Abstract
The prediction of treatment response in many neuropsychiatric disorders would be facilitated by easily accessible biomarkers. Using flow cytometry, we herein demonstrate correlations between early reductions of p11 levels in Natural Killer (NK) cells and monocytes and antidepressant response to citalopram in patients with major depressive disorder (MDD).
Keywords: S100A10, Annexin II light chain, serotonin, biomarker, depression, p11, antidepressant
The multifunctional protein p11 amplifies serotonin receptor-mediated signaling (1,2) and regulates gene transcription (3,4). P11 levels are reduced in neurons of the frontal cortex, nucleus accumbens and hippocampus from depressed individuals and suicide victims (1,5). Neuronal p11 levels are also reduced in animal models of depression (1), but are up-regulated by various antidepressant treatments, including selective serotonin reuptake inhibitors (SSRIs) (1,6). P11 knockout (KO) mice show a depression-like phenotype and reduced behavioral improvements and neurogenesis in response to antidepressant regimens (6,7). Conversely, overexpression of p11 in mice mimics the behavioural phenotype seen after antidepressant treatment (1). Here, we examined the possibility that p11 in white blood cells could serve as a biomarker of antidepressant response using the SSRI, citalopram.
After giving informed consent, 26 patients with MDD in a current major depressive episode were recruited at the National Institute of Mental Health (for details see Supplementary information). Their average (SD) age was 36.9±10.4 and there were 11 females and 15 males. The patients were screened and diagnosed using DSM-IV and SCID criteria. Severity of depression was assessed using the Montgomery–Åsberg Depression Rating Scale (MADRS) and the Quick Inventory of Depressive Symptomatology-C16 (QIDS). Subjects were given a daily dosage of citalopram, doses were increased over time (see Supplementary information), but were held constant for the week before blood was drawn. Subjects were evaluated on a weekly basis for eight weeks. Peripheral blood mononuclear cells (PBMCs) were prepared at baseline, and following two and eight weeks of citalopram treatment; 14 patients had all three sets of blood collected. A separate group of nine patients with MDD were recruited at UT Southwestern in Dallas and assessed using the Inventory of Depressive Symptomatology (IDS) (for details see Supplementary information). They were treated with citalopram and had blood collected at baseline, and following one and twelve weeks; 6 patients had all three sets of blood collected. The protocols were approved by local IRBs. The PBMC samples were coded at the NIMH and UT Southwestern, respectively. All subsequent analysis and quantifications of p11 were done blind at Karolinska Institutet. PBMCs were isolated and stored at −80°C in 90% FCS/10% DMSO. For analysis, PBMCs were thawed, washed in PBS, permeabilised and incubated for 30 min at +4°C with anti-human p11 (148; 2.5 µg/ml; BD Biosciences, Stockholm) or isotype control mouse IgG1 monoclonal antibodies. Bound antibody was detected by PE-conjugated anti-mouse antibody (Dako, Glostrup). To distinguish between NK cells, monocytes, and T cells, surface staining (CD3, CD14 and CD56; BD Biosciences) was performed after blocking of unbound anti-mouse antibody using 1% normal mouse serum (Sigma, Stockholm). After labeling, cells were subjected to flow cytometry analysis to detect p11 levels in gated populations of white blood cells (Fig S1). Analyses were done using FlowJo software (Tree Star Inc, Ashland). Statistical analyses used Pearson’s correlation test and determined 95% confidence intervals (GraphPad Prism5, La Jolla).
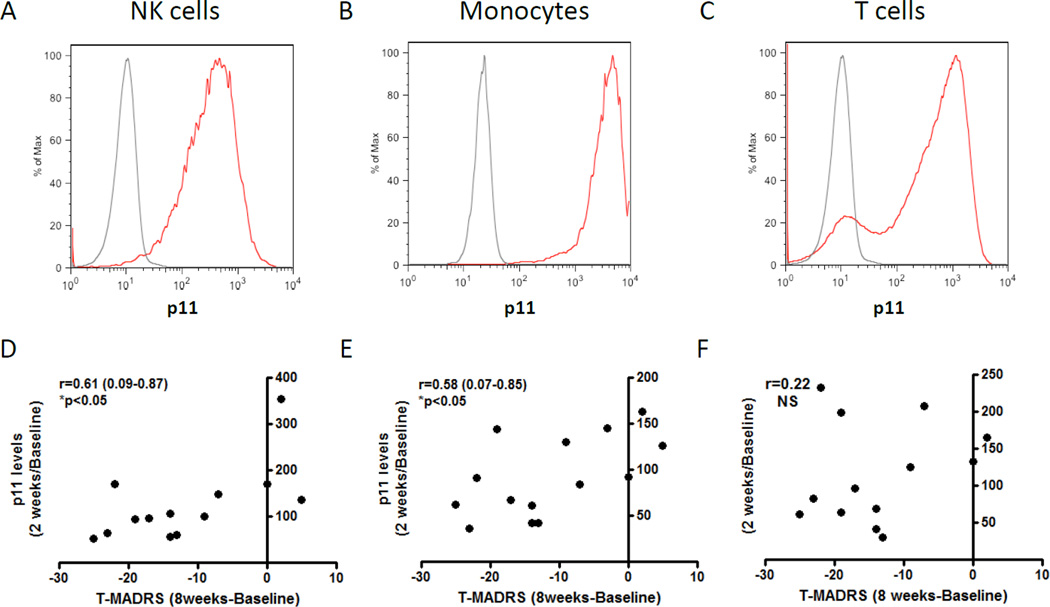
Flow cytometry analysis of PBMCs showed that p11 was present in essentially all CD3−CD56+ NK cells (Fig 1A), all CD14+ monocytes (Fig 1B) and most CD3+ T cells (Fig 1C), with monocytes having an order of magnitude higher levels. Clinical evaluations showed that most patients in the NIMH cohort responded to citalopram (see Supplementary Information). P11 was reduced in NK cells and monocytes after two weeks of citalopram and this reduction correlated with clinical improvements on MADRS (Fig 1; r=0.61 (0.09–0.87) and r=0.58 (0.07–0.85); p<0.05) and QIDS (Fig S2; r=0.60 (0.08–0.87) and r=0.61 (0.12–0.86); p<0.05) as assessed after eight weeks of citalopram. Correlations were likewise found when comparing p11 reductions and clinical improvements after eight weeks of citalopram (data not shown). Similarly, p11 was reduced in NK cells and monocytes after one week of citalopram in patients recruited at UT Southwestern and this reduction correlated with clinical improvements on IDS (Fig S3; r=0.87 (0.22–0.98) and r=0.81 (0.01–0.98); p<0.05) as assessed after twelve weeks of citalopram. Taken together, the data thus suggest that early reductions of p11 levels in NK cells and monocytes in response to citalopram predict the likelihood of a later antidepressive response. Baseline p11 levels did not correlate with baseline MADRS or citalopram-induced improvements on the MADRS (Fig S4).
Figure 1. p11 in NK cells, monocytes, and T cells and its regulation by citalopram in depressed individuals.
Representative flow cytometry analysis of PBMCs with primary antibodies towards p11 (red lines) or IgG1 isotype control (grey lines) demonstrates specific p11 expression in essentially all (A) CD56+CD3− NK cells and all (B) CD14+monocytes and in most (C) CD3+ T cells. (D–F) Graphs showing median fluorescence intensity of p11 (percentage of baseline values) in (D) NK cells, (E) monocytes, and (F) T cells after two weeks of citalopram treatment and correlations to improvements in total MADRS score after eight weeks of citalopram treatment. Dots represent individual patients. Statistical analyses used Pearson’s correlation test and determined 95% confidence intervals. Statistical significance was defined as P<0.05.
Since animal studies have shown that antidepressants increase p11 in neurons in the brain (4), it appears paradoxical that a decrease in p11 in white blood cells should be associated with antidepressant response. Future studies will need to examine whether antidepressants regulate p11 in microglia, which, like monocytes, are derived from myeloid precursors. Future clinical studies, using citalopram and other antidepressants with larger sample sizes, need to be carried out. If such studies confirm the results of the present investigation, early analyses of p11 levels would guide clinicians in deciding whether treatment with a particular antidepressant should continue or whether it would be better to switch to another antidepressant therapy.
Supplementary Material
Acknowledgements
This study was supported in part by the Intramural Research Program of the National Institute of Mental Health (DM, DFI, ER, MN, AM, HM, CAZ), National Institutes of Health (IRP-NIMH-NIH), by NIMH MH090963 (PG), JPB Foundation (PG), Fisher Center for Alzheimer’s research Foundation (PG, PS) and by the Swedish Medical Research Council (PS).
Drs. Svenningsson and Greengard are coinventors on an issued patent for usage of p11 to study depression and antidepressant responses. This patent was licensed by The Rockefeller University to Intracellular Therapies Inc., for which Drs Svenningsson and Greengard serve as consultants. Dr. Manji is an employee at Johnson & Johnson. Dr. Trivedi is or has been an advisor/consultant to several pharmaceutical companies (listed in the supplementary material). Dr. Zarate is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government.
Footnotes
Conflict of Interest:
The other authors declare no conflicts of interest.
REFERENCES
- 1.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 2.Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh YS, Gao P, Lee KW, Ceglia I, Seo JS, Zhang X, et al. Cell. 2013;152:831–843. doi: 10.1016/j.cell.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. Nat Rev Neurosci. 2013;14:673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, et al. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Biol Psychiatry. 2010;67:1048–1056. doi: 10.1016/j.biopsych.2010.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.