Abstract
Purpose
Invariant natural killer T (iNKT) cells might play an important role in asthma pathogenesis in humans. Our previous study found no difference in the number of blood iNKT cells between asthma patients and controls. However, few studies have examined the function of blood iNKT cells in human asthma.
Methods
Twenty asthma patients and eight controls were included in this study. Blood iNKT cells were identified using double staining with anti-Vα24 and anti-Vβ11 monoclonal antibodies (mAbs) or with 6B11 and anti-Vβ11 mAbs. Intracellular IL-4, IL-10, and IFN-γ cytokines were stained in blood iNKT cells using their respective mAbs and isotypes. In addition, their relationships with clinical parameters were analyzed.
Results
The number of Vα24+Vβ11+ iNKT cells or 6B11+Vβ11+ iNKT cells did not differ between asthma patients and controls. However, among Vα24+Vβ11+iNKT cells, the proportion of IL-4+iNKT cells was increased in asthma patients compared to controls (7.0±3.0% vs 0.5%±0.4%, P<0.05). There were no differences in the proportions of IL-10+or IFN-γ+iNKT cells between the groups. The proportion of IL-4+ cells among 6B11+Vβ11+iNKT cells inversely correlated with FEV1, expressed as a percentage predicted value in asthma patients (Rs=-0.64, P<0.05, n=19).
Conclusions
Blood iNKT cells are thought to be Th2-like, and IL-4-producing iNKT cells may be associated with lung function in human asthma.
Keywords: Asthma, Natural killer T cell, Th2 cytokines
INTRODUCTION
Asthma is characterized by chronic airway inflammation, airway hyperresponsiveness (AHR), and reversible airflow obstruction.1 It has been reported that natural killer T (NKT) cells might play an important role in asthma pathogenesis.2 NKT cells are unique CD1d-restricted T cells with the surface markers of NK cells, and they act as a bridge between innate and acquired immune responses. NKT cells secrete large amounts of Th1 or Th2 cytokines immediately following activation, which may affect dendritic cells, NK cells, B cells, and conventional CD4+/CD8+ T cells.3 Invariant NKT (iNKT) cells express very limited numbers of T-cell receptors (TCR): Vα24-Jα18 for α chain and Vβ11 for β chain in humans. They respond to α-galactosylceramide (α-GalCer) and synthetic glycolipids from the marine sponge. iNKT cells have a Vα24+Vβ11+ double positive population. However, iNKT cells can be identified with different markers. In the TCR α chain, the Vα24 segment is joined with Jα18 in a germ-line configuration, resulting in an invariant complementarity-determining-region (CDR) 3 loop. 6B11 is a monoclonal antibody directed against the CDR3 loop. Thus, the 6B11+Vβ11+ double-positive population can also be iNKT cells. Recently, iNKT cells have been reported to play essential roles in the development of ovalbumin-induced,4 α-GalCer-induced,5 or ozone-induced6 AHR in a mouse model of asthma. Non-invariant NKT cells may also function in the development of allergen-induced AHR.7
It has been reported that iNKT cells are increased in the airways of patients with asthma8; however, other studies have not observed increased iNKT cells in asthma patient airways.9 Recently, the number of iNKT cells in induced sputum from patients with asthma and eosinophilic bronchitis have been analyzed. iNKT cells were found to be associated with the development of eosinophilic airway inflammation,10 suggesting the contribution of iNKT cells to eosinophilic airway inflammation in human asthma. Additionally, it has been reported that the number of peripheral blood iNKT cells decreases during asthma exacerbation and is inversely associated with eosinophilic airway inflammation,11 suggesting that blood iNKT cells might be mobilized to the airways and lungs during human asthma exacerbation. Two studies10,11 suggest that iNKT cells in blood and airways might behave like Th2-like iNKT cells in patients with asthma. In contrast, another study determined the number of iNKT cells in the blood of stable asthma patients and found an inverse association between blood iNKT cells and atopic indexes, such as total serum IgE levels and atopy score on skin prick testing,12 indicating that blood iNKT cells might behave similarly to Th1-like iNKT cells in patients with asthma. Thus, it remains unclear whether blood iNKT cells are Th1-like or Th2-like type in human asthma. Recently, iNKT cells were classified into various subsets: Th1-like, Th2-like, Th17-like, and IL-10-producing iNKT cells.13 Thus, iNKT cells may behave differently in various allergic and autoimmune diseases.
In the present study, we aimed to determine whether blood iNKT cells function as Th1-like or Th2-like cells in patients with asthma, which may facilitate NKT cell-based immunotherapy for treatment of human asthma in the future.
MATERIALS AND METHODS
Subjects
Twenty asthma patients and eight controls were included in this study. Asthma was defined by the presence of reversible airflow obstruction (increases in FEV1 ≥12% and≥200 mL after bronchodilator test)14 or AHR in patients with typical asthmatic symptoms. AHR was diagnosed if the concentration of methacholine that provoked a 20% decrease in FEV1 (PC20) was less than 25 mg/mL. Patient data, including spirometry, bronchodilator tests, methacholine bronchial provocation tests, skin prick tests, total serum IgE levels, blood eosinophils, and medications, were obtained from medical records. The level of asthma control and asthma treatment steps were classified according to the Global Initiative for Asthma Guidelines.15 Normal controls had no symptoms or history of allergic diseases, including asthma, rhinoconjunctivitis, and atopic dermatitis. All subjects agreed to participate in the study and provided written consent. The Ethical Review Board of Chonnam National University Hospital approved this study.
Spirometry
Spirometry was performed using a spirometer (Spiro Analyzer ST-250, Fukuda Sangyo, Tokyo, Japan). Three reasonably reproducible efforts, with less than 5% difference between the two best, were required. FEV1 was measured, and values for the best effort were used in each case.
Skin prick tests and atopy
A standardized skin prick test was performed using 12 common aeroallergens (Dermatophagoides pteronyssinus, D. farinae, cat, dog, Alternaria tenuis, trichophyton, cockroach, alder, birch, orchard, mugwort, and ragweed; Allergopharma, Reinbek, Germany) as previously described.12 A subject was classified as atopic if any allergen caused 3 mm or larger wheal compared to the negative controls.
Flow cytometric analyses of iNKT cells and intracellular cytokines
Heparinized blood was layered over an equal volume of Lymphoprep™ (Axis-Shield PoC AS, Oslo, Norway) solution, which was centrifuged at 2,000 rpm for 30 minutes. Peripheral blood mononuclear cells (PBMCs) were isolated from the interface and used to determine the number of iNKT cells, as described previously.12
PBMCs were incubated at 4℃ for 30 minutes with fluorochrome-conjugated monoclonal antibodies, including PE-6B11 (BD Biosciences, San Jose, CA, USA) or PE-anti-TCRvα24 (Beckman Coulter, Marseille, France) and APC-anti-TCRvβ11 (BD Biosciences). PE-mouse IgG1 (BD Biosciences) and APC-mouse IgG1 (BD Biosciences) were used for isotype control antibodies. For intracellular staining of iNKT cells, fixation and permeabilization were performed using Cytofix/Cytoperm kits (eBiosciences) according to the manufacturer's instructions. These cells were incubated with FITC-IL-4 mAb (BD Biosciences), FITC-IFN-γ mAb (BD Biosciences), or FITC-IL-10 mAb (BD Biosciences). IgG1 isotype control (BD Biosciences) was used. After gating the lymphocytes, the frequency of Vα24+Vβ11+ or 6B11+Vβ11+ iNKT cells was expressed as a percentage of lymphocytes. Intracellular IL-4+, IFN-γ+ or IL-10+ cells were presented as a percentage of Vα24+Vβ11+ or 6B11+Vβ11+ iNKT cells (Fig. 1).
Fig. 1.
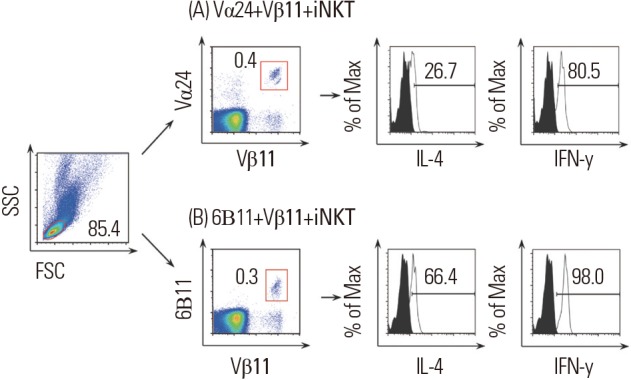
Representative flow cytometry analyses of intracellular IL-4 and IFN-γ cytokines in Vα24+Vβ11+ iNKT cells (A) or 6B11+Vβ11+ iNKT cells (B).
Statistical analyses
Data are expressed as the means±standard error of the mean. To examine differences between the 2 groups, Student's t-tests for continuous variables were used. Pearson's chi-square tests were used to determine the associations between categorical variables. Correlations between parameters were analyzed using Spearman's rank-correlation coefficient (Rs) (SPSS 17.0 for Windows; SPSS Inc., Chicago, IL, USA). Values of P less than 0.05 were considered to indicate statistical significance.
RESULTS
Characteristics of the subjects
Asthma patients were diagnosed by positive methacholine bronchial provocation tests in 10 (50.0%) subjects and by significant reversible airway obstruction in 10 (50.0%) subjects. Asthma patients were older than the controls, but there were no differences in gender, body mass index, smoking habits, and the presence of atopy. Most asthma patients had accompanying rhinitis symptoms (Table 1). Mean values of FEV1, total serum IgE, and blood eosinophils in subjects are shown in Table 1. Asthma was controlled in 18 (90.0%) patients and uncontrolled in 2 (10.0%) patients. Asthma medications were step 1 in 2 (10.0%) patients, step 3 in 1 (5.0%) patient, step 4 in 15 (75.0%) patients, and step 5 in 2 (10.0%) patients. Eighteen patients have been taking regular anti-asthma medications, including inhaled corticosteroids, long-acting β2 agonists, leukotriene receptor antagonists, and/or sustained-released theophylline. The average time taking the medications was 22.7±4.5 months.
Table 1.
Characteristics of the subjects
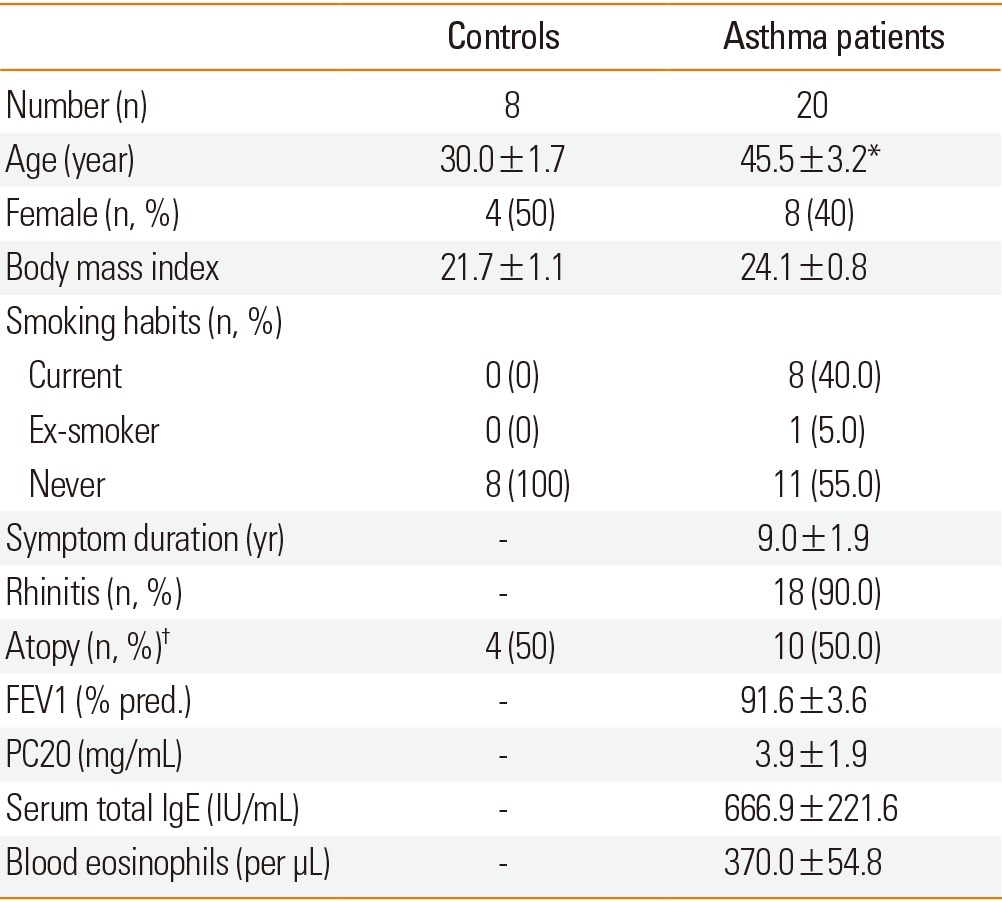
*P<0.001 compared to the control. FEV1: forced expiratory volume in one second, PC20: concentration of methacholine that provoked a 20% decrease in FEV1. †Subjects was classified as atopic if any allergen caused a wheal with a diameter of 3 mm or larger than that yielded by saline.
Intracellular cytokine production from iNKT cells
The number of Vα24+Vβ11+ iNKT cells (0.2±0.1% vs 0.2±0.1%, P>0.05) and 6B11+Vβ11+ iNKT cells [0.2±0.1% (n=19) vs 0.07±0.01% (n=7), P>0.05] did not differ between asthma patients and controls, as reported previously.12
Intracellular cytokines were determined in Vα24+Vβ11+ iNKT cells. The frequency of IL-4+ cells was higher in the asthma group compared to controls (Fig. 2). However, the frequency of IFN-γ+cells or IL-10+cells did not differ between the 2 groups (Table 2).
Fig. 2.
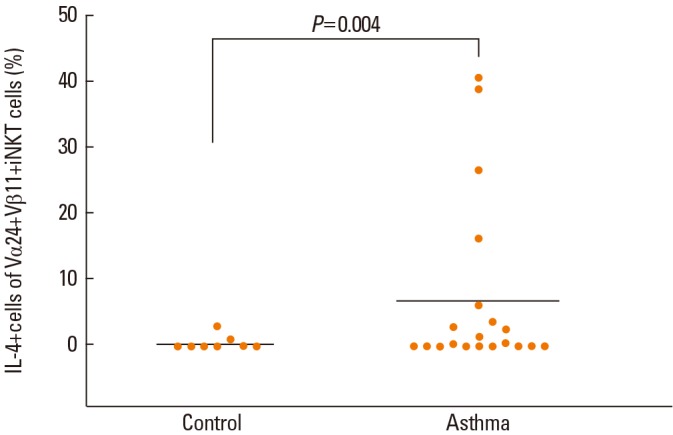
Comparison of the proportion of IL4+ cells in Vα24+Vβ11+ iNKT cells between asthma subjects (n=20) and controls (n=8).
Table 2.
Intracellular IL-4, IFN-γ, and IL-10 production by blood iNKT cells
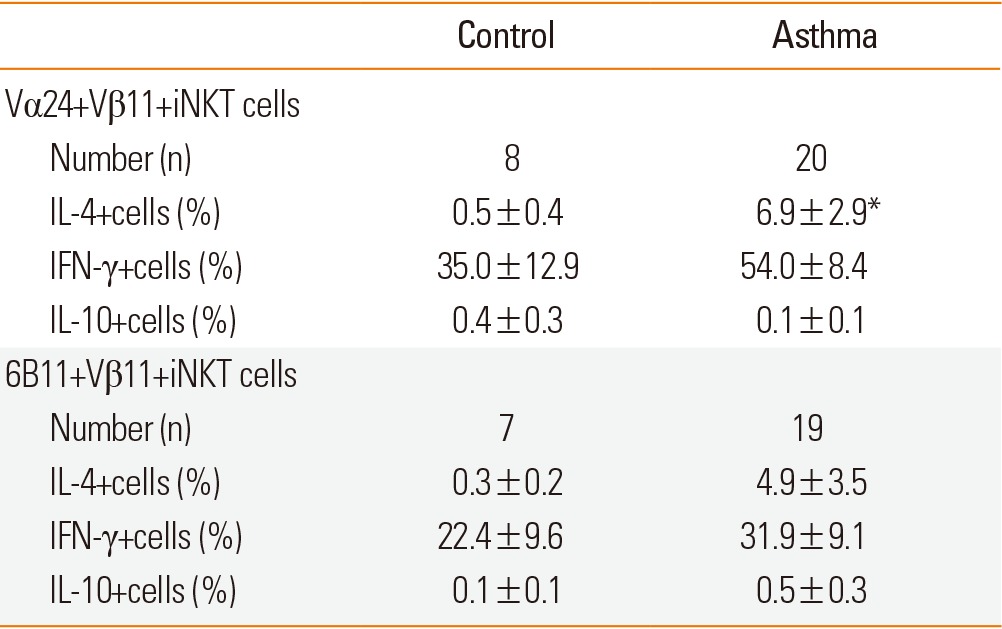
*P<0.05 compared to the control. The proportion of cytokine-stained cells was analyzed after gating on Vα24+Vβ11+ iNKT cells or 6B11+Vβ11+ iNKT cells, as shown in Fig. 1.
We also analyzed intracellular cytokine production from 6B11+Vβ11+iNKT cells. IL-4 production from iNKT cells was increased in asthma patients compared to controls; however, the difference was not significant. IFN-γ or IL-10 production did not differ between asthma patients and controls (Table 2).
The relationship between IL4+ iNKT cells and clinical parameters
Because IL-4 production from blood iNKT cells was increased in patients with asthma, we determined whether IL-4+ iNKT cells were related to the clinical parameters. The proportion of total IL-4+ cells from 6B11+Vβ11+ iNKT cells was inversely correlated with FEV1 expressed as a percentage predicted value in asthma patients (Rs=-0.64, P<0.05, Fig. 3). The proportion of IL-4+cells from Vα24+Vβ11+ iNKT cells was higher in asthma patients on step 4 and 5 medications (8.0±3.4%, n=17) compared to those on step 1 and 3 medications (1.1±0.9%, n=3); however, the difference was not significant (P=0.406).
Fig. 3.
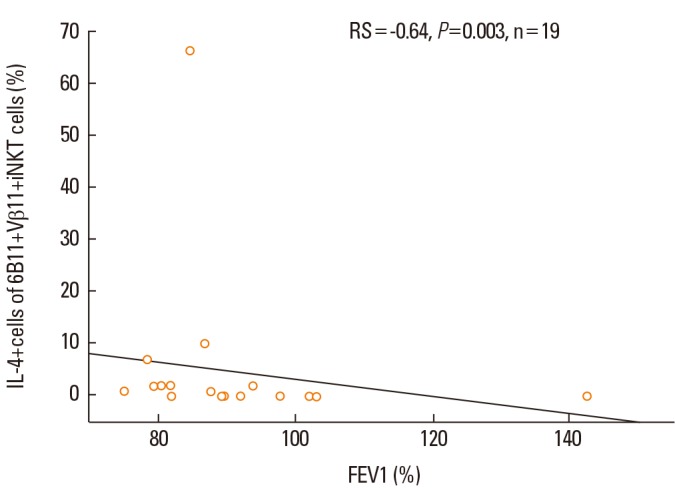
Relationship between the proportion of IL-4+ cells in 6B11+Vβ11+ iNKT cells and FEV1, expressed as percentage predicted values, in asthma patients.
DISCUSSION
Although the number of iNKT cells was similar, the present study showed that blood iNKT cells produced more IL-4 in asthma patients compared to normal controls. Additionally, the number of IL-4-producing blood iNKT cells was inversely related to lung function in subjects with asthma. Our findings suggest that blood iNKT cells may belong to the Th2-like subset and thereby contribute to lung function in human asthma.
It has been reported that the number of blood iNKT cells is inversely related to atopic indices in asthmatics.12 Based on the present findings, blood Th2-like iNKT cells may migrate to the lungs and airways in asthma patients with more severe airway atopic or allergic inflammations. Thus, the number may be decreased in patients with higher atopic indexes. Additionally, blood iNKT cells have been shown to be reduced during acute asthma exacerbations compared with convalescence.11 This phenomenon could be explained by blood Th2-like iNKT cells moving to the lungs as the acute exacerbation episode proceeds. Blood iNKT cells were restored to normal ranges during convalescence. Blood iNKT cells have been reported to be reduced in number in asthma patients with sputum eosinophilia.11 Thus, in patients with asthma, all blood and lung iNKT cells may be of the Th2-like subset, which may contribute to eosinophilic airway inflammation. In another study, sputum iNKT cells were reported to be associated with eosinophilic airway inflammation.10 iNKT cells were found to be increased in lung tissue after allergen challenges in patients with asthma.16 IL-4 production from α-GalCer-stimulated blood iNKT cells was increased in asthma patients compared to controls.17 In children with asthma, increased IL-4 secretion from iNKT cells has been observed during acute exacerbations compared to those with stable asthma or normal controls.18 These human studies also suggest that blood iNKT cells in asthmatics may be of the Th2-like subset, which is consistent with our findings. Th2-like iNKT cells express IL-17RB and have been reported to contribute to AHR development in an IL-25-dependent manner in the lungs.19
Regarding IFN-γ from blood iNKT cells, we did not find any significant differences between asthma patients and controls, which is consistent with the findings by Yan-Ming et al.17 Carpio-Pedroza et al. reported less IFN-γ production from iNKT cells during acute exacerbations of asthmatics.18 In asthma, IFN-γ production from blood iNKT cells may be normal or reduced.17,18
In the present study, the frequency of IL-10-producing iNKT cells was not altered in subjects with asthma. However, Yan-Ming et al. reported that IL-10 production from iNKT cells was reduced in asthma patients.17 Bosma et al. determined the frequency of blood IL-10-producing iNKT cells from patients with systemic lupus erythematosus and found the frequency was increased compared to controls.20 Although the role of IL-10-producing iNKT cells in asthma is not known, they might play a regulatory role in asthma pathogenesis, similar to regulatory T cells in asthma and allergic diseases. iNKT cells may include Foxp3-expressing subsets.13 However, further studies are needed.
Th2-like iNKT cells, as well as conventional Th2 cells, are assumed to play an important role in asthma pathogenesis. It is possible that Th2-like iNKT cells contribute to the severity of asthma. The present study showed that Th2-like iNKT cells were related to lung function. Additionally, Th2-like iNKT cells tended to be increased in patients taking more anti-asthma medications. Because more medications might correlate with more severe asthma, Th2-like iNKT cells might be increased in patients with severe asthma.
Based on the previous studies,8,10,12 it is unlikely that anti-asthma medications reduce IL-4 production by iNKT cells. In contrast, blood Th2-like iNKT cells were increased in patients taking more asthma medications. Recently, asthma treatment have focused on conventional Th2 cells rather than iNKT cells. However, a new therapeutic agent that modulates iNKT cells could be effective for the control of severe asthma, particularly asthma refractory to inhaled corticosteroids and leukotriene receptor antagonists. It has been reported that allergen-specific immunotherapy restores IL-4 and IL-10 production from iNKT cells to normal ranges in house-dust-mite-sensitized asthma patients.17
iNKT cells can be defined by staining as either the Vα24+Vβ11+ or 6B11+Vβ11+ population. However, it is possible that there are minor differences between Vα24+Vβ11+ iNKT cells and 6B11+ Vβ11+ iNKT cells. Vα24+ cells may include non-invariant and non-CD1d-restricted Vα24+ T cells. Thus, Vα24+ staining could lead to overestimation of iNKT cell numbers compared to 6B11+ staining. Va24+Vβ11+ staining may be more sensitive and less specific than 6B11+Vβ11+ staining for identifying iNKT cells. Further studies are needed to elucidate which form of iNKT cells plays a more important role in asthma pathogenesis.
Collectively, our data showed that blood iNKT cells are likely Th2-like and may be associated with lung function in human asthma.
ACKNOWLEDGMENTS
This study was supported by a grant (CRI12002-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Umetsu DT, DeKruyff RH. A role for natural killer T cells in asthma. Nat Rev Immunol. 2006;6:953–958. doi: 10.1038/nri1968. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 4.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 5.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh YI, Kim HY, Meyer EH, Pichavant M, Akbari O, Yasumi T, Savage PB, Dekruyff RH, Umetsu DT. Activation of nonclassical CD1d-restricted NK T cells induces airway hyperreactivity in beta 2-microglobulin-deficient mice. J Immunol. 2008;181:4560–4569. doi: 10.4049/jimmunol.181.7.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlström J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 9.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedmann PS, Djukanovic R. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 10.Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010;153:239–248. doi: 10.1159/000314364. [DOI] [PubMed] [Google Scholar]
- 11.Koh YI, Shim JU, Wi J, Kwon YE. The role of natural killer T cells in the pathogenesis of acute exacerbation of human asthma. Int Arch Allergy Immunol. 2012;158:131–141. doi: 10.1159/000330908. [DOI] [PubMed] [Google Scholar]
- 12.Koh YI, Shim JU, Wi JO, Han ER, Jin NC, Oh SH, Park CK, Park DJ. Inverse association of peripheral blood CD4(+) invariant natural killer T cells with atopy in human asthma. Hum Immunol. 2010;71:186–191. doi: 10.1016/j.humimm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 15.Global Initiative For Asthma. Global strategy for asthma management and prevention. Bethesda: national Institutes of Health/National Heart, Lung, and Blood Institute, Global Initiative For Asthma; 2006. [Google Scholar]
- 16.Reynolds C, Barkans J, Clark P, Kariyawasam H, Altmann D, Kay B, Boyton R. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J Allergy Clin Immunol. 2009;124:860–862. doi: 10.1016/j.jaci.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Yan-ming L, Lan-fang C, Chen L, Ya-qin L, Wei C, Wen-ming Z. The effect of specific immunotherapy on natural killer T cells in peripheral blood of house dust mite-sensitized children with asthma. Clin Dev Immunol. 2012;2012:148262. doi: 10.1155/2012/148262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpio-Pedroza JC, Vaughan G, del Rio-Navarro BE, del Río-Chivardí JM, Vergara-Castañeda A, Jiménez-Zamudio LA, Morales-Flores A, Rodríguez-Moreno G, Ruiz-Tovar K, Fonseca-Coronado S, Gonçalves Rossi LM, Escobar-Gutiérrez A. Participation of CD161(+) and invariant natural killer T cells in pediatric asthma exacerbations. Allergy Asthma Proc. 2013;34:84–92. doi: 10.2500/aap.2013.34.3619. [DOI] [PubMed] [Google Scholar]
- 19.Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, Taniguchi M. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C. Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36:477–490. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


