diary Day × Medication Group × Genotype Group | −0.008 | 0.004 | 0.040 | −0.016 | −0.001 |
| Desire to Drink | ||||||
|---|---|---|---|---|---|---|
| 1 | Diary Day | −0.007 | 0.001 | <0.001 | −0.009 | −0.005 |
| Sex | 0.073 | 0.116 | 0.528 | −0.156 | 0.302 | |
| Medication Group | −0.161 | 0.113 | 0.156 | −0.384 | 0.062 | |
| Genotype Group | 0.007 | 0.114 | 0.949 | −0.218 | 0.233 | |
| 2 | Diary Day × Genotype group | −0.002 | 0.002 | 0.504 | −0.006 | 0.003 |
| Diary Day × Medication group | −0.004 | 0.002 | 0.110 | −0.008 | 0.001 | |
| Medication group × Genotype group | −0.040 | 0.231 | 0.862 | −0.497 | 0.417 | |
| 3 | Diary Day × Medication group × Genotype Group | −0.009 | 0.004 | 0.050 | −0.018 | −0.000 |
Note: Diary day (coded 1-84), Sex (coded 0=female, 1=male), Medication Group (coded 0=placebo, 1=topiramate) and Genotype Group (coded 0=A-allele carrier, 1=C-allele homozygote); Baseline Heavy Drinking=proportion of heavy drinking days during the 90 days prior to treatment.
Note: Diary day (coded 1-84), Sex (coded 0=female, 1=male), Medication Group (coded 0=placebo, 1=topiramate) and Genotype Group (coded 0=A-allele carrier, 1=C-allele homozygote); Baseline Heavy Drinking=proportion of heavy drinking days during the 90 days prior to treatment.
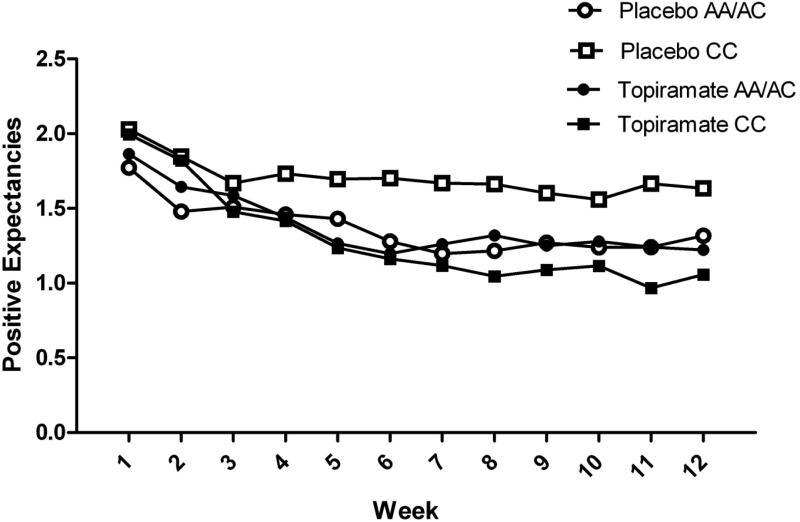
Figure 1. Positive alcohol expectancies regarding the effects of alcohol by study week and medication and genotype groups.
During the last week of treatment, rs2832407*C-allele homozygotes treated with topiramate had lower daily mean values for positive alcohol expectancies than those receiving placebo (p=0.003). No such effect was seen in rs2832407*A-allele carriers (p=0.92).
We found a significant study day × medication group × genotype group interaction in predicting both positive alcohol expectancies and desire to drink. The form of the interaction for positive alcohol expectancies is shown in Figure 1 (the pattern for desire to drink was similar), where it can be seen that, among C-allele homozygotes, topiramate-treated patients showed the largest decline in expectancies over the 12 weeks. Probing of the 3-way interactions indicated that there was a significant medication group × study day interaction among C-allele homozygotes for positive alcohol expectancies (b= −0.009, SE=0.003, p=0.003, 95%CI: −0.015, −0.003) and for desire to drink (b= −0.008, SE=0.003, p=0.012, 95%CI: −0.015, −0.002). Topiramate-treated patients showed greater declines in both subjective measures during treatment. In contrast, the medication group × study day interactions for expectancies (b= −0.001, SE=0.003, p=0.746, 95%CI: −0.006, 0.004) and for desire to drink (b= 0.002, SE=0.003, p=0.949, 95%CI: −0.006, 0.006) were not significant among A-allele carriers, i.e., there was not differential change in these outcomes by medication group.
Indirect effects
Because the topiramate × genotype interaction significantly predicted (a) mean levels of drinking during treatment and (b) changes in both alcohol expectancies and desire to drink (i.e., study day-outcome slopes), we examined whether the effects on drinking were, in part, mediated by the degree of change in the two subjective measures during treatment. We specified a multilevel SEM with indirect effects of the topiramate group × genotype group interaction through the study-day-expectancy slopes and the study day-desire to drink slopes on mean drinking level across the 12 treatment weeks. Neither of these indirect effects was significant (p=0.23 and p=0.58, respectively).
DISCUSSION
In this study, we analyzed daily data on measures of drinking, positive alcohol expectancies, and desire to drink obtained using IVR. We found an interaction of medication group by genotype group on drinking level, with the greatest reduction seen among topiramate-treated individuals with the CC genotype. This finding is consistent with the results of our prior analyses based on Timeline Follow-back measures of drinking days and heavy drinking days, which were obtained at weekly or biweekly intervals during treatment (Kranzler et al. 2014). Although the rate of decline in daily drinking level in the present study appears to be similar to effects on both drinking days and heavy drinking days in Kranzler et al. (2014), in the present study the interaction with time did not reach significance. This null effect might be a product of random variability introduced by the different methods of assessment, or alternatively, IVR assessment, compared to TLFB, might have been more sensitive to the early treatment effects of topiramate for rs2832407*C homozygotes, which persisted throughout the treatment period.
We also found that, among rs2832407*C homozygotes, topiramate treatment produced the greatest reductions in the expected positive effects of drinking and desire to drink during the treatment period. There were no such effects of medication in the A-allele carriers. This is the first study of the effects of topiramate on alcohol expectancies, either as a main or pharmacogenetic effect. Exploratory analyses failed to support the hypothesis that the effect on alcohol expectancies mediated the effect of topiramate on daily drinking in rs2832407*C homozygotes.
The effects that we observed on desire to drink are consistent with those reported by Johnson et al. (2008), who found greater reductions in the topiramate group in obsessional thoughts and compulsions about using alcohol (measured with the OCDS) than with placebo. The similarity of findings from the two studies (despite different approaches to the measurement of “craving”) may underscore the impact of motivation to reduce or stop drinking, as patients in both of these studies were seeking treatment for heavy drinking. In contrast, Miranda et al. (2008) found no effect of topiramate on alcohol craving in non-treatment-seeking subjects, which they measured both during the dosage adjustment period (with the OCDS) and, subsequently, in a laboratory setting (with the AUQ). Despite discrepancies in the observed effects on desire to drink or craving for alcohol, all three studies showed significantly greater reductions in drinking with topiramate treatment than with placebo.
Evidence of parallel effects of medication group × genotype group on measures of daily drinking, desire to drink, and positive alcohol expectancies notwithstanding, exploratory analyses showed no support for indirect, i.e., mediating, effects. One possible explanation for the lack of mediation is that our estimates of the indirect effects were attenuated by noise in the observed change trajectories (i.e., study day slopes), which we specified as mediators. Hasking et al. (2011) found support for a model in which alcohol expectancies were related to drinking motives, which were proximal predictors of drinking behavior. Because our daily survey did not include measures of drinking motives, we are unable to examine the causal chain shown by these authors to predict drinking behavior. Future research that incorporates all of these measures could help to elucidate the cognitive or other subjective path by which topiramate treatment in rs2832407*C-allele homozygotes reduces daily drinking. In addition to further elucidation of the cognitive and subjective aspects of the findings reported here, patients could be genotyped for rs2832407 prior to being randomized to treatment with topiramate or placebo. The genotypes could then be used to stratify or enrich the randomization with potential responders, which could allow the identification of other moderators of topiramate treatment. These findings could also be reverse translated by testing GluK1 knockout mice, which would be expected to be non-responsive to the reduction in drinking produced by topiramate. Finally, it would be of interest to develop compounds with greater specificity than topiramate for the GluK1-containing kainate receptor to yield greater efficacy and tolerability than topiramate.
Figure 2. Drinking level by study week and medication and genotype groups.
During the last week of treatment, rs2832407*C-allele homozygotes treated with topiramate had lower daily mean drinks/day than those receiving placebo (p<0.001). No such effect was seen in rs2832407*A-allele carriers (p=0.71).
ACKNOWLEDGMENTS
Supported by NIH grants P60 AA03510, K24 AA13736, P50 AA12870, and M01 RR06192. Staff members of the Clinical Research and Evaluation Unit of the University of Connecticut Alcohol Research Center and the Center for Studies of Addiction of the University of Pennsylvania Perelman School of Medicine were instrumental in the conduct of the study.
Footnotes
STATEMENT OF INTEREST
SA, RF, HT, JG, and JC have no disclosures to make. HRK has been a consultant or advisory board member with Alkermes, Lilly, Lundbeck, Pfizer, and Roche. He has also received honoraria from the Alcohol Clinical Trials Initiative (ACTIVE) of the American Society of Clinical Psychopharmacology, which is supported by Lilly, Lundbeck, AbbVie and Pfizer.
REFERENCES
- Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–65. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–6. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Dagne G, Brown C, Howe G. Hierarchical modeling of sequential behavior data: Examining complex association patterns in mediation models. Psychol Methods. 2007;12:298–316. doi: 10.1037/1082-989X.12.3.298. [DOI] [PubMed] [Google Scholar]
- Fromme K, Stroot EA, Kaplan D. Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychol Assessment. 1993;5:19–26. [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41:S10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Grotmol KS, Vaglum P, Ekeberg Ø , Gude T, Aasland OG, Tyssen R. Alcohol expectancy and hazardous drinking: a 6-year longitudinal and nationwide study of medical doctors. Eur Addict Res. 2010;16:17–22. doi: 10.1159/000253860. [DOI] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Yang BZ, Kranzler HR, Stein MB, Shriver MD, Gelernter J. Measurement of admixture proportions and description of admixture structure in different U.S. populations. Hum Mutat. 2009;30:1299–309. doi: 10.1002/humu.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasking P, Lyvers M, Carlopio C. The relationship between coping strategies, alcohol expectancies, drinking motives and drinking behaviour. Addict Behav. 2011;36:479–87. doi: 10.1016/j.addbeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology. 2013;229:539–54. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Addolorato G, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM, the Topiramate for Alcoholism Advisory Board and the Topiramate for Alcoholism Study Group Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med. 2008;168:1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, Fromme K. A review of expectancy theory and alcohol consumption. Addiction. 2001;96:57–72. doi: 10.1046/j.1360-0443.2001.961575.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Abu-Hasaballah K, Tennen H, Feinn R, Young K. Using daily interactive voice response technology to measure drinking and related behaviors in a pharmacotherapy study. Alcohol Clin Exp Res. 2004;28:1060–1064. doi: 10.1097/01.alc.0000130806.12066.9c. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Covault J, Tennen H. Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol. 2013;18:193–201. doi: 10.1111/j.1369-1600.2012.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Covault J. 5-HTTLPR genotype and daily negative mood moderate the effects of sertraline on drinking intensity. Addict Biol. 2013;18:1024–31. doi: 10.1111/adb.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment of heavy drinkers: Moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014 Feb 14; doi: 10.1176/appi.ajp.2013.13081014. doi: 10.1176/appi.ajp.2013.13081014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Burian L, Covault J. Association of markers in the 3' region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res. 2009;33:925–930. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh BC, Stacy AW. Alcohol outcome expectancies: Scale construction and predictive utility in higher order confirmatory models. Psychol Assessment. 1993;5:216–229. [Google Scholar]
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12-week, randomized, placebo-controlled trial. Drug Alcohol Depend. 2013;133:440–6. doi: 10.1016/j.drugalcdep.2013.06.032. [DOI] [PubMed] [Google Scholar]
- McDonald R, Rogawski M. Cellular effects of antiepileptic drugs, in Epilepsy: A Comprehensive Textbook. Second Edition Lippincott, Williams, & Wilkins; Philadelphia: 2006. [Google Scholar]
- Miranda R, Jr., MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Nicolai J, Moshagen M, Demmel R. Patterns of alcohol expectancies and alcohol use across age and gender. Drug Alcohol Depend. 2012;126:347–53. doi: 10.1016/j.drugalcdep.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Weiss R, Miller W, Donovan D, Ernst D, Rounsaville B. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. NIAAA; Bethesda: 2004. COMBINE Monograph Series, Volume 2. DHHS Publication No. NIH 04-5289 2004. [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–233. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ. Drinking habits and expectancies about alcohol's effects for self versus others. J Consult Clin Psych. 1983;51:752–756. doi: 10.1037//0022-006x.51.5.752. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health. 1995;85:823–828. doi: 10.2105/ajph.85.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawayama T, Yoneda J, Tanaka K, Shirakawa N, Sawayama E, Ikeda T, Higuchi S, Miyaoka H. The predictive validity of the Drinking-Related Cognitions Scale in alcohol-dependent patients under abstinence-oriented treatment. Subst Abuse Treat Prev Policy. 2012;7:17. doi: 10.1186/1747-597X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultured neurons. Epilepsia. 2000;41(1):S45–47. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline Follow-back: a technique for assessing self-reported alcohol consumption. In: Clifton Allen J., editor. Measuring Alcohol Consumption. Humana Press; 1992. pp. 41–65. 1992. [Google Scholar]
- Sommer BR, Mitchell EL, Wroolie TE. Topiramate: Effects on cognition in patients with epilepsy, migraine headache and obesity. Ther Adv Neurol Disord. 2013;6:211–27. doi: 10.1177/1756285613481257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000 Genomes Project Consortium. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner MJ, Walker LS, Greene JW. Relation of alcohol expectancies to changes in problem drinking among college students. Arch Pediat Adol Med. 1995;149:733–9. doi: 10.1001/archpedi.1995.02170200023003. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41:S17–20. [PubMed] [Google Scholar]
- Zamboanga BL, Horton NJ, Leitkowski LK, Wang SC. Do good things come to those who drink? A longitudinal investigation of drinking expectancies and hazardous alcohol use in female college athletes. J Adolescent Health. 2006;39:229–36. doi: 10.1016/j.jadohealth.2005.11.019. [DOI] [PubMed] [Google Scholar]