Abstract
Purpose
The clinical benefit of combined intraoperative magnetic resonance imaging (iMRI) and endoscopy for transsphenoidal pituitary adenoma resection has not been completely characterized. This study assessed the impact of microscopy, endoscopy, and/or iMRI on progression-free survival, extent of resection status (gross-, near-, and subtotal resection), and operative complications.
Methods
Retrospective analyses were performed on 446 transsphenoidal pituitary adenoma surgeries at a single institution between 1998 and 2012. Multivariate analyses were used to control for baseline characteristics, differences during extent of resection status, and progression-free survival analysis.
Results
Additional surgery was performed after iMRI in 56/156 cases (35.9 %), which led to increased extent of resection status in 15/156 cases (9.6 %). Multivariate ordinal logistic regression revealed no increase in extent of resection status following iMRI or endoscopy alone; however, combining these modalities increased extent of resection status (odds ratio 2.05, 95 % CI 1.21–3.46) compared to conventional transsphenoidal microsurgery. Multivariate Cox regression revealed that reduced extent of resection status shortened progression-free survival for near- versus gross-total resection [hazard ratio (HR) 2.87, 95 % CI 1.24–6.65] and sub- versus near-total resection (HR 2.10; 95 % CI 1.00–4.40). Complication comparisons between microscopy, endoscopy, and iMRI revealed increased perioperative deaths for endoscopy versus microscopy (4/209 and 0/237, respectively), but this difference was non-significant considering multiple post hoc comparisons (Fisher exact, p = 0.24).
Conclusions
Combined use of endoscopy and iMRI increased pituitary adenoma extent of resection status compared to conventional transsphenoidal microsurgery, and increased extent of resection status was associated with longer progression-free survival. Treatment modality combination did not significantly impact complication rate.
Keywords: Endoscopy, Extent of resection, Intraoperative MRI, Pituitary adenoma, Progression-free survival
Introduction
For patients undergoing transsphenoidal surgery for pituitary adenomas, even large series from some of the most experienced centers have reported gross-total resection rates of only 46–78 % [1–3]. In recent years, operative methods have evolved in an effort to increase extent of resection, tumor visualization, and post-operative patient comfort. Conventional transsphenoidal techniques such as microsurgery, fluoroscopy, and the sublabial approach are being replaced at some centers by endoscopy, endonasal approaches, and stereotactic neuronavigation. The use of endoscopy for transsphenoidal pituitary surgery has become common due to the potential advantages of increased field-of-view, high-definition clarity, and studies suggesting resection benefits over microscopic approaches [3–8]; however, other studies have demonstrated no difference in efficacy between modalities [2, 9, 10]. Also, many surgeons continue to prefer conventional transsphenoidal microsurgical techniques due to familiarity with the procedure and concern for a potential increase in complications using endoscopy. Studies have also demonstrated increased adenoma resection with the use of low-field (<1.0 Tesla (T) field strength) [11–17] and high-field (>1.0 T field strength) [18–25] intraoperative magnetic resonance imaging (iMRI), but skepticism persists among some surgeons about whether the potential patient benefit justifies the increased operative time, cost, and complexity [18, 26, 27].
In addition to controversy regarding the independent use of endoscopy or iMRI for pituitary adenoma resection, few studies have evaluated the combined effect of these modalities on patient outcomes [15, 28], and thus far, rigorous outcomes analysis comparing newer with traditional techniques is limited. Furthermore, few studies have quantified the effect of increased extent of resection status on progression-free survival [1]. Therefore, the principle aims of this retrospective study were (1) to determine the independent and combined effect of endoscopy and high-field iMRI on extent of resection status; (2) to measure the impact of baseline patient and tumor characteristics, as well as extent of resection status on post-operative progression-free survival; and (3) to assess the effect of endoscopy and iMRI on surgical complication rates.
Materials and methods
Patient selection
The investigations utilized an Institutional Review Board approved prospective and retrospective brain tumor database containing extensive demographic, pre-, peri- and post-operative data from over 3,000 surgeries performed since 1993 at our institution. Over 600 cases used a high-field iMRI device since implementation in April 2008, of which 183 were pituitary adenoma resections. The database also contains 263 cases of pituitary adenoma resection without iMRI (200 before and 63 after iMRI installation). The decision to use iMRI in these latter cases was based upon the surgeon’s preference and iMRI availability.
General exclusion criteria
Of the initial 446 cases, general exclusion criteria related to case heterogeneity and adequacy of follow-up were applied for the extent of resection status and progression-free survival analyses. Heterogeneity was limited by removing cases performed for non-resection purposes [i.e., biopsies and cyst aspirations (2 iMRI, 6 non-iMRI)], cases with patients younger than 18 (5 iMRI, 0 non-iMRI) and older than 80 years (1 iMRI, 8 non-iMRI), and operations performed by surgeons with low case counts [<5 cases (12 iMRI, 13 non-iMRI)]. Surgeries for older patients were often performed without iMRI to diminish operative time in consideration of frequent co-morbidities. Finally, of the remaining cases, those deemed to have inadequate follow-up (no post-operative MRI scans and/or relevant hormone level measurement [18 iMRI, 19 non-iMRI]) were excluded.
Intraoperative magnetic resonance imaging
The iMRI suites utilized have ceiling mounted rails (IMRIS, Inc., Winnipeg, Ontario, Canada) for moving an Espree™ high-field 1.5 T MRI device (Siemens, Malvern, Pennsylvania) between either of two operating rooms [26, 29–32]. Surgeons used standard instruments when the magnet was in storage and the patient was outside the 5-gauss line. After maximum tumor resection, surgical instruments and other ferromagnetic objects were moved to the outer perimeter of the room, safety checks were completed, the movable iMRI device was moved into the operating room, and iMRI sequences were obtained. One iMRI case received two intraoperative scanning sessions; the remaining 155 iMRI cases received only one scan. Additional resection was performed after integration of iMRI scans into the navigation system if safely accessible residual tumor was identified by the neuroradiologist and neurosurgeon.
Extent of resection status
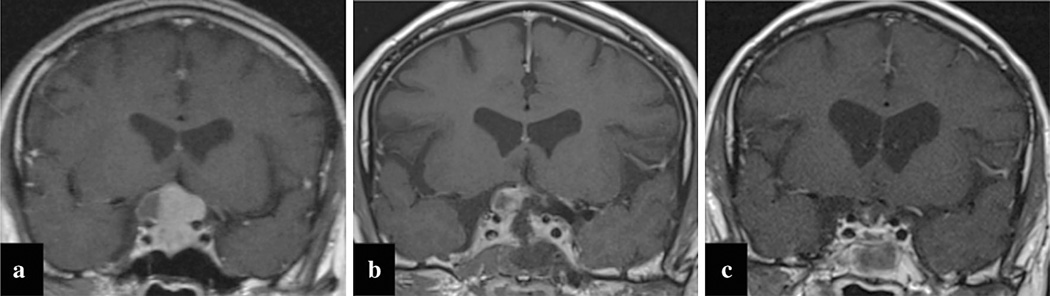
Pre-operative MRI sequences included T1 with (Fig. 1a) and without gadolinium, T2, and 3-dimensional volumetric neuronavigation studies. Tumor size in maximum diameter and cavernous sinus invasion were determined from radiology reports and scan review. Intra- and post-operative scans (Fig. 1b,c)were classified as (1) gross-total resection when no residual tumor was identified; (2) near-total resection when imaging showed small potential areas of residual tumor, or (3) sub-total resection when definite tumor was identified [33, 34]. Functional tumors accounted for 39.9 % of cases (146/ 366, Table 1). The mean time from surgery to post-operative follow-up MRI for the iMRI and non-iMRI cohorts was 5.22 ± 3.41 and 4.98 ± 5.25 months, respectively.
Fig. 1.
Representative coronal MRI slices on a single patient at different time points. The pre-operative image (a) demonstrates suprasellar extension of a pituitary adenoma. The intra-operative image (b) shows residual tumor consistent with a sub-total resection. The post-operative image (c) shows the final increased extent of resection status achieved after removal of residual tumor identified on iMRI
Table 1.
Baseline, tumor and operative characteristics before and after general exclusion
| Covariates | Total n (%)/ mean (std) |
Non-excluded n (%)/ mean (std) |
Excluded n (%)/ mean (std) |
|---|---|---|---|
| Total | 446 (100) | 366 (100) | 80 (100) |
| Female | 238 (53.4) | 200 (54.6) | 38 (47.5) |
| Previous pituitary adenoma resection |
78 (17.5) | 70 (19.1) | 8 (10.0) |
| Functional tumor | 183 (41.0) | 146 (39.9) | 37 (46.3) |
| Cavernous sinus Extension |
169 (37.9) | 140 (38.3) | 29 (36.3) |
| Apoplexy | 40 (9.0) | 30 (8.2) | 10 (12.5) |
| Atypical pathology Treatment combination |
52 (11.7) | 49 (13.4) | 3 (3.8)** |
| Non-iMRI + Microscopy |
190 (42.6) | 159 (43.4) | 31 (38.8) |
| Non-iMRI + Endoscopy |
73 (16.4) | 51 (13.9) | 22 (27.5) |
| iMRI + Microscopy | 47 (10.5) | 41 (11.2) | 6 (7.5) |
| iMRI + Endoscopy | 136 (30.5) | 115 (31.4) | 21 (26.3) |
| Age (years) | 48.7 (15.5) | 48.4 (14.2) | 50.3 (20.2) |
| BMI (kg/m2) | 32.1 (8.2) | 31.8 (7.6) | 33.2 (10.5) |
| Tumor size largest dimension (mm) |
22.3 (12.6) | 22.5 (12.7) | 21.2 (12.2) |
Atypical pituitary adenoma cases were more likely to be in the non-excluded than the excluded group (p <0.01). No other significant difference between groups were noted for all other baseline, tumor, and operative characteristics
Data are mean (standard deviation) or number (%)
Pearson’s Chi squared and Student’s t test were used for significance testing as appropriate
p < 0.05;
p < 0.01;
p < 0.001
Endocrine evaluation
Functional tumor status and biochemical remission were defined by the treating endocrinologist using consensus guidelines available at the time of follow-up [35–37]. Adrenocorticotropic hormone (ACTH) secreting tumors were diagnosed by documentation of hypercortisolism (with elevated 24-h urinary free cortisol levels, an abnormal low dose dexamethasone suppression test, and/or elevated late-night salivary cortisol levels) in the setting of a normal or high serum ACTH level and confirmed by inferior petrosal sinus sampling in patients with small tumors and in patients with no visible tumor on MRI. Prolactin (PRL) secreting adenomas were diagnosed by either elevation in hormone levels (PRL > 200 ng ml) or diffuse immunohistological positivity for prolactinoma for patients on bromocriptine/cabergoline treatment. Thyroid stimulating hormone (TSH) secreting adenomas were diagnosed by elevation in relevant hormone levels, while the diagnosis of growth hormone (GH) secreting adenomas was based on an elevated IGF-1 level adjusted for age and gender (± an abnormal GH suppression test) in patients with signs and symptoms of acromegaly. Biochemical remission criteria for ACTH, PRL, and TSH secreting adenomas were defined by post-operative normalization of hypercortisolemia, hyperprolactinemia, and hyperthyroidism respectively. For GH secreting adenomas, biochemical remission was defined by normalization of IGF-1 3 months after surgery and in some instances suppression of GH during a glucose tolerance test.
Post-operative treatment and progression
Post-operative treatment type (i.e., further surgery, radiation, or hormone suppressive medication) and months elapsed from surgery were recorded as appropriate. In addition to general exclusion criteria, cases were excluded from progression and progression-free survival analysis if treated for residual tumor as a result of testing performed on the first follow-up assessment or if they failed to achieve biochemical remission over the available follow-up (105/ 366). For the 261 cases followed expectantly, radiographic progression was defined as new regions of tumor on follow-up MRI in the months and years after surgery. Biochemical progression was defined for all secretory types by meeting diagnostic criteria in the post-operative period.
Complications
Perioperative complications were assessed for all 446 cases (Fig. 2). Specific complications included death, permanent diabetes insipidus (DI), deep vein thrombosis (DVT), meningitis, pulmonary embolism (PE), surgical wound infection, cerebral infarction, arterial injury requiring endovascular intervention and cerebrospinal fluid (CSF) leak (intra- or post-operative). Cases receiving an additional procedure for CSF leak (i.e., lumbar drain, post-operative surgical repair) were identified. Complication rates were compared for iMRI versus non-iMRI cases; endoscopy versus microscopy cases; endoscopy with iMRI versus conventional transsphenoidal microsurgery cases; and cases receiving versus not receiving additional resection following iMRI.
Fig. 2.
Case counts and exclusion criteria for analysis of complications, extent of resection status, and progression-free survival
Statistical analysis
Statistical analyses were performed with biostatistician assistance using SPSS version 19 (IBM Corp., Armonk, NY). All reported p-values were two-sided with a threshold for statistical significance defined as <0.05. Kaplan–Meier plots and log-rank testing were used to assess the impact of factors on progression-free survival. Cox proportional-hazards regression was used to determine univariate and multivariate hazard-ratios (HR) for baseline characteristics relative to progression-free survival. Inclusion of covariates in the multivariate Cox models was based on univariate analysis achieving p < 0.15. An ordinal logistic regression model (constructed using a general linear model) was used to determine univariate and multivariate odds-ratios (OR) for baseline characteristics relative to three possible extent of resection statuses (gross-, near-, and sub-total resection). Wald Chi squared testing was used for significance testing of multivariate model outputs. Continuous variables were z-score standardized prior to regression model insertion; thus, a one standard deviation increase in these variables produced a one unit increase in the OR and HR.
Results
Patient characteristics
The non-excluded study cohort used for extent of resection status analysis was composed of 339 patients (185 female, 154 male) who received 366 transsphenoidal resections. The patient and tumor characteristics were similar between excluded and non-excluded cases, except atypical adenoma cases were less likely to be excluded (p < 0.05, Table 1; Fig. 2). Case counts comparing iMRI versus non-iMRI; endoscopy versus microscopy; and endoscopy with iMRI versus conventional transsphenoidal microsurgery are listed in Table 2. The mean age for all patients at the time of surgery was 48.3 ± 14.2 years (range 19.0–79.5 years). Clinical and immunohistochemical evaluation of the 128 unique patients with functional tumor (146 cases) revealed 55 ACTH, 54 GH, 16 PRL, and 3 TSH secreting adenoma operations.
Table 2.
Baseline patient and tumor characteristics by treatment type for the 366 cases after general exclusions
| Covariates | Total n (%)/mean (std) |
Non-iMRI n (%)/mean (std) |
iMRI n (%)/mean (std) |
Microscopy n (%)/mean (std) |
Endoscopy n (%)/mean (std) |
Non- iMRI + Microscopy n (%)/mean (std) |
iMRI + Endoscopy n (%)/mean (std) |
|---|---|---|---|---|---|---|---|
| Total | 366 (100) | 210 (100) | 156 (100) | 200 (100) | 166 (100) | 159 (100) | 115 (100) |
| Female | 200 (54.6) | 111 (52.9) | 89 (57.1) | 108 (54.0) | 92 (55.4) | 80 (50.3) | 61 (53.0) |
| Previous pituitary adenoma resection |
70 (19.1) | 36 (17.1) | 34 (21.8) | 31 (15.5) | 39 (23.5) | 26 (16.4) | 29 (26.2) |
| Functional tumor | 146 (39.9) | 80 (38.1) | 66 (42.3) | 82 (41.0) | 64 (38.6) | 60 (37.7) | 44 (38.3) |
| Cavernous sinus extension |
140 (38.3) | 85 (40.5) | 55 (35.3) | 79 (39.5) | 61 (36.7) | 66 (41.5) | 42 (36.5) |
| Apoplexy | 30 (8.2) | 17 (8.1) | 13 (8.3) | 10 (5.0) | 20 (12.0)* | 7 (4.4) | 10 (8.7) |
| Atypical pathology | 49 (13.4) | 29 (13.8) | 20 (12.8) | 30 (15.0) | 19 (11.4) | 24 (15.1) | 14 (12.2) |
| Age (years) | 48.4 (14.2) | 48.2 (14.9) | 48.6 (13.4) | 48.2 (14.7) | 48.75 (13.6) | 48.4 (15.3) | 49.0 (13.7) |
| BMI (kg/m2) | 31.8 (7.6) | 32.3 (7.9) | 31.3 (7.1) | 31.8 (6.6) | 31.9 (8.7) | 31.8 (6.9) | 31.1 (7.6) |
| Tumor size largest dimension (mm) |
22.5 (12.7) | 23.3 (12.6) | 21.3 (12.7) | 22.8 (11.9) | 22.2 (13.6) | 23.3 (12.0) | 21.5 (13.2) |
Pituitary apoplexy cases were more likely to have received endoscopic than microscopic transsphenoidal resection (p < 0.05). No other significant differences between groups were noted for all other baseline patient and tumor characteristics
Pearson’s Chi squared and Students t-test were used for significance testing as appropriate
p < 0.05;
p < 0.01;
p < 0.001
Extent of resection status analysis—iMRI dependent
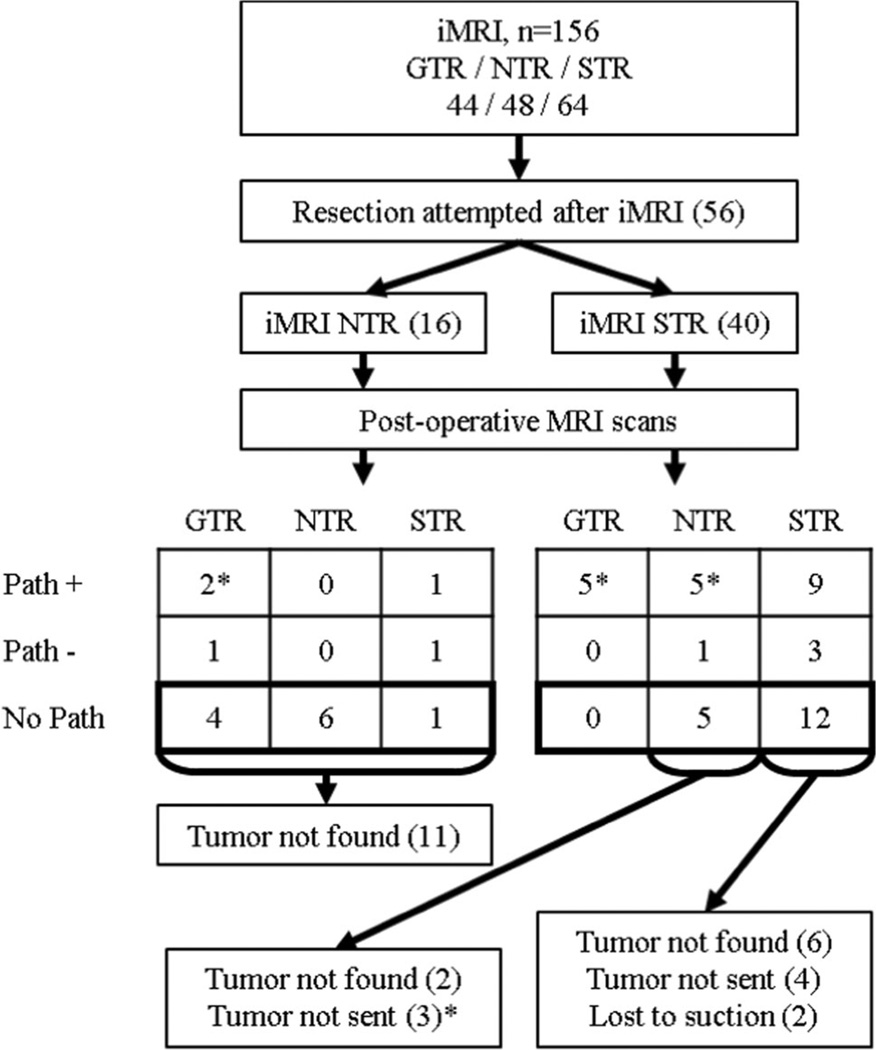
Fifty-six of the 156 iMRI cases (35.9 %) remaining after general exclusion had additional tumor resection attempted after iMRI. Reasons for no attempted resection after iMRI included gross-total resection (44), near-total resection with no definitively resectable tumor (23), and near/subtotal resection with residual tumor not safe for further resection (33). Figure 3 illustrates the dispersion of iMRI cases undergoing additional tumor resection after iMRI. Additional tissue was removed after iMRI in 36/56 cases receiving additional surgery, with resection locations including the sella turcica (21), suprasellar region (7), combined sella/suprasellar (6), pterygopalatine fossa (1), and the third ventricle (1). Pathological specimens were obtained in 28/56 cases (50.0 %) that received further tumor resection after iMRI, and 22/29 of these cases (75.9 %) where positive for adenoma on microscopic analysis.
Fig. 3.
Flow diagram illustrating the extent of resection status (gross-, near-, and sub-total resection; GTR, NTR, and STR, respectively) noted on intraoperative MRI (iMRI), followed by pathologic evaluation and post-operative MRI in cases that received additional resection after iMRI. Fifteen cases had extent of resection status improvement (gross- vs. near- vs. sub-total resection) attributable to additional resection after iMRI (cases denoted with asterisk). Ten of these cases increased from sub-total resection on iMRI to either near-total resection (five cases) or gross-total resection (five cases) on postoperative MRI with pathology positive for adenoma (Path+). Two of these cases increased from near-total resection on iMRI to gross-total resection on post-operative MRI and had Path+. Three of these cases increased from sub-total resection to near-total resection, but had no pathology results (No Path); however, additional adenomatous tissue was removed per the operative notes. Cases with extent of resection status improvement with pathology negative for adenoma (Path-; two cases) or no tumor found during additional resection after iMRI (six cases) were considered false positive iMRI results in this analysis
Increased extent of resection status (gross- versus near-versus sub-total resection) attributable to additional resection after iMRI was shown in 15/156 of iMRI cases (9.6 %), 12 with positive pathology and 3 with suspicious tissue removed without pathology (Fig. 3). Anatomical locations of residual tumor on iMRI that lead to further resection and a higher extent of resection status included the sella turcica (11), suprasellar region (2), and a combination of both regions (2).
Chi squared testing demonstrated an association between lower extent of resection status and factors such as previous adenoma resection, functional tumors, and cavernous sinus invasion (Table 3). Univariate regression analysis revealed associations with a lower extent of resection status for increased tumor size, previous pituitary adenoma resection, non-functional tumors, and cavernous sinus invasion (Table 4, right). Multivariate regression analysis revealed lower extent of resection status associations for increased tumor size, previous pituitary adenoma resection, cavernous sinus invasion, and the absence of pituitary apoplexy. The combination of endoscopy with iMRI was associated with more favorable extent of resection status than conventional transsphenoidal microsurgery on both univariate (OR 1.83, 95 % CI 1.17–2.87, p < 0.01) and multivariate regression analysis (OR 2.05, 95 % CI 1.21–3.46, p < 0.01), and was associated with a higher odds of increased extent of resection status than either endoscopy without iMRI or microscopy with iMRI (Table 4, right).
Table 3.
Extent of resection status by characteristic
| Covariates | Total | Gross-total resection |
Near-total resection |
Sub-total resection |
|---|---|---|---|---|
| Total [n (%)] | 366 (100) | 119 (33.4) | 91 (24.9) | 156 (42.6) |
| Previous pituitary adenoma resection [n (%)] | 70 (100) | 11 (15.7) | 14 (20.0) | 45 (64.3)*** |
| Functional tumor [n (%)] | 146 (100) | 62 (51.7) | 30 (33.0) | 54 (34.8)** |
| Cavernous sinus extension [n (%)] | 140 (100) | 16 (11.4) | 26 (18.6) | 98 (70.0)*** |
| Apoplexy [n (%)] | 30 (100) | 13 (43.3) | 7 (23.3) | 10 (33.3) |
| Atypical pathology [n (%)] | 49 (100) | 9 (18.4) | 15 (30.6) | 25 (51.0) |
| Treatment combination | ||||
| Non-iMRI + Microscopy [n (%)] | 159 (100) | 44 (27.7) | 33 (20.8) | 82 (51.6) |
| Non-iMRI + Endoscopy [n (%)] | 51 (100) | 18 (35.3) | 14 (27.5) | 19 (37.3) |
| iMRI + Microscopy [n (%)] | 41 (100) | 15 (36.6) | 9 (22.0) | 17 (41.5) |
| iMRI + Endoscopy [n (%)] | 115 (100) | 42 (36.5) | 35 (30.4) | 38 (33.3) |
Significant differences in extent of resection status were noted of resection status were noted for recurrent tumors (p < 0.001), functional tumors (p < 0.01), and cavernous sinus invading tumors (p <0.001)
Pearson’s Chi squared and Students t-test were used for significance testing as appropriate
p < 0.05;
p < 0.01;
p < 0.001
Table 4.
Predictors of increased extent of resection status: Univariate and multivariate ordinal logistic regression models
| Covariates (n = 366) | iMRI-independent—iMRI or 1st post-op of MRI |
iMRI-dependent—1st post-op MRI |
||
|---|---|---|---|---|
| Univariate OR (95 % CI) |
Multivariate OR (95 % CI) |
Univariate OR (95 % CI) |
Multivariate OR (95 % CI) |
|
| Age (standardized)a | 0.80 (0.66, 0.96)* | 1.00 (0.79, 1.28) | 0.85 (0.70, 1.03)† | 0.99 (0.78, 1.26) |
| BMI (standardized)a | 1.16 (0.96, 1.40)† | 0.97 (0.78, 1.20) | 1.21 (0.99, 1.47)† | 1.06 (0.85, 1.33) |
| Tumor size (standardized)a | 0.31 (0.23, 0.40)*** | 0.36 (0.26, 0.51)*** | 0.38 (0.30, 0.48)*** | 0.43 (0.31, 0.59)*** |
| Previous pituitary adenoma resection |
0.35 (0.20, 0.59)*** | 0.27 (0.14, 0.51)*** | 0.33 (0.19, 0.55)*** | 0.26 (0.14, 0.48)*** |
| Functional tumor | 2.38 (1.60, 3.54)*** | 1.03 (0.59, 1.82) | 1.71 (1.15, 2.53)* | 0.77 (0.44, 1.33) |
| Cavernous sinus extension | 0.13 (0.08, 0.21)*** | 0.21 (0.13, 0.36)*** | 0.15 (0.09, 0.23)*** | 0.22 (0.13, 0.36)*** |
| Apoplexy | 1.25 (0.64, 2.45) | 2.42 (1.10, 5.33)* | 1.58 (0.79, 3.18) | 2.52 (1.13, 5.62)* |
| Atypical pathology | 0.72 (0.41, 1.27) | 1.50 (0.75, 3.01) | 0.58 (0.33, 1.01)† | 1.16 (0.60, 2.26) |
| Treatment combination | ||||
| Non-iMRI + Microscopy | Ref | Ref | Ref | Ref |
| Non-iMRI + Endoscopy | 1.42 (0.97, 2.08)† | 1.48 (0.91, 2.39)† | 1.78 (0.99, 3.19)† | 1.65 (0.82, 3.31)† |
| iMRI + Microscopy | – | – | 1.53 (0.80, 2.93) | 1.42 (0.60, 3.33) |
| iMRI + Endoscopy | – | – | 1.83 (1.17, 2.87)** | 2.05 (1.21, 3.46)** |
Similar associations were noted between covariates and extent of resection status for the iMRI-independent and iMRI dependent analyses. The combination of iMRI and endoscopy was more effective than either iMRI or endoscopy alone in achieving increased extent of resection status
Analysis performed on 366 cases remaining after general exclusion. Multivariate models also controlled for primary surgeon; however, odds ratios for this covariate are not presented due to inherent lack of generalizability
95 % CI 95 % confidence interval, OR odds ratio, ref reference category
Standardization was performed by conversion of continuous variables to z-scores
p < 0.15;
p < 0.05;
p < 0.01;
p < 0.001
Extent of resection status analysis—iMRI independent
The extent of resection status achieved by endoscopy alone (i.e., independent of post-iMRI resection) was captured by analyzing iMRI scans in the iMRI cohort and post-operative MRI scans in non-iMRI cohort (Table 4, left). Univariate regression analysis revealed that increased age, increased tumor size, previous pituitary adenoma resection, non-functional tumor status, and cavernous sinus invasion were associated with a lower extent of resection status. Multivariate regression analysis revealed similar associations as univariate analysis for increased tumor size, previous pituitary adenoma resection, and cavernous sinus invasion with a lower extent of resection status. Differences noted in multivariate compared to univariate regression analysis were an association between pituitary apoplexy and increased extent of resection status, and no extent of resection status association with age or functional tumor status. Endoscopy showed a trend towards increased extent of resection status compared to microscopy on univariate (OR 1.42, 95 % CI 0.97–2.08) and multivariate regression analysis (OR 1.48, 95 % CI 0.91–2.39), but these results were not statistically significant.
Post-operative treatment and progression analysis
Following surgery, 103/366 cases (28.1 %) were treated for residual tumor including 48 with stereotactic radiosurgery only, 14 with conventional external beam radiotherapy only, 5 with hormone suppressive medication only, 13 with additional surgical resection only, and 23 with a combination of radiotherapy, surgery and/or medication. Failure to achieve biochemical remission within the available follow-up was noted in 13/128 functional adenoma patients (10.2 %), 11 received post-operative adjuvant therapy and 2 elected to be followed expectantly. Median follow-up for cases failing to achieve remission post-operatively was 15.5 months (mean 22.3 ± 19.8 months, range 8.7–79.2 months). No difference was noted in biochemical remission rate between iMRI and non-iMRI cases (55/62 [88.7 %] vs. 63/66 [95.5 %], p = 0.62) or between endoscopic and microscopic surgery cases (53/58 [91.4 %] vs. 65/70 [92.9 %], p = 0.93).
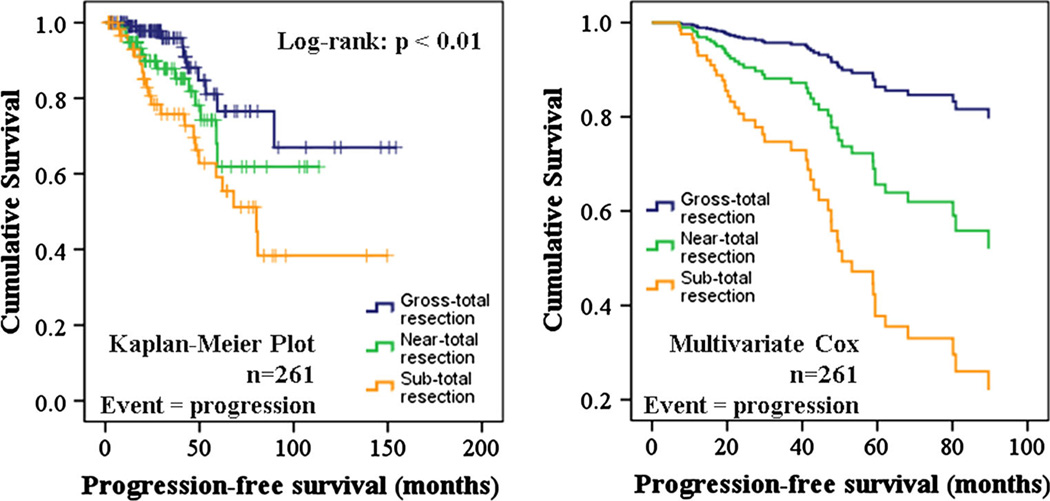
Forty-five of the 261 cases (17.2 %) followed expectantly (i.e. no further surgery, radiation, or hormone suppressing medications at initial follow-up), including those that achieved biochemical remission post-operatively exhibited tumor progression (median follow-up time = 32.2 months; range 2–154 months). These 261 cases were less likely to have received previous pituitary adenoma resection and more likely to have an increased extent of resection status than the 105 excluded cases. These cases were also more likely to have tumors that were functional, cavernous sinus invading, or atypical (Table 5). Log-rank testing revealed a significant difference in progression-free survival for different extent of resection statuses (p < 0.01, Fig. 4, left). Univariate Cox regression revealed a significantly shorter progression-free survival for cases that received previous pituitary adenoma resection (HR 2.34, 95 % CI 1.09–5.06, p <0.05) and for younger aged patients (HR 0.72, 95 % CI 0.55–0.95, p < 0.05). Multivariate Cox regression revealed that successively worse extent of resection statuses was associated with a shorter progression-free survival [near- vs. gross-total resection (HR 2.87, 95 % CI 1.24–6.65, p <0.05); sub- versus near-total resection (HR 2.10, 95 % CI 1.00–4.40, p < 0.05); Table 6; Fig. 4, right]. Older age (HR 0.60, 95 % CI 0.43–0.83, p <0.01) and functional tumor status (HR 2.16, 95 % CI 1.09–4.30, p < 0.05) were associated with longer progression-free survival, while previous pituitary adenoma resections (HR 1.85 95 % CI 0.82–4.18, p < 0.15) showed a trend towards shorter progression-free survival. Based on these results suggesting a strong association between greater extent of resection status and longer progression-free survival, as well as the more limited follow-up time for iMRI cases (median 25.6 months; range 2–64 months) versus non-iMRI cases (median 47.7 months; range 2–154 months), extent of resection status was considered a reasonable surrogate marker of clinical outcome following transsphenoidal resection.
Table 5.
Progression rate by characteristic
| Covariates | Treatment for residual/no biochemical remission |
Progression |
||||
|---|---|---|---|---|---|---|
| Total n (%) |
Yes n (%) |
No n (%) |
Total n (%) |
Yes n (%) |
No n (%) |
|
| Total [n (%)] | 366 (100) | 105 (100) | 261 (100) | 261 (100) | 45 (100) | 216 (100) |
| Previous pituitary adenoma resection [n (%)] | 70 (19.1) | 40 (38.1) | 30 (11.5)*** | 30 (11.5) | 8 (17.8) | 22 (10.2) |
| Functional tumor [n (%)] | 146 (39.9) | 54 (51.4) | 92 (35.2)* | 92 (35.2) | 18 (40.0) | 74 (34.3) |
| Cavernous sinus extension [n (%)] | 140 (38.3) | 65 (61.9) | 75 (28.7)*** | 75 (28.7) | 16 (35.6) | 59 (27.3) |
| Apoplexy [n (%)] | 30 (8.2) | 8 (7.6) | 22 (8.4) | 22 (8.4) | 2 (4.4) | 20 (9.3) |
| Atypical pathology [n (%)] | 49 (13.4) | 24 (22.9) | 25 (9.6)*** | 25 (9.6) | 5 (11.1) | 20 (9.3) |
| Post-op extent of resection status | ||||||
| Gross-total resection [n (%)] | 119 (32.5) | 2 (1.9) | 117 (44.8) | 117 (44.8) | 10 (22.2) | 107 (49.5) |
| Near-total resection [n (%)] | 91 (24.9) | 14 (13.3) | 77 (29.5) | 77 (29.5) | 14 (31.1) | 63 (29.2) |
| Sub-total resection [n (%)] | 156 (42.6) | 88 (83.8) | 67 (25.7) | 67 (25.7) | 21 (46.7) | 46 (21.3) |
| Treatment combination | ||||||
| Non-iMRI + Microscopy [n (%)] | 159 (43.4) | 54 (51.4) | 105 (40.2) | 105 (40.2) | 26 (57.8) | 79 (36.6) |
| Non-iMRI + Endoscopy [n (%)] | 51 (13.9) | 9 (8.6) | 42 (16.1) | 42 (16.1) | 10 (22.2) | 32 (14.8) |
| iMRI + Microscopy [n (%)] | 41 (11.2) | 13 (12.4) | 28 (10.7) | 28 (10.7) | 0 (0.0) | 28 (13.0) |
| iMRI + Endoscopy [n (%)] | 115 (31.4) | 29 (27.6) | 86 (33.0) | 86 (33.0) | 9 (20.0) | 77 (35.6) |
Cases were more likely to receive treatment for residual tumor or fail biochemical remission if recurrent tumors (p <0.001), functional tumors (p < 0.05), cavernous sinus invading tumors (p < 0.001), atypical tumors (p < 0.001), or lower extent of resection status (p< 0.001). Extent of resection status and treatment modality combination had a significant effect on post-operative progression (p<0.001)
Pearson’s Chi squared test was used for significance testing
p < 0.05;
p < 0.01;
p < 0.001
Fig. 4.
Survival analyses depicting progression-free survival for different extent of resection statuses. Kaplan– Meier plot (left) and multivariate Cox regression (right) of cases followed expectantly after surgery (i.e., no additional surgery, radiation, or hormone suppressive medication) showed a significantly longer progression-free survival for greater extent of resection status
Table 6.
Predictors of shorter progression-free survival: Univariate and multivariate Cox proportional hazards regression models
| Covariates (n = 261) | Univariate HR (95 % CI) |
Multivariate HR (95 % CI) |
|---|---|---|
| Age (standardized)a | 0.72 (0.55, 0.95)* | 0.60 (0.43, 0.83)** |
| BMI (standardized)a | 1.18 (0.89, 1.56) | – |
| Tumor size (standardized)a | 0.96 (0.69, 1.35) | – |
| Previous pituitary adenoma resection |
2.34 (1.09, 5.06)* | 1.85 (0.82, 4.18)† |
| Functional tumor | 1.80 (0.98, 3.28)† | 2.16 (1.09, 4.30)* |
| Cavernous sinus extension | 1.16 (0.63, 2.14) | – |
| Apoplexy | 0.61 (0.15, 2.51) | – |
| Atypical pathology | 1.08 (0.42, 2.73) | – |
| Extent of resection statusb | ||
| Gross-total resection | Ref | Ref |
| Near-total resection | 1.93 (0.86, 4.35)† | 2.87 (1.24, 6.65)* |
| Sub-total resection | 1.68 (0.85, 3.31)† | 2.10 (1.00, 4.40)* |
Univariate Cox regression revealed significantly greater hazard ratios of progression for cases with young age at the time of surgery and recurrent tumors. Multivariate Cox regression of covariates approaching significance on univariate testing revealed significantly greater hazard ratios of progression for cases with younger age at the time of surgery and successive lowering of extent of resection statuses
Analysis performed on 261 cases not treated with additional surgery, radiation or hormone suppressing medications after surgery and achieved biochemical remission
95 % CI 95 % confidence interval, HR hazard ratio, ref reference category
Standardization was performed by conversion of continuous variables to z-scores
Comparisons for extent of resection status were gross- versus near-total resection and near- versus sub-total resection
p < 0.15;
p < 0.05;
p < 0.01;
p < 0.001
Surgical complications
Assessment of the 446 total transsphenoidal cases identified four perioperative deaths and four perioperative arterial injuries receiving endovascular intervention. Causes of death included (1) hemorrhage and infarction in a 77-year-old with a large non-functioning macroadenoma who underwent an endovascular procedure for an anterior cerebral artery injury that occurred during dissection of suprasellar tumor; (2) multi-organ failure in a 86-year-old with apoplexy who required an endovascular procedure for an internal carotid artery injury; (3) an apparent pulmonary embolism in a 33-year-old morbidly obese patient (body weight = 168 kg, BMI = 54.9 kg/m2) with Cushing’s Disease, multiple endocrine neoplasm type 1 (MEN-1), and multiple medical comorbidities; and (4) sepsis and multi-organ failure in a 37-year-old morbidly obese patient (body weight = 208 kg, BMI = 85.5 kg/m2) with Cushing’s Disease and multiple medical comorbidities. None of the cases with perioperative death received iMRI, but in one case a planned iMRI was deferred due to an arterial injury that required endovascular control. Four perioperative deaths occurred following endoscopy (1.9 %), while none occurred following microscopic resection. These results were significant on direct comparison in a 2 × 2 contingency table (Fisher exact, p = 0.04), but were non-significant after accounting for the numerous post hoc complication comparisons (e.g., CSF leak, DI, death, etc.) between microscopy and endoscopy (p = 0.24). Cases receiving iMRI and endoscopy trended towards an increased likelihood of intraoperative CSF leak than microscopy cases without iMRI (p <0.10). No other differences in complication rates were noted for iMRI versus non-iMRI cases; endoscopy versus microscopy cases; endoscopy with iMRI versus conventional transsphenoidal microsurgery; or cases receiving versus not receiving additional resection after iMRI (Table 7).
Table 7.
Frequency of specific post-operative complications by treatment type
| Covariates | Total n(%) |
Non-iMRI n(%) |
iMRI n(%) |
Microscopy n(%) |
Endoscopy n(%) |
Non-iMRI + Microscopy n(%) |
iMRI + Endoscopy n(%) |
Add resect after iMRI n(%) |
No add resect after iMRI n(%) |
| Total | 446 (100) | 263 (100) | 183 (100) | 237 (100) | 209 (100) | 190 (100) | 136 (100) | 66 (100) | 117 (100) |
| Permanent DI | 22 (4.9) | 12 (4.6) | 10 (5.5) | 12 (5.1) | 10 (4.8) | 9 (4.7) | 7 (5.1) | 3 (4.5) | 7 (6.0) |
| DVT | 7 (1.6) | 5 (1.9) | 2(1.1) | 3 (1.3) | 4 (1.9) | 2(1.1) | 1 (0.7) | 0 (0.0) | 2 (1.7) |
| Meningitis | 6(1.3) | 4 (1.5) | 2(1.1) | 4 (1.7) | 2 (1.0) | 3 (1.6) | 1 (0.7) | 2 (3.0) | 0 (0.0) |
| PE | 5(1.1) | 4 (1.5) | 1 (0.5) | 3 (1.3) | 2 (1.0) | 2(1.1) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| CVA | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Wound infectiona | 1 (0.2) | 1 (0.4) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CSF leakb | |||||||||
| Total | 78 (17.5) | 43 (16.3) | 35 (19.1) | 37 (15.6) | 41 (19.6) | 29 (15.3) | 32 (23.5)† | 16 (24.2) | 21 (17.9) |
| Procedure | 68 (15.2) | 34 (12.9) | 34 (18.6) | 30 (12.7) | 38 (18.2) | 24 (12.6) | 28 (20.6)† | 14 (21.2) | 19 (16.2) |
| Additional surgery | 16 (3.6) | 6 (2.3) | 10 (5.5) | 6 (2.5) | 10 (4.8) | 4 (2.1) | 8 (5.9) | 2 (3.0) | 8 (6.8) |
| Arterial injuryc | 4 (0.9) | 4 (1.5)† | 0 (0.0) | 1 (0.4) | 3 (1.4) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Deathc | 4 (0.9) | 4 (1.5)† | 0 (0.0) | 0 (0.0) | 4 (1.9)* | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Perioperative mortality was significantly greater for cases receiving endoscopic compared to microscopic transsphenoidal resection (p < 0.05). All other comparisons between microscopy, endoscopy, and/or intraoperative MRI showed no significant differences
Fisher’s exact test compared each complication between treatment types in adjacent columns, and was repeated comparing all complications to account for alpha inflation from multiple comparisons
Add resect additional resection after iMRI, CSF cerebrospinal fluid, DI diabetes insipidus, DVT deep vein thrombosis, PE pulmonary embolus, Perm permanent
One patient had an abdominal adipose donor site infection that resolved following debridement and a 10-day course of oral antibiotics
Any CSF leak occurring either intra- or post-operatively. Procedures for CSF leak included lumbar drain placement and/or operative repair
One patient intended for iMRI treatment had a perioperative artery injury with a subsequent endovascular procedure for hemostasis that precluded iMRI scanning and ultimately resulted in death
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001
Extended operation time
Median total surgical time (first incision to closure) for iMRI cases was 277 min and median total time for iMRI scanning (operation stop to restart) was 80 min. Additional iMRI time was from pre-iMRI preparation (median 17 min), scanning and interpretation (median 45 min), and post-iMRI preparation (median 13 min). Median total operating room time (in-room to out-of-room time) was 127 min greater for the 156 iMRI cases (378 min) compared to times available for 121 available non-iMRI cases (250 min).
Discussion
This retrospective study was designed to determine whether endoscopy and iMRI offer independent and/or combined improvement in patient outcomes following transsphenoidal pituitary adenoma resection. Prior publications by Theodosopolous et al. [28], Anand et al. [11], and Schwartz et al. [15] have demonstrated complementary efficacy of endoscopy and iMRI; however, these were relatively small studies with limited analytical evaluation. Furthermore, these previous studies did not perform detailed statistical analysis of cases treated with endoscopy and/or iMRI in comparison to those treated with traditional methods. Results of the present study support two general conclusions including: (1) iMRI and endoscopy have a complementary role in improving extent of resection status and (2) increased extent of resection status (gross- vs. near-vs. sub-total resection) lengthens progression-free survival. More aggressive resection with iMRI and endoscopy may be associated with increased complications related to resection technique (i.e., arterial injury and CSF leak), however, low event counts, multiple post hoc comparisons, and complication rarity limit this analysis.
The primary objective of this investigation was to assess the clinical impact of endoscopy and/or iMRI use during transsphenoidal resection of pituitary adenomas. A substantial challenge to performing this analysis was that many cases (103/366) received additional treatment (i.e. additional surgery, radiation, and/or hormone suppressing medications) following the first post-operative MRI/endocrine evaluation, and 2/366 failed to achieve biochemical remission and declined additional therapy. Additional postoperative therapy by definition prohibited inclusion in progression-free survival analysis, as additional therapies significantly impact progression-free survival independent of surgery with endoscopy and/or iMRI. To assess the “natural history” of pituitary adenomas after transsphenoidal resection, all cases that did not undergo further treatment (additional surgery, radiation, or hormone suppressing medications) after initial postoperative assessments were followed for tumor progression or recurrence. This included 77/91 cases identified with near-total resection, 67/156 cases with sub-total resection, and 117/119 cases with gross-total resection (and biochemical remission for functional tumors). The two gross-total resection cases excluded from the progression-free survival analysis had a recurrent ACTH-secreting adenoma and a GH-secreting adenoma, respectively, that failed to achieve biochemical remission despite no tumor identified radiographically. Selection bias in the progression-free survival analysis cohort was noted against cases with suspected poor prognostic factors (i.e., previous pituitary adenoma resection, functional tumors, cavernous sinus invasion, and atypical adenoma); therefore, the estimated hazard ratios for these covariates are likely lower than actual values. Cases with lower extent of resection status were also more likely to receive post-operative adjuvant treatment and be excluded from progression-free survival analysis, which suggests that the impact of extent of resection status in the Cox regression is likely a conservative estimate.
The reported long tumor volume doubling time reported for untreated (mean 337–980 days) [38, 39] and recurrent pituitary adenomas (mean 1,836 days) [40] impacted two features of this study. First, to capture the efficacy of endoscopy (i.e., independent of iMRI), extent of resection status was defined using scans at different time points for iMRI and non-iMRI cases. In iMRI cases, extent of resection status was defined on iMRI scans, while in non-iMRI cases, the first post-operative MRI scan was used. Imaging at these time points were considered comparable due to the short mean follow-up intervals (5.22 ± 3.41 months) relative to the long tumor doubling time. Similar odds ratios for endoscopy without iMRI in both multivariate ordinal logistic regression extent of resection status models (iMRI-independent OR 1.48, 95 % CI 0.91–2.41 vs. iMRI-dependent OR 1.65, 95 % CI 0.82–3.31) strengthen this assumption. These results also suggest that the pre-iMRI resection “aggressiveness” was not significantly altered due to the knowledge of having an additional resection opportunity after iMRI. Second, the relatively short overall follow-up time for iMRI cases (25.6 months) limited direct observation of many potential tumor progression events; however, Cox proportional hazards analysis showed a strong association between extent of resection status and progression-free survival (Fig. 4). Based on these findings and prior work by Chang [1] also demonstrating this association, we concluded that extent of resection status could be used in this analysis as a suitable surrogate measure of clinical benefit. Numerous groups have reported the percentage of cases receiving increased extent of resection status for pituitary adenoma resections attributable to high-field iMRI [41]. The largest study to date analyzing 1.5T iMRI was conducted by Nimsky et al., [22] who prospectively evaluated 85 non-functional pituitary macroadenoma cases determined eligible for complete resection pre-operatively. Results showed 36 cases (42 %) had possible residual tumor on iMRI, with 21 of these achieving gross-total resection after additional post-iMRI resection, increasing the gross-total resection rate in this selected sample of cases by 24 % (58 % on iMRI to 82 % on post-op MRI). Other groups have begun to report on experiences with 3.0T iMRI [19, 23, 42]. Netuka et al. [19] have reported results of 49 cases intended for gross-total resection receiving 3.0T iMRI and endoscopy, with results demonstrating an increase in gross-total resection rate of 22.4 % (69.4 % on iMRI to 91.8 % on post-op MRI). Additionally, Pamir et al. [23] have reported on 29 cases intended for complete resection with 3.0T iMRI, and noted a 13.8 % increase in gross-total resection. In the present investigation, 56/156 iMRI cases (35.9 %) had iMRI findings that led to additional surgery, and 15/156 (9.6 %) had increased extent of resection status with positive pathology results attributable to additional resection after iMRI.
Prior studies have not included detailed comparisons between non-iMRI and iMRI resections, or multivariate analyses evaluating the effect of covariates such as cavernous sinus invasion, tumor size, and prior surgery on extent of resection and progression-free survival. In a 2013 study, Coburger et al. [25] retrospectively compared cases with versus without high-field iMRI, and showed that iMRI led to improved extent of resection in planned gross total resection cases, lower tumor volumes in planned sub-total resection cases, and a lower incidence of intrasellar tumor. In the present study, factors known to influence extent of resection such as previous resection, tumor size, and cavernous sinus invasion had significant effects in both the univariate and multivariate extent of resection models. Pituitary apoplexy was significantly associated with increased extent of resection status, as has been reported in other studies, which suggest that the necrosis and hemorrhagic changes associated with apoplexy facilitate easier tumor removal [43, 44]. The combined use of iMRI and endoscopy led to a significant increase in extent of resection compared to conventional transsphenoidal microsurgery when controlling for these other prognostic factors.
Any clinical benefit offered by endoscopy and iMRI through greater extent of resection status must be balanced against the potential increase in complication rate. A number of large case series have evaluated complication rates for endoscopic [45, 46] and microscopic [47, 48] transsphenoidal pituitary adenoma resection. Recent work by Halvorsen et al. [49] revealed low morbidity and mortality associated with both microscopic and endoscopic transsphenoidal resection, with no difference in complication rates between these modalities. The perioperative mortality rate in this series of cases (4/466, 0.9 %) is comparable to other larger series (0.3–1.2 %) [45, 50]. All four perioperative deaths occurred following endoscopic resection. Two deaths were related to intracranial arterial injuries during resection, which could potentially be attributable to resection technique. The remaining two deaths were from pulmonary embolism and multi-organ failure associated with significant co-morbidities from Cushing’s disease. When considering the limited theoretical association between endoscopy and the cause of death in the latter two cases, as well as the multiple comparisons made during these complication assessments (i.e., the numerous complication types compared among multiple treatment groups), it is possible that the significantly higher rate of death after surgery performed with endoscopy is a spurious finding. Nonetheless, the surgeon must balance the goals of maximal resection with potentially greater risks associated with more aggressive resection.
The most common minor complication was an intraoperative CSF leak (78/466 cases; 16.7 %) and the most common major complication was post-operative CSF leak requiring surgical correction (16/466 cases; 3.4 %). A trend towards a significantly higher intraoperative CSF leak rate was noted for cases that received endoscopy and iMRI (28/ 136 cases, 20.6 %) compared to microscopy only (24/190 cases, 12.6 %; p < 0.10) when not accounting for multiple comparisons. No significant difference in the rate of postoperative CSF leak was noted for endoscopy (10/209 cases; 4.8 %) compared to microscopic resection (6/237 cases; 2.5 %). In contrast to our study, a meta-analysis by DeKlotz et al. [51] revealed a small but significantly higher post-operative CSF leak rate for microscopic compared to endoscopic pituitary adenoma resections (7 vs. 5 %, respectively). Interpretation of these results is limited, since included studies were case series prone to selection bias. The results from our study are also prone to a degree of selection bias, since treatment options were not assigned randomly or compared after systematic matching. Survey work by Ciric et al. [52] revealed that surgeons with less experience were more likely to report a higher number of complications during pituitary adenoma resection than more experienced surgeons. Tumor characteristics (i.e., size, extension, and dural invasion) and other operative characteristics such as closure technique also contribute to modify the risk of intra- and post-operative CSF leak.
To our knowledge, no iMRI studies to date have reported perioperative complications associated with scanning or the ferromagnetic environment. Additional resection after iMRI could conceivably result in operative complication; however, prior studies have not reported an increased rate. The series of 106 non-functioning pituitary adenoma by Nimsky et al. [22] reported two major complications including a death from pulmonary embolism and a CSF leak requiring reoperation. In the present study, no complications were directly attributable to the iMRI environment, patient scanning, or additional resection after iMRI. In addition, complications potentially increased by longer anesthesia time (i.e., DVT, PE) were not increased for the iMRI group (median 80 min increase); however, increased anesthesia time does pose a theoretical risk for patients with significant co-morbidities or old age.
Principle differences of the present study compared to prior work are the inclusion of all cases regardless of pre-operatively tumor size, functional status, or resectability; inclusion of near-total resection as a distinct extent of resection status; and evaluation of the independent extent of resection status impact of endoscopy and iMRI. All cases that received iMRI were included in our analysis, since despite the large body of work published on high-field iMRI, there is limited guidance as to which subsets of patients with pituitary adenomas are most likely to have increased resection with the use of iMRI. Imaging of the post-operative sellar region can be challenging, even using high-field MRI, and quite often small abnormal foci are not definitive for residual tumor. The high percentage of such scans in this study (30.2 % for iMRI and 24.9 % for the first post-operative MRI) prompted the definition of a “near-total” extent of resection status, which was found to have a statistically significant pattern of progression-free survival compared to gross- and sub-total resection (Fig. 4). When accounting for near-total resection, additional resection after iMRI was found to be directly responsible for increased extent of resection status in 15/156 cases (9.6 %, Fig. 3), which was less than the 24 % reported by Nimsky et al., [22] likely due in part to a lack of case selection in the present study. Of note, in our study additional surgery was performed after the iMRI scan in 56/156 cases (35.9 %) of the iMRI cases, but only in 15/56 was the extent of resection status improved to a higher level (i.e., near- or gross-total resection), which was demonstrated to have a progression-free survival benefit. Decreased tumor residual volume, even if it does not increase extent of resection status, may still offer patients benefit from decreasing the volume targeted for radiosurgery, which could decrease the radiation risk and increase the potential for radiation efficacy. Multivariate analysis of post-operative outcome (i.e., extent of resection status) including cases that did not receive iMRI was used to eliminate potential biases associated with the knowledge of iMRI availability, as well as to evaluate the independent impact of endoscopy and iMRI. Some have argued that a “second chance” to complete tumor removal may result in less aggressive initial (i.e., pre-iMRI scan) resections. Others have countered that the immediate performance evaluation offered by iMRI provides additional motivation to accomplish gross-total resection, and may lead to more aggressive initial resections and technique refinement for subsequent cases. These potential biases, along with case heterogeneity, limit interpretation of prior reported analyses, which define iMRI efficacy as the percentage of cases that had increased extent of resection status to gross-total resection.
Principle study limitations
Although data was collected in a combined prospective and retrospective manner, analysis was retrospective. Suspected confounding covariates were addressed by multivariate statistical modeling; however, other unappreciated confounding variables may persist. The impact of neuronavigation and the endonasal approach were not considered in the extent of resection status models due to collinearity and overlapping theoretical benefit with endoscopy and iMRI (i.e., updating of neuronavigation with iMRI scans). Surgeon experience at the time of surgery was also not considered due to the strong association with the implementation of the new techniques. Selection bias was introduced by the case exclusion process, particularly for the progression-free survival analysis where cases were excluded for residual tumor treatment and failed biochemical remission. The most effective method to analyze the impact of different combinations of surgical technologies on pituitary adenoma extent of resection status would be a prospective randomized controlled trial. A randomized controlled trial has not been performed that tests iMRI resection efficacy for pituitary adenoma; however, both 5-ALA and iMRI use during malignant glioma resection have been shown to improve extent of resection status using this a randomized prospective study design [53, 54].
The length of follow-up available for analysis was limited given the potential for delayed recurrence of pituitary adenoma and the implementation of new technologies (i.e. endoscopy and iMRI). Time-to-event analysis (i.e., Kaplan–Meier analysis and Cox regression) accounted for early censoring and difference in follow-up interval between cases, but it is certainly possible that delayed recurrences were missed. It was; however, apparent and mechanistically reasonable that extent of resection status was a strong and independent predictor of post-operative progression-free survival, and we believe the use of this as a surrogate marker for clinical benefit was justified. Perhaps more challenging is the assumption that progression-free survival is a robust marker of clinical effectiveness. A more comprehensive measure would account for the patient-specific efficacy and harm associated with surgery and available non-invasive post-operative adjuvants such as fractionated radiation, stereotactic radiosurgery, and hormone suppressive medication. Decision analysis and cost-effectiveness comparison may be more useful techniques for identifying true clinical benefit, as well as determining which patients receive the most benefit from endoscopy or iMRI.
Despite these limitations, this study of a large series of cases with pituitary adenomas treated with a variety of methodologies adds to our understanding of the potential impact of these advanced surgical techniques on patient outcomes. Longer follow-up of larger series of patients may shed additional light on which patients might realize the most benefit from the use of these techniques. This study suggests that the combination of endoscopy and iMRI can significantly improve the extent of resection status and therefore progression-free survival for patients undergoing transsphenoidal resection for pituitary adenomas. Further analysis to assess the most appropriate, efficient, and safest application of these technologies is warranted.
Conclusions
Statistical analysis revealed that the combined used of endoscopy and iMRI for transsphenoidal resection of pituitary adenomas increased extent of resection status compared to conventional microsurgery without iMRI when controlling for baseline and tumor characteristics. Increased extent of resection status (gross- vs. near- vs. sub-total resection) was strongly associated with longer disease-specific progression-free survival. No significant difference in complication rates were noted in comparisons including microscopy, endoscopy, and iMRI; however, further study is need to determine if more aggressive resection is associated with an increase in resection-related complications.
Acknowledgments
We would like to thank Feng Gao, PhD and J. Phillip Miller, PhD from the Department of Biostatistics at Washington University in St. Louis for his advice regarding the statistical methods performed in this study. Furthermore, we would like to thank Bridget McCullough & Stan Goddard our MRI technologists, and Kathy Draege our neurosurgical operating room charge nurse and our entire operating room staff. These individuals enable the safe completion of these surgical procedures in the complex iMRI environment
Michael Chicoine and John Evans received funding from IMRIS Inc. for an unrestricted educational grant that has helped support the iMRI database and outcomes analysis. Peter Sylvester received grant support from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1 TR000448 and TL1 TR000449.
Footnotes
Conflict of interest The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Peter T. Sylvester, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA
John A. Evans, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA
Gregory J. Zipfel, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA
Richard A. Chole, Getz Department of Otolaryngology, Head and Neck Surgery, Washington University School of Medicine, St. Louis, MO, USA
Ravindra Uppaluri, Getz Department of Otolaryngology, Head and Neck Surgery, Washington University School of Medicine, St. Louis, MO, USA.
Bruce H. Haughey, Getz Department of Otolaryngology, Head and Neck Surgery, Washington University School of Medicine, St. Louis, MO, USA
Anne E. Getz, Getz Department of Otolaryngology, Head and Neck Surgery, Washington University School of Medicine, St. Louis, MO, USA
Julie Silverstein, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA; Department of Internal Medicine/Endocrinology, Metabolism and Lipid Research, Washington University School of Medicine, St. Louis, MO, USA.
Keith M. Rich, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA
Albert H. Kim, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA
Ralph G. Dacey, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA
Michael R. Chicoine, Email: chicoinem@wudosis.wustl.edu, Department of Neurosurgery, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8057, St. Louis, MO, USA.
References
- 1.Chang EF, Zada G, Kim S, et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736–745. doi: 10.3171/JNS/2008/108/4/0736. [DOI] [PubMed] [Google Scholar]
- 2.Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic transsphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2011;36:212–220. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 3.Tabaee A, Anand VK, Barro´n Y, et al. Endoscopic pituitary surgery: a systematic review and meta-analysis. J Neurosurg. 2009;111:545–554. doi: 10.3171/2007.12.17635. [DOI] [PubMed] [Google Scholar]
- 4.Cho D-Y, Liau W-R. Comparison of endonasal endoscopic surgery and sublabial microsurgery for prolactinomas. Surg Neurol. 2002;58:371–375. doi: 10.1016/s0090-3019(02)00892-3. [DOI] [PubMed] [Google Scholar]
- 5.Chole RA, Lim C, Dunham B, et al. A novel transnasal transsphenoidal speculum: a design for both microscopic and endoscopic transsphenoidal pituitary surgery. J Neurosurg. 2011;114:1380–1385. doi: 10.3171/2010.11.JNS101167. [DOI] [PubMed] [Google Scholar]
- 6.D’Haens J, Van Rompaey K, Stadnik T, et al. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: a retrospective comparison with traditional trans-sphenoidal microsurgery in the same institution. Surg Neurol. 2009;72:336–340. doi: 10.1016/j.surneu.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin N, Eisenberg AA, Cohan P, et al. Value of endoscopy for maximizing tumor removal in endonasal trans-sphenoidal pituitary adenoma surgery. J Neurosurg. 2013;118:613–620. doi: 10.3171/2012.11.JNS112020. [DOI] [PubMed] [Google Scholar]
- 8.Messerer M, De Battista JC, Raverot G, et al. Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus. 2011;30:E11. doi: 10.3171/2011.1.FOCUS10308. [DOI] [PubMed] [Google Scholar]
- 9.Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:843–849. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starke RM, Raper DMS, Payne SC, et al. Endoscopic versus microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013;98:1–10. doi: 10.1210/jc.2013-1036. [DOI] [PubMed] [Google Scholar]
- 11.Anand VK, Schwartz TH, Hiltzik DH, Kacker A. Endo-scopic transsphenoidal pituitary surgery with real-time intraoperative magnetic resonance imaging. Am J Rhinol. 2006;20:401–405. doi: 10.2500/ajr.2006.20.2877. [DOI] [PubMed] [Google Scholar]
- 12.Berkmann S, Fandino J, Zosso S, et al. Intraoperative magnetic resonance imaging and early prognosis for vision after transsphenoidal surgery for sellar lesions. J Neurosurg. 2011;115:518–527. doi: 10.3171/2011.4.JNS101568. [DOI] [PubMed] [Google Scholar]
- 13.Fahlbusch R, Ganslandt O, Buchfelder M, et al. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381–390. doi: 10.3171/jns.2001.95.3.0381. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach R, du Mesnil de Rochemont R, Gasser T, et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63:272–275. doi: 10.1227/01.NEU.0000312362.63693.78. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz TH, Stieg PE, Anand VK. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery. 2006;58:44–51. doi: 10.1227/01.neu.0000193927.49862.b6. [DOI] [PubMed] [Google Scholar]
- 16.Vitaz TW, Inkabi KE, Carrubba CJ. Intraoperative MRI for transsphenoidal procedures: short-term outcome for 100 consecutive cases. Clin Neurol Neurosurg. 2011;113:731–735. doi: 10.1016/j.clineuro.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Wu JS, Shou XF, Yao CJ, et al. Transsphenoidal pituitary macroadenomas resection guided by PoleStar N20 low-field intraoperative magnetic resonance imaging: comparison with early postoperative high-field magnetic resonance imaging. Neurosurgery. 2009;65:61–63. doi: 10.1227/01.NEU.0000348549.26832.51. [DOI] [PubMed] [Google Scholar]
- 18.Fahlbusch R, Keller B, Ganslandt O, et al. Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol. 2005;153:239–248. doi: 10.1530/eje.1.01970. [DOI] [PubMed] [Google Scholar]
- 19.Netuka D, Masopust V, Belsan T, et al. One year experience with 3.0 T intraoperative MRI in pituitary surgery. Acta Neurochir Suppl. 2011;109:3–5. doi: 10.1007/978-3-211-99651-5_24. [DOI] [PubMed] [Google Scholar]
- 20.Nimsky C, Ganslandt O, Fahlbusch R. Comparing 0.2 tesla with 1.5 tesla intraoperative magnetic resonance imaging analysis of setup, workflow, and efficiency. Acad Radiol. 2005;12:1065–1079. doi: 10.1016/j.acra.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Nimsky C, Ganslandt O, Von Keller B, et al. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology. 2004;233:67–78. doi: 10.1148/radiol.2331031352. [DOI] [PubMed] [Google Scholar]
- 22.Nimsky C, Keller B, Ganslandt O, et al. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. 2006;58:105–114. doi: 10.1227/01.neu.0000243289.98791.05. [DOI] [PubMed] [Google Scholar]
- 23.Pamir MN, Peker S, Ozek MM, Dincer A. Intraoperative MR imaging: preliminary results with 3 tesla MR system. Acta Neurochir Suppl. 2006;98:97–100. doi: 10.1007/978-3-211-33303-7_13. [DOI] [PubMed] [Google Scholar]
- 24.Szerlip NJ, Zhang Y-CC, Placantonakis DG, et al. Transsphenoidal resection of sellar tumors using high-field intraoper-ative magnetic resonance imaging. Skull Base. 2011;21:223–232. doi: 10.1055/s-0031-1277262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coburger J, Konig R, Seitz K, et al. Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg. 2014;120:346–356. doi: 10.3171/2013.9.JNS122207. [DOI] [PubMed] [Google Scholar]
- 26.Chicoine MR, Lim CC, Evans JA, et al. Implementation and preliminary clinical experience with the use of ceiling mounted mobile high field intraoperative magnetic resonance imaging between two operating rooms. Acta Neurochir Suppl. 2011;109:97–102. doi: 10.1007/978-3-211-99651-5_15. [DOI] [PubMed] [Google Scholar]
- 27.Hall WA, Kowalik K, Liu H, et al. Costs and benefits of intraoperative MR-guided brain tumor resection. Acta Neurochir Suppl. 2003;85:137–142. doi: 10.1007/978-3-7091-6043-5_19. [DOI] [PubMed] [Google Scholar]
- 28.Theodosopoulos PV, Leach J, Kerr RG, et al. Maximizing the extent of tumor resection during transsphenoidal surgery for pituitary macroadenomas: can endoscopy replace intraoperative magnetic resonance imaging? J Neurosurg. 2010;112:736–743. doi: 10.3171/2009.6.JNS08916. [DOI] [PubMed] [Google Scholar]
- 29.Chicoine RM, Evans AJ, Wippold JF, et al. Comparison of intraoperative and postoperative MRI for endoscopic transsphenoidal resection of pituitary macroadenomas. Skull Base. 2011;21:A064. [Google Scholar]
- 30.Haydon DH, Chicoine MR, Dacey RG. The impact of high-field-strength intraoperative magnetic resonance imaging on brain tumor management. Neurosurgery. 2013;60:92–97. doi: 10.1227/01.neu.0000430321.39870.be. [DOI] [PubMed] [Google Scholar]
- 31.Leuthardt EC, Lim CCH, Shah MN, et al. Use of movable high-field-strength intraoperative magnetic resonance imaging with a wake craniotomies for resection of gliomas: preliminary experience. Neurosurgery. 2011;69:194–205. doi: 10.1227/NEU.0b013e31821d0e4c. [DOI] [PubMed] [Google Scholar]
- 32.Shah MN, Leonard JR, Inder G, et al. Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr. 2012;9:259–264. doi: 10.3171/2011.12.PEDS11227. [DOI] [PubMed] [Google Scholar]
- 33.De Paiva Neto MA, Vandergrift A, Fatemi N, et al. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin Endocrinol. 2010;72:512–519. doi: 10.1111/j.1365-2265.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- 34.Di Maio S, Cavallo LM, Esposito F, et al. Extended endoscopic endonasal approach for selected pituitary adenomas: early experience. J Neurosurg. 2011;114:345–353. doi: 10.3171/2010.9.JNS10262. [DOI] [PubMed] [Google Scholar]
- 35.Arnaldi G, Angeli A, Atkinson A, et al. Diagnosis and complications of Cushing’s Syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 36.Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol. 2006;65:265–273. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 37.Giustina A, Chanson P, Bronstein MD, et al. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141–3148. doi: 10.1210/jc.2009-2670. [DOI] [PubMed] [Google Scholar]
- 38.Ekramullah SM, Saitoh Y, Arita N, et al. The correlation of Ki-67 staining indices with tumour doubling times in regrowing non-functioning pituitary adenomas. Acta Neurochir. 1996;138:1449–1455. doi: 10.1007/BF01411125. [DOI] [PubMed] [Google Scholar]
- 39.Green VL, Atkin SL, Speirs V, et al. Cytokine expression in human anterior pituitary adenomas. Clin Endocrinol. 1996;45:179–185. doi: 10.1046/j.1365-2265.1996.d01-1554.x. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Hongo K, Tada T, et al. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg. 2003;98:359–365. doi: 10.3171/jns.2003.98.2.0359. [DOI] [PubMed] [Google Scholar]
- 41.Buchfelder M, Schlaffer S-M. Intraoperative magnetic resonance imaging during surgery for pituitary adenomas: pros and cons. Endocrine. 2012;42:483–495. doi: 10.1007/s12020-012-9752-6. [DOI] [PubMed] [Google Scholar]
- 42.Lang MJ, Kelly JJ, Sutherland GR. A moveable 3-Tesla intraoperative magnetic resonance imaging system. Neurosurgery. 2011;68:168–179. doi: 10.1227/NEU.0b013e3182045803. [DOI] [PubMed] [Google Scholar]
- 43.Koutourousiou M, Gardner PA, Fernandez-Miranda JC, et al. Endoscopic endonasal surgery for giant pituitary adenomas: advantages and limitations. J Neurosurg. 2013;118:621–631. doi: 10.3171/2012.11.JNS121190. [DOI] [PubMed] [Google Scholar]
- 44.Losa M, Mortini P, Barzaghi R, et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008;108:525–532. doi: 10.3171/JNS/2008/108/3/0525. [DOI] [PubMed] [Google Scholar]
- 45.Berker M, Hazer DB, Yu¨cel T, et al. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary. 2012;15:288–300. doi: 10.1007/s11102-011-0368-2. [DOI] [PubMed] [Google Scholar]
- 46.Mortini P, Losa M, Barzaghi R, et al. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005;56:1222–1233. doi: 10.1227/01.neu.0000159647.64275.9d. [DOI] [PubMed] [Google Scholar]
- 47.Frank G, Pasquini E, Farneti G, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83:240–248. doi: 10.1159/000095534. [DOI] [PubMed] [Google Scholar]
- 48.Kabil MS, Eby JB, Shahinian HK. Fully endoscopic endonasal vs. transseptal transsphenoidal pituitary surgery. Minim Invasive Neurosurg. 2005;48:348–354. doi: 10.1055/s-2005-915635. [DOI] [PubMed] [Google Scholar]
- 49.Halvorsen H, Ramm-Pettersen J, Josefsen R, et al. Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir (Wien) Epub before print. 2013 doi: 10.1007/s00701-013-1959-7. [DOI] [PubMed] [Google Scholar]
- 50.Jane JJ, Laws E. The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001;193:651–659. doi: 10.1016/s1072-7515(01)01101-2. [DOI] [PubMed] [Google Scholar]
- 51.DeKlotz TR, Chia SH, Lu W, et al. Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope. 2012;122:511–518. doi: 10.1002/lary.22479. [DOI] [PubMed] [Google Scholar]
- 52.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 54.Stummer W. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]