Abstract
Objective:
Adolescent marijuana use continues to increase in prevalence as harm perception declines. Better understanding of marijuana’s impact on neurodevelopment is crucial. This prospective study aimed to investigate cortical thickness and neurocognitive performance before and after 28 days of monitored abstinence in adolescent marijuana and alcohol users.
Method:
Subjects (N = 54; >70% male) were adolescent marijuana users (ages 15–18 years) with regular alcohol use (MJ + ALC; n = 24) and non-using controls (CON; n = 30) who were compared before and after 4 weeks of sequential urine toxicology to confirm abstinence. Participants underwent magnetic resonance imaging, neuropsychological assessment, and substance use assessment at both time points. Repeated-measures analysis of covariance was used to look at the main effects of group, time, and Group × Time interactions on cortical thickness and neurocognitive functioning. Bivariate correlations estimated associations between cortical thickness, substance use severity, and cognitive performance.
Results:
Marijuana users showed thicker cortices than controls in the left entorhinal cortex (ps < .03) before and after monitored abstinence, after adjusting for lifetime alcohol use. More lifetime marijuana use was linked to thinner cortices in temporal and frontal regions, whereas more lifetime alcohol use and heavy episodic drinking episodes was linked to thicker cortices in all four lobes (ps < .05). Age of onset of regular marijuana use was positively related to cortical thickness (ps < .03).
Conclusions:
Adolescent alcohol and marijuana use may be linked to altered longer-term neurodevelopmental trajectories and compromised neural health. Cortical thickness alterations and dose-dependent associations with thickness estimates were observed both before and after monitored abstinence and suggest neural differences continue to persist 28 days after cessation of marijuana use. Neural recovery may be identified with longer follow-up periods; however, observed changes related to use severity could have implications for future psychosocial outcomes.
Adolescence is an important developmental period associated with significant increases in alcohol and marijuana use (Brown et al., 2008). Sixty-eight percent of American 12th graders endorse lifetime alcohol use, with more than 20% reporting recent heavy episodic drinking (i.e., ≥5 drinks on one occasion) (Johnston et al., 2014). Marijuana is commonly used in conjunction with alcohol (Terry-McElrath et al., 2013), as 45% endorse lifetime use of marijuana by 12th grade (Johnston et al., 2014).
High rates of adolescent alcohol and marijuana use are concerning, given the significant neurodevelopment in gray and white matter during this period. Gray matter, consisting of neurons and glial cells, increases in volume during childhood and decreases during early adolescence. Gray matter reduction (including extensive cortical thinning) may represent the pruning of excess synapses, white matter encroachment, and/or changes in the extracellular matrix (Blakemore, 2012; Paus, 2005; Stiles and Jernigan, 2010). White matter continues to increase linearly throughout adolescence, likely driven by progressive axonal myelination (Gerber et al., 2009; Giedd, 2004; Gogtay et al., 2004; Simmonds et al., 2014). Both processes are associated with more efficient cognitive development, and any neurotoxic insults during these crucial processes could have long-lasting implications for cognitive development (Jacobus and Tapert, 2013, 2014).
Cross-sectional neuropsychological findings show that heavy marijuana and alcohol users perform worse on tests of psychomotor speed, complex attention, story memory, and planning and sequencing abilities, even after a month of abstinence (Medina et al., 2007), and show deficits on tests of verbal and visual memory (Solowij et al., 2011; Thoma et al., 2011). Longitudinal examinations have found that subtle deficits in cognitive functioning may remit with abstinence to some degree; however, this may not be the case for all aspects of cognitive functioning (Hanson et al., 2010). Early initiation of marijuana use in particular may have long-lasting consequences (Meier et al., 2012) in sustained attention, executive functioning (Fontes et al., 2011), impulse control (Gonzalez et al., 2012; Gruber et al., 2011, 2012a, 2012b), and verbal memory (Dougherty et al., 2013; Solowij et al., 2011).
Underlying brain structural changes, particularly cortical thinning, may help explain the cognitive abnormalities found in alcohol- and marijuana-using teens. Cross-sectional structural magnetic resonance imaging (MRI) studies have found thinner cortices in prefrontal and insular regions and thicker cortices in posterior regions in marijuana-using adolescents when compared to controls (Lopez-Larson et al., 2011). It remains unclear if structural differences exist before marijuana use. Although structural neuroimaging studies show marijuana-related effects on tissue (Jacobus et al., 2013b), smaller orbitofrontal volumes at age 12 predict marijuana initiation by age 16, suggesting cross-sectional findings may be partially attributable to premorbid structural brain differences (Cheetham et al., 2012). Perhaps an interaction of vulnerability and exposure leads to continued use and poorer neural and cognitive outcomes. Several functional neuroimaging studies have shown aberrations in brain response patterns and cerebral blood flow in marijuana- and alcohol-using adolescents, which may partially be explained by aberrant cortical thinning (Jacobus et al., 2012; Jager et al., 2010; Lopez-Larson et al., 2012; Schweinsburg et al., 2005, 2008, 2010; Tapert et al., 2007). Abstinence may aid in tissue recovery to some extent and diminish the observed marijuana-related differences in neural functioning (Jacobus et al., 2012). However, studies suggest deficits persist even after a month (Padula et al., 2007; Tapert et al., 2007).
The aims of this study were to examine the impact of heavy marijuana and alcohol use on cortical thickness in adolescents before and after 28 days of monitored abstinence. We hypothesized that heavy marijuana and alcohol users, compared to controls, would demonstrate thicker cortices across independent standardized neuroanatomical cortical regions (Desikan et al., 2006) at both baseline (pre-abstinence) and follow-up (post-abstinence) because of altered neurodevelopmental trajectories (e.g., interference with regressive events such as cortical thinning). We did not expect considerable within-group brain change in cortical thickness after 4 weeks of monitored abstinence in either group. Nevertheless, this has not been previously explored in the literature to our knowledge, and we predicted that, along with a between-group effect, substance users might demonstrate greater improvement in cortical thickness (subtle thinning), along with neurocognitive performance, during 1 month of abstinence from marijuana use.
Method
Participants
Adolescents (N = 54) were recruited from local San Diego schools and included 24 heavy marijuana users who regularly used alcohol (MJ + ALC; lifetime marijuana episodes > 200) and 30 control teens (CON) with minimal substance use histories (lifetime marijuana episodes < 7) (Table 1). Teens were recruited for marijuana use; however, the majority of users also reported heavy lifetime alcohol use (Jacobus et al., 2012). Comprehensive screening interviews were administered to adolescents and parents/guardians; adolescents provided assent for their own participation, and guardians were required to provide consent in accordance with the University of California, San Diego Human Research Protections Program. Exclusionary criteria were history of a Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition, Text Revision (American Psychiatric Association, 2000), Axis I disorder other than alcohol or cannabis use disorder; psychoactive medications; learning disability or mental retardation; neurological condition (e.g., migraine) or traumatic brain injury with loss of consciousness greater than 2 minutes; prenatal alcohol or other drug exposure; premature birth; left handedness; and nonfluency in English.
Table 1.
Participant characteristics at baseline, unless otherwise noted
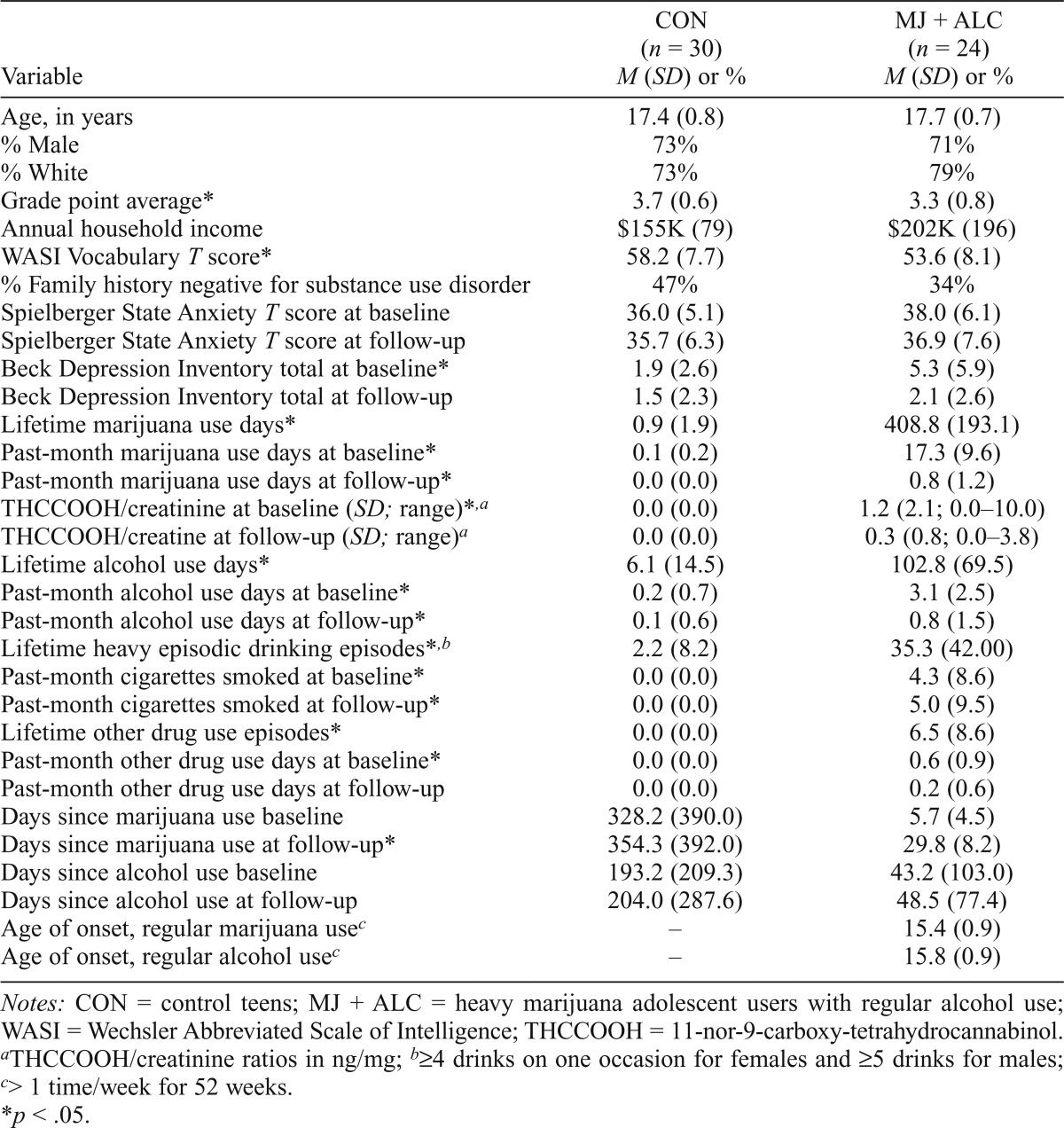
| Variable | CON (n = 30) M (SD) or % | MJ + ALC (n = 24) M (SD) or % |
| Age, in years | 17.4 (0.8) | 17.7 (0.7) |
| % Male | 73% | 71% |
| % White | 73% | 79% |
| Grade point average* | 3.7 (0.6) | 3.3 (0.8) |
| Annual household income | $155K (79) | $202K (196) |
| WASI Vocabulary T score* | 58.2 (7.7) | 53.6 (8.1) |
| % Family history negative for substance use disorder | 47% | 34% |
| Spielberger State Anxiety T score at baseline | 36.0 (5.1) | 38.0 (6.1) |
| Spielberger State Anxiety T score at follow-up | 35.7 (6.3) | 36.9 (7.6) |
| Beck Depression Inventory total at baseline* | 1.9 (2.6) | 5.3 (5.9) |
| Beck Depression Inventory total at follow-up | 1.5 (2.3) | 2.1 (2.6) |
| Lifetime marijuana use days* | 0.9 (1.9) | 408.8 (193.1) |
| Past-month marijuana use days at baseline* | 0.1 (0.2) | 17.3 (9.6) |
| Past-month marijuana use days at follow-up* | 0.0 (0.0) | 0.8 (1.2) |
| THCCOOH/creatinine at baseline (SD; range)*,a | 0.0 (0.0) | 1.2 (2.1; 0.0–10.0) |
| THCCOOH/creatine at follow-up (SD; range)a | 0.0 (0.0) | 0.3 (0.8; 0.0–3.8) |
| Lifetime alcohol use days* | 6.1 (14.5) | 102.8 (69.5) |
| Past-month alcohol use days at baseline* | 0.2 (0.7) | 3.1 (2.5) |
| Past-month alcohol use days at follow-up* | 0.1 (0.6) | 0.8 (1.5) |
| Lifetime heavy episodic drinking episodes*,b | 2.2 (8.2) | 35.3 (42.00) |
| Past-month cigarettes smoked at baseline* | 0.0 (0.0) | 4.3 (8.6) |
| Past-month cigarettes smoked at follow-up* | 0.0 (0.0) | 5.0 (9.5) |
| Lifetime other drug use episodes* | 0.0 (0.0) | 6.5 (8.6) |
| Past-month other drug use days at baseline* | 0.0 (0.0) | 0.6 (0.9) |
| Past-month other drug use days at follow-up | 0.0 (0.0) | 0.2 (0.6) |
| Days since marijuana use baseline | 328.2 (390.0) | 5.7 (4.5) |
| Days since marijuana use at follow-up* | 354.3 (392.0) | 29.8 (8.2) |
| Days since alcohol use baseline | 193.2 (209.3) | 43.2 (103.0) |
| Days since alcohol use at follow-up | 204.0 (287.6) | 48.5 (77.4) |
| Age of onset, regular marijuana usec | – | 15.4 (0.9) |
| Age of onset, regular alcohol usec | – | 15.8 (0.9) |
Notes: CON = control teens; MJ + ALC = heavy marijuana adolescent users with regular alcohol use; WASI = Wechsler Abbreviated Scale of Intelligence; THCCOOH = 11 -nor-9-carboxy-tetrahydrocannabinol.
THCCOOH/creatinine ratios in ng/mg;
≥4 drinks on one occasion for females and ≥5 drinks for males;
> 1 time/week for 52 weeks.
p < .05.
Participants received neuroimaging and neuropsychological, substance use, and mental health assessment at baseline and follow-up (see Jacobus et al., 2012, for procedure timeline). Participants were asked to refrain from using all intoxicants for 28 days and underwent biweekly urine toxicology screening (nine total) to examine 11-nor-9-carboxy-tetrahydrocannabinol (THCCOOH)/creatinine excretion ratios for confirmation of completing the marijuana abstinence protocol (Smith et al., 2009). New cannabis use was determined by dividing each THCCOOH normalized to creatinine concentration collected by the previously collected specimen (urine 2 / urine 1) and factoring in time intervals between collections, per Huestis and Cone recommendations for determining new cannabis use as a function of time (Huestis and Cone, 1998; Smith et al., 2009). A change in metabolite/creatinine ratios greater than 1.5 is considered new use. Eight of our 24 users reported ≤4 days of light use within the first week of the abstinence period; however, we included them as abstinent given their decreasing THCCOOH/creatinine ratios (see Table 1 for values). Breath alcohol analysis was given at each in-person appointment (nine times over 28 days) to help affirm abstinence from alcohol; we did not have any positive breath-alcohol-analysis tests. For those individuals successfully completing the protocol and demonstrating decreasing THCCOOH ratios, self-reported alcohol use over the 28 days was ≤5.
Measures
Neuropsychological battery.
A comprehensive battery was administered at both baseline and follow-up covering the well-established domains of complex attention, processing speed, learning and memory, spatial processing, and executive functioning (Lezak et al., 2004). Twenty-five variables were examined from 10 tests: (a) complex attention: California Verbal Learning Test-II Trial 1 and Trial 1–5 Total Recall (CVLT-II; Delis et al., 2001); Wechsler Adult Intelligence Scale–Third Edition (WAIS-III; Wechsler, 1997b) Arithmetic, and Digit Span Total, Forward and Backward subtests; (b) processing speed: WAIS-III Digit Symbol; Delis–Kaplan Executive Function System (D-KEFS) Trail Making Test, Visual Scanning, Number Sequencing, Letter Sequencing, and Motor Speed subtests (Delis and Kaplan, 2000); (c) verbal memory: Wechsler Memory Scale–Third Edition (WMS-III; Wechsler, 1997a) Logical Memory I, II, and Recognition; CVLT-II Short and Long Delay Free and Cued Recall, CVLT-II Recognition (Delis et al., 2001); (d) visuospatial functioning: Rey–Osterrieth Complex Figure (Rey-O, baseline) and Taylor Complex Figure (Taylor, follow-up) Copy and Delay Accuracy (Corwin and Bylsma, 1993; Hubley and Tremblay, 2002); Wechsler Abbreviated Scale of Intelligence (WASI) Block Design subtest (Wechsler, 1999); and (e) executive functioning: D-KEFS Trail Making Test Number-Letter Switching, Verbal Letter Fluency, and Color-Word Interference subtests (Inhibition and Inhibition-Switching; Delis and Kaplan, 2000). Scaled scores corrected for age were used for all tests, excluding the Rey-O and Taylor Complex Figures, Logical Memory Recognition subtest, and the Digit Span Forward and Backward subtests.
Composite categories were developed for each of the five domains to reduce the number of dependent variables for correlations with cortical thickness. Individual z scores were created for each subject, neuropsychological variable, and time point based on the whole sample of adolescents and corresponding standard deviations (N = 54). Next, z scores within each domain were averaged to create the final composite z score for each of the five domains at each time point (Jacobus et al., 2013a; Medina et al., 2007). A global composite score was created for baseline and follow-up by averaging all five composite domains. Cronbach’s α coefficients were used to assess internal consistency (α > .50).
Substance use and mental health assessment.
The Customary Drinking and Drug Use Record was used to assess quantity and frequency of lifetime alcohol, marijuana, cigarette, and other drug use (Brown et al., 1998). The Timeline Followback was used to assess self-reported substance use (e.g., alcohol, marijuana) in the 28 days before each scan session (Sobell and Sobell, 1992).
Emotional functioning and demographics.
The Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001; Shaffer et al., 1996) was administered to youth and parents at the screening interview to identify and exclude those individuals with Axis I disorders other than alcohol or cannabis use disorder. The Beck Depression Inventory (Beck, 1978) and Spielberger State Trait Anxiety Inventory (Spielberger et al., 1970) assessed depression and state anxiety. The Family History Assessment Module (Rice et al., 1995) assessed family history of psychiatric and substance use disorders. Parental income and grade point average were collected during a clinical interview before the baseline imaging session. The WASI Vocabulary subtest was included as an estimate of premorbid intellectual functioning (Wechsler, 1999).
Procedures
Cortical thickness acquisition and processing.
All scans were acquired on a 3.0 Tesla CXK4 short bore Excite-2 magnetic resonance system (General Electric, Milwaukee, WI) with an eight-channel phase array head coil at the University of California San Diego Center for Functional MRI. Subjects were asked to remain awake and still in the scanner while a high-resolution T1-weighted anatomical spoiled gradient recall scan was acquired (echo time / repetition time = minimum full; field of view = 24 cm; resolution = 1 mm3, 170 continuous slices).
The neuroimaging software FreeSurfer, which is well documented and freely available (Version 5.1, surfer.nmr.mgh.harvard.edu), was used for cortical surface reconstruction and thickness estimates (Dale et al., 1999; Fischl et al., 1999). The initial cross-sectional processing involves motion correction and averaging of T1 weighted images, removal of nonbrain tissue and transformation to standardized space, segmentation of subcortical white and deep gray matter structures, intensity normalization, and tessellation of the gray/white matter boundary. Local MRI intensity gradients then guide a surface deformation algorithm to place smooth borders where the greatest shift in intensity defines transition to other tissue classes (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 1999, 2004); this procedure allows for quantification of submillimeter group differences (Fischl and Dale, 2000).
Cortical thickness was calculated as the closest distance from the gray/white matter boundary to the gray matter/cerebral spinal fluid boundary at each vertex on the cortical surface (Fischl and Dale, 2000). Validity of the cortical thickness measurement procedures has been verified using manual measurements and histological analysis (Kuperberg et al., 2003; Rosas et al., 2002; Salat et al., 2004). Test–retest reliability across scanners and field strengths has been shown using these standardized procedures (Han et al., 2006; Reuter et al., 2012).
Following cross-sectional processing of all time points, data were next fed through the longitudinal processing stream in FreeSurfer (Reuter et al., 2012). This approach extracts reliable volume and thickness estimates by creating an unbiased within-subject template space and image from the two cross-sectionally processed time points (baseline and follow-up) using a consistent robust inverse registration method (Reuter et al., 2010). Processing steps such as Talairach transforms, atlas registration, and spherical surface maps and parcellations are initialized with common information from the within-subject template, increasing reliability and statistical power (Reuter et al., 2012).
One rater (JJ), blind to participant characteristics, followed the reconstruction and longitudinal edit procedures to identify and correct any errors made during the cortical reconstruction process. This involved verification of the automated skull stripping and a coronal plane slice-by-slice inspection of the gray/white and gray/cerebral spinal fluid surfaces. Modifications to the surfaces were made as necessary to correct for tissue misclassifications (e.g., residual dura matter classified as cortex). No editing was necessary following the longitudinal processing, although all longitudinal runs were checked for quality.
Following inspection, an automated parcellation procedure divided each hemisphere into 34 independent cortical regions based on gyral and sulcal features (Desikan et al., 2006; Fischl et al., 2004). Cortical thickness estimates averaged over each parcellation region were extracted for statistical analyses in SPSS.
Data analysis
Demographic comparisons.
Analysis of variance (ANOVA) and chi-square tests were run between groups to evaluate differences on demographic variables and to identify appropriate covariates for subsequent analysis.
Cortical thickness measurement.
Repeated-measures analysis of covariance (ANCOVA) examined main effects of time, group, and Group × Time interactions on cortical thickness values for 34 independent standard neuroanatomical cortical regions (Desikan et al., 2006) in each hemisphere. Intracranial volume was included as a covariate. Marijuana and alcohol are commonly used concomitantly (Johnston et al., 2014; Midanik et al., 2007; Substance Abuse and Mental Health Services Administration, 2013; Terry-McElrath et al., 2013). In fact, more than 80% of marijuana users in our studies report regular alcohol use. Given the literature on the deleterious effects of alcohol on neurodevelopment (Jacobus and Tapert, 2013) and between group differences known to exist in this sample, lifetime alcohol use was specified a priori and statistically adjusted for in follow-up post hoc ANCOVAs examining group differences at each time point for regions in which a main effect of group or interaction was identified. Secondary analyses further evaluating the impact of substance use on cortical thickness included exploratory bivariate correlations between substance use variables (i.e., lifetime marijuana use, lifetime alcohol use, heavy episodic drinking episodes [≥4 drinks on one occasion for females and ≥5 drinks for males], and age of initiation) and cortical thickness estimates at each time point in the user group (n = 24).
Neuropsychological performance.
Repeated-measures ANCOVA was used to evaluate neurocognitive performance over time with alcohol as a covariate for each neurocognitive subtest. Bivariate correlations between neurocognitive performance (global domain scores to reduce the number of correlations) and cortical thickness estimates were explored; if significant associations were identified, relationships between cortical thickness in that region and individual domain scores were examined within each group to identify correlates driving the relationship. Associations between age of onset of alcohol and marijuana use and neuropsychological outcome data (global domain scores) were also explored in the user group.
Results
Groups did not statistically differ on any demographic characteristics with the exception of grade point average and WASI Vocabulary T score (ps < .05), despite both groups falling within the average range. MJ + ALC reported slightly more depression-type symptoms compared to controls at baseline; however, neither group fell within the clinically significant range, and this difference was not found at follow-up. Groups differed on lifetime marijuana, alcohol, and lifetime other drug use episodes, as anticipated (ps < .05, see Table 1).
Main effect of group on cortical thickness measurement
A main effect of group status on cortical thickness estimates was found in four regions (controlling for intracranial volume) in which MJ + ALC demonstrated significantly thicker cortices compared to controls: (a) left caudal anterior cingulate cortex, F(1, 51) = 9.6, p < .01; (b) left entorhinal cortex, F(1, 51) = 8.3, p < .01; (c) left lingual gyrus, F(1, 51) = 4.4, p = .04; and (d) left pericalcarine cortex, F(1, 51) = 4.1, p = .04. In no region did controls demonstrate thicker cortices than MJ + ALC.
Post hoc analyses revealed group differences at both baseline and follow-up (MJ + ALC with thicker cortices) in the left entorhinal cortex persisted after both lifetime alcohol use in addition to intracranial volume were controlled for (ps ≤ .03). Between-group differences did not persist in the left caudal anterior cingulate, left lingual gyrus, or left pericalcarine cortex after covarying for alcohol use (ps > .05). Lifetime alcohol use was not significant in the post hoc models (ps > .10)
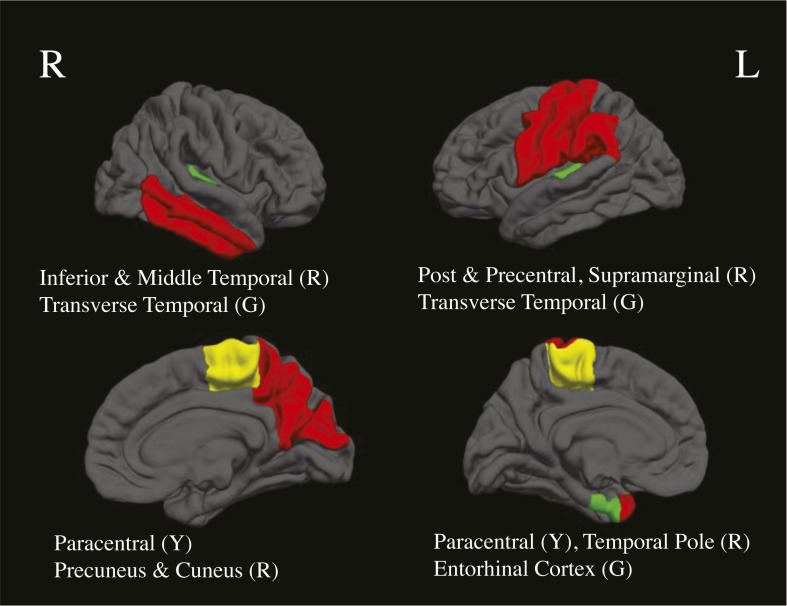
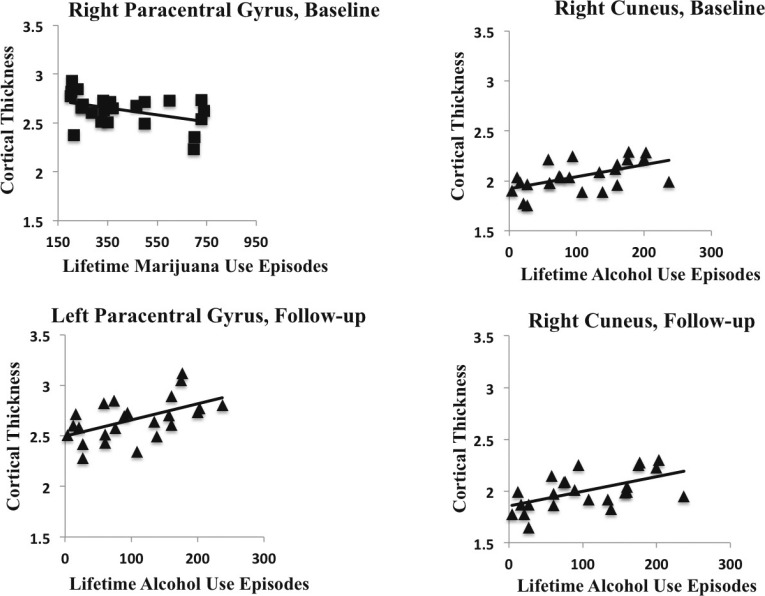
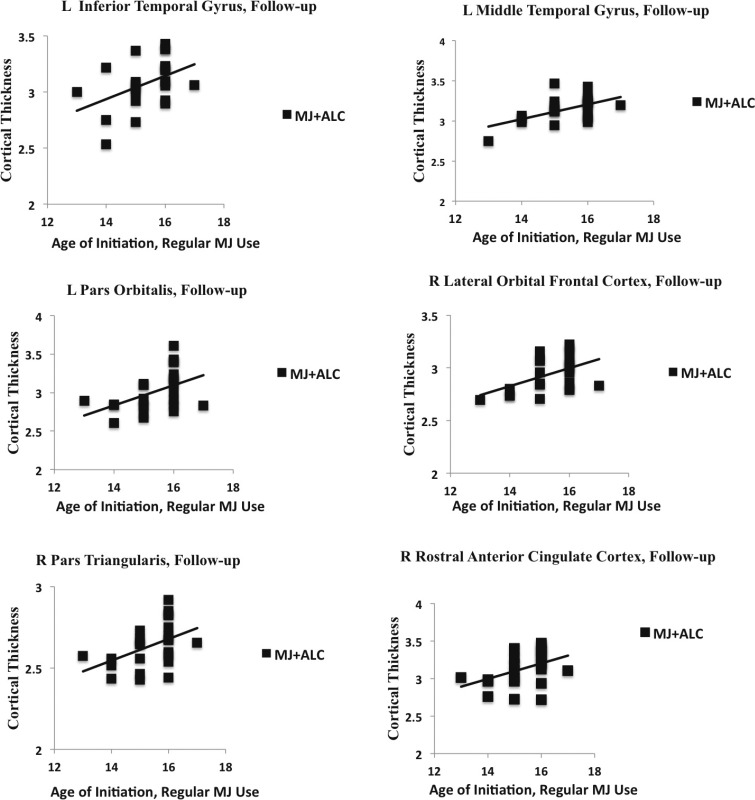
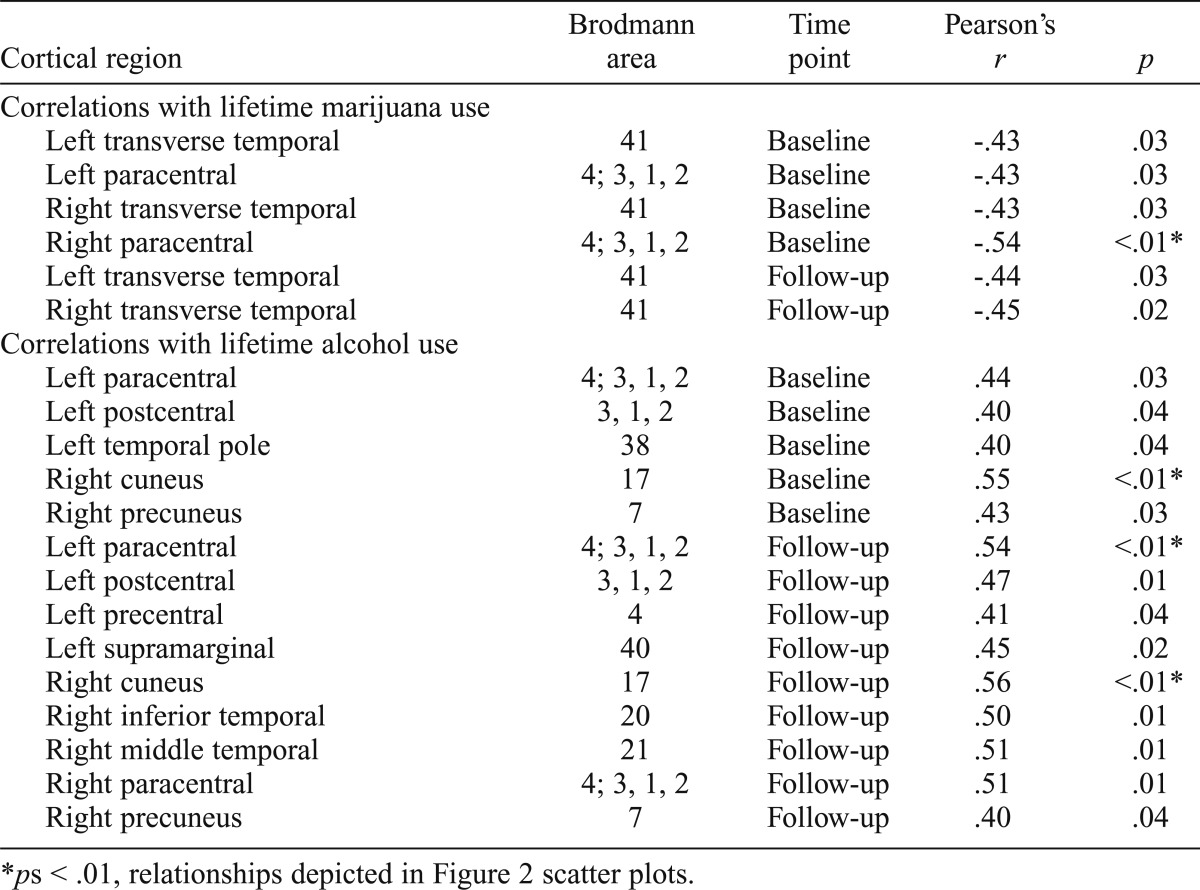
In substance users (n = 24), negative correlations were found between lifetime marijuana use and cortical thickness in the left and right transverse temporal cortex at both baseline and follow-up, and the left and right paracentral lobule at baseline (ps < .05); decreased cortical thickness estimates were associated with more reported lifetime marijuana use (Table 2, Figures 1 and 2). Relationships did not change holding lifetime alcohol use constant (rs > -.40, ps < .05). We found that age of onset of regular marijuana use was positively related to cortical thickness, in that initiation of regular use at a younger age was linked to thinner cortices at follow-up in the left inferior temporal gyrus (r =.43, p = .03), left middle temporal gyrus (r = .53, p < .01), left pars orbitalis (r = .45, p = .02), right lateral orbital frontal cortex (r = .48, p = .01), right pars triangularis (r = .54, p = .02), and right rostral anterior cingulate cortex (r = .42, p = .03) (Figure 3).
Table 2.
Dose-dependent associations with cortical thickness in the user group (n = 24)
| Cortical region | Brodmann area | Time point | Pearson’s r | p |
| Correlations with lifetime marijuana use | ||||
| Left transverse temporal | 41 | Baseline | -.43 | .03 |
| Left paracentral | 4; 3, 1, 2 | Baseline | -.43 | .03 |
| Right transverse temporal | 41 | Baseline | -.43 | .03 |
| Right paracentral | 4; 3, 1, 2 | Baseline | -.54 | <.01* |
| Left transverse temporal | 41 | Follow-up | -.44 | .03 |
| Right transverse temporal | 41 | Follow-up | -.45 | .02 |
| Correlations with lifetime alcohol use | ||||
| Left paracentral | 4; 3, 1, 2 | Baseline | .44 | .03 |
| Left postcentral | 3, 1, 2 | Baseline | .40 | .04 |
| Left temporal pole | 38 | Baseline | .40 | .04 |
| Right cuneus | 17 | Baseline | .55 | <.01* |
| Right precuneus | 7 | Baseline | .43 | .03 |
| Left paracentral | 4; 3, 1, 2 | Follow-up | .54 | <.01* |
| Left postcentral | 3, 1, 2 | Follow-up | .47 | .01 |
| Left precentral | 4 | Follow-up | .41 | .04 |
| Left supramarginal | 40 | Follow-up | .45 | .02 |
| Right cuneus | 17 | Follow-up | .56 | <.01* |
| Right inferior temporal | 20 | Follow-up | .50 | .01 |
| Right middle temporal | 21 | Follow-up | .51 | .01 |
| Right paracentral | 4; 3, 1, 2 | Follow-up | .51 | .01 |
| Right precuneus | 7 | Follow-up | .40 | .04 |
ps < .01, relationships depicted in Figure 2 scatter plots.
Figure 1.
Cortical regions representing between-group (entorhinal cortex) and dose-dependent bivariate associations with marijuana (green; G), dose-dependent bivariate associations with alcohol (red; R), and dose-dependent bivariate associations with both substances (yellow; Y), at baseline and follow-up, ps < .05
Figure 2.
Scatter plots depicting dose-dependent relationships with lifetime marijuana (negative) and alcohol (positive) and cortical thickness in the user group only (n = 24, ps < .01)
Figure 3.
Scatter plots depicting associations between age of initiation of regular marijuana use and cortical thickness in the user group at follow-up (n = 24)
Interestingly, several positive correlations were found for users (n = 24) between lifetime alcohol use and cortical thickness at baseline and follow-up in areas including the left paracentral, postcentral, precentral, and supramarginal cortices; the left temporal pole; the right cuneus and precuneus; and the right inferior temporal gyrus (ps < .05; see Table 2, Figures 1 and 2). Increased cortical thickness estimates were associated with more reported lifetime alcohol use. Relationships did not change holding marijuana use constant (rs > .41, ps < .05). In the areas in which we saw positive correlations with lifetime alcohol use (Table 2), positive relationships with number of heavy drinking episodes, defined as ≥4 drinks on one occasion for females and ≥5 drinks for males, was observed in the left paracentral (r = .54, p < .01; r = .61, p < .01), right cuneus (r = .57, p < .01, r = .63, p < .01), and right precuneus (r = .45, p = .03, r = .52, p = .01) cortex at baseline and follow-up, and the left postcentral (r = .54, p < .01) and right inferior temporal (r = .42, p = .02) cortex at follow-up. Relationships did not change holding marijuana use constant (rs > .43, ps < .05). We did not see associations with cortical thickness and age of initiation of regular alcohol use.
Main effect of time on cortical thickness measurement
A main effect of time (i.e., decreasing cortical thickness for both ALC + MJ and CON) was found in seven regions, including the left and right fusiform gyrus, left postcentral gyrus, left and right temporal pole, right entorhinal cortex, and right superior parietal cortex, F(1, 52) = 4.6–8.4, ps ≤ .03. We did not see any significant Group × Time interactions (ps > .10).
Neuropsychological performance
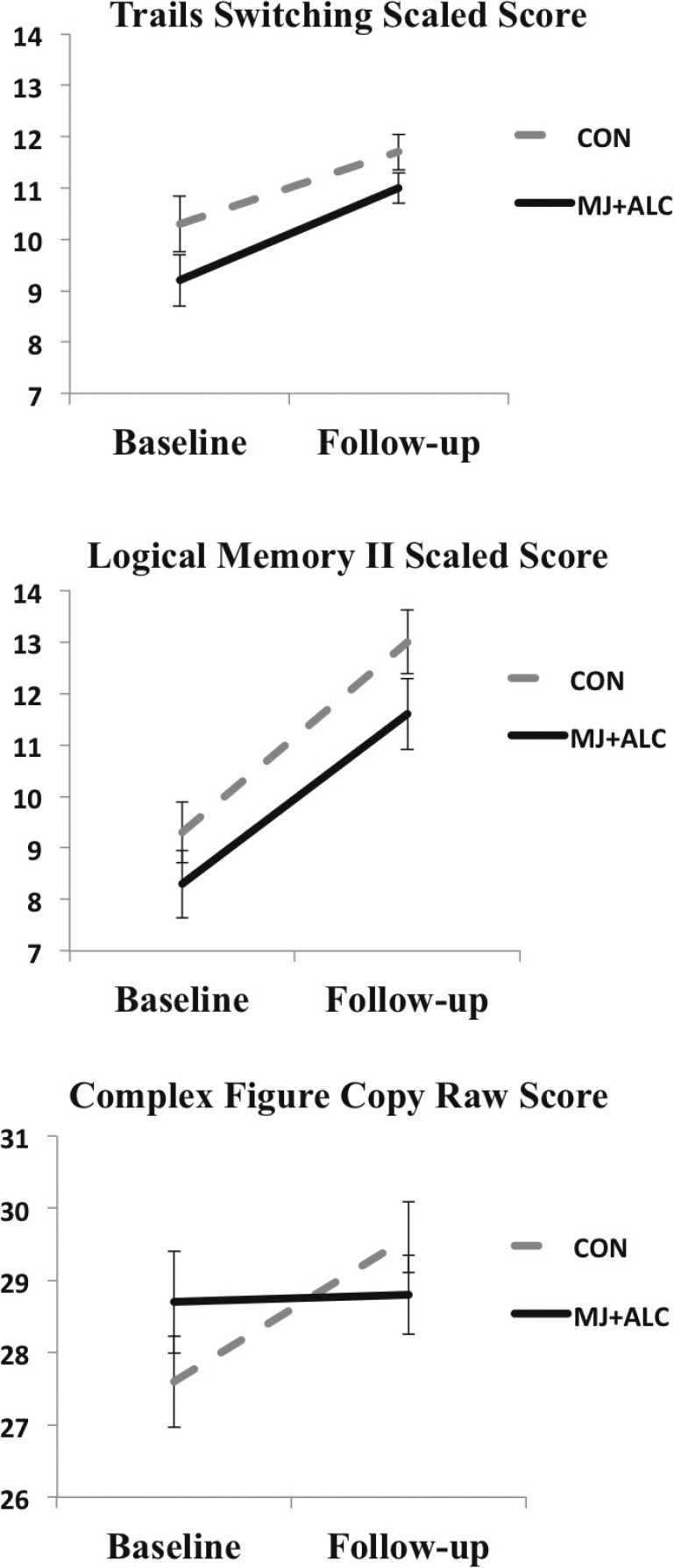
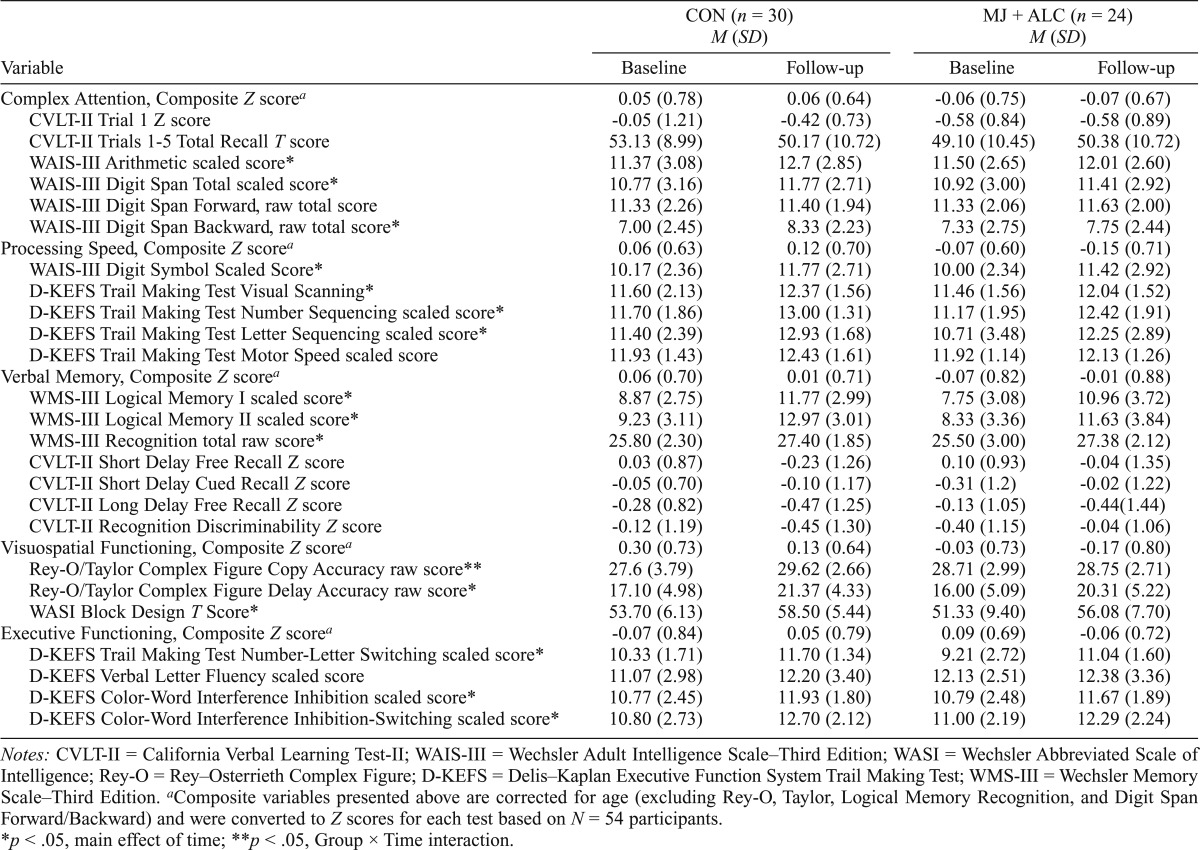
When lifetime alcohol use was controlled for, repeated-measures ANCOVA found a main effect of time (increasing performance from baseline to follow-up) for the vast majority of neurocognitive measures (15 of 25 neurocognitive tests) as expected, including the Complex Figure Delay, Logical Memory I, II, and Recognition, Color-Word Inhibition and Switching subtests, Trail Making Test Visual Scanning, Number Sequencing, Letter Sequencing, and Switching subtests, Block Design, Digit-Symbol Coding, Arithmetic, and Digit Span Backward and Total subtests, F(1, 52) = 5.2–103.4, ps < .03 (Table 3). A Group × Time interaction was found for Complex Figure Copy condition F(1, 52) = 4.8, p = .03 (Table 3, Figure 4), as improved performance was seen for controls but not for MJ + ALC. No group effects were observed with or without controlling for alcohol (ps > .20).
Table 3.
Descriptive statistics of neuropsychological composite variables by group
| CON (n = 30) M (SD) |
MJ + ALC (n = 24) M (SD) |
|||
| Variable | Baseline | Follow-up | Baseline | Follow-up |
| Complex Attention, Composite Z scorea | 0.05 (0.78) | 0.06 (0.64) | -0.06 (0.75) | -0.07 (0.67) |
| CVLT-II Trial 1 Z score | -0.05 (1.21) | -0.42 (0.73) | -0.58 (0.84) | -0.58 (0.89) |
| CVLT-II Trials 1-5 Total Recall T score | 53.13 (8.99) | 50.17 (10.72) | 49.10 (10.45) | 50.38 (10.72) |
| WAIS-III Arithmetic scaled score* | 11.37 (3.08) | 12.7 (2.85) | 11.50 (2.65) | 12.01 (2.60) |
| WAIS-III Digit Span Total scaled score* | 10.77 (3.16) | 11.77 (2.71) | 10.92 (3.00) | 11.41 (2.92) |
| WAIS-III Digit Span Forward, raw total score | 11.33 (2.26) | 11.40 (1.94) | 11.33 (2.06) | 11.63 (2.00) |
| WAIS-III Digit Span Backward, raw total score* | 7.00 (2.45) | 8.33 (2.23) | 7.33 (2.75) | 7.75 (2.44) |
| Processing Speed, Composite Z scorea | 0.06 (0.63) | 0.12 (0.70) | -0.07 (0.60) | -0.15 (0.71) |
| WAIS-III Digit Symbol Scaled Score* | 10.17 (2.36) | 11.77 (2.71) | 10.00 (2.34) | 11.42 (2.92) |
| D-KEFS Trail Making Test Visual Scanning* | 11.60 (2.13) | 12.37 (1.56) | 11.46 (1.56) | 12.04 (1.52) |
| D-KEFS Trail Making Test Number Sequencing scaled score* | 11.70 (1.86) | 13.00 (1.31) | 11.17 (1.95) | 12.42 (1.91) |
| D-KEFS Trail Making Test Letter Sequencing scaled score* | 11.40 (2.39) | 12.93 (1.68) | 10.71 (3.48) | 12.25 (2.89) |
| D-KEFS Trail Making Test Motor Speed scaled score | 11.93 (1.43) | 12.43 (1.61) | 11.92 (1.14) | 12.13 (1.26) |
| Verbal Memory, Composite Z scorea | 0.06 (0.70) | 0.01 (0.71) | -0.07 (0.82) | -0.01 (0.88) |
| WMS-III Logical Memory I scaled score* | 8.87 (2.75) | 11.77 (2.99) | 7.75 (3.08) | 10.96 (3.72) |
| WMS-III Logical Memory II scaled score* | 9.23 (3.11) | 12.97 (3.01) | 8.33 (3.36) | 11.63 (3.84) |
| WMS-III Recognition total raw score* | 25.80 (2.30) | 27.40 (1.85) | 25.50 (3.00) | 27.38 (2.12) |
| CVLT-II Short Delay Free Recall Z score | 0.03 (0.87) | -0.23 (1.26) | 0.10 (0.93) | -0.04 (1.35) |
| CVLT-II Short Delay Cued Recall Z score | -0.05 (0.70) | -0.10 (1.17) | -0.31 (1.2) | -0.02 (1.22) |
| CVLT-II Long Delay Free Recall Z score | -0.28 (0.82) | -0.47 (1.25) | -0.13 (1.05) | -0.44 (1.44) |
| CVLT-II Recognition Discriminability Z score | -0.12 (1.19) | -0.45 (1.30) | -0.40 (1.15) | -0.04 (1.06) |
| Visuospatial Functioning, Composite Z scorea | 0.30 (0.73) | 0.13 (0.64) | -0.03 (0.73) | -0.17 (0.80) |
| Rey-O/Taylor Complex Figure Copy Accuracy raw score** | 27.6 (3.79) | 29.62 (2.66) | 28.71 (2.99) | 28.75 (2.71) |
| Rey-O/Taylor Complex Figure Delay Accuracy raw score* | 17.10 (4.98) | 21.37 (4.33) | 16.00 (5.09) | 20.31 (5.22) |
| WASI Block Design T Score* | 53.70 (6.13) | 58.50 (5.44) | 51.33 (9.40) | 56.08 (7.70) |
| Executive Functioning, Composite Z scorea | -0.07 (0.84) | 0.05 (0.79) | 0.09 (0.69) | -0.06 (0.72) |
| D-KEFS Trail Making Test Number-Letter Switching scaled score* | 10.33 (1.71) | 11.70 (1.34) | 9.21 (2.72) | 11.04 (1.60) |
| D-KEFS Verbal Letter Fluency scaled score | 11.07 (2.98) | 12.20 (3.40) | 12.13 (2.51) | 12.38 (3.36) |
| D-KEFS Color-Word Interference Inhibition scaled score* | 10.77 (2.45) | 11.93 (1.80) | 10.79 (2.48) | 11.67 (1.89) |
| D-KEFS Color-Word Interference Inhibition-Switching scaled score* | 10.80 (2.73) | 12.70 (2.12) | 11.00 (2.19) | 12.29 (2.24) |
Notes: CVLT-II = California Verbal Learning Test-II; WAIS-III = Wechsler Adult Intelligence Scale–Third Edition; WASI = Wechsler Abbreviated Scale of Intelligence; Rey-O = Rey–Osterrieth Complex Figure; D-KEFS = Delis–Kaplan Executive Function System Trail Making Test; WMS-III = Wechsler Memory Scale–Third Edition.
Composite variables presented above are corrected for age (excluding Rey-O, Taylor, Logical Memory Recognition, and Digit Span Forward/Backward) and were converted to Z scores for each test based on N = 54 participants.
p < .05, main effect of time;
p < .05, Group × Time interaction.
Figure 4.
Neuropsychological performance (N = 54), showing a main effect of time (Trails, Logical Memory) and Group × Time interaction (Complex Figure), ps ≤ .03
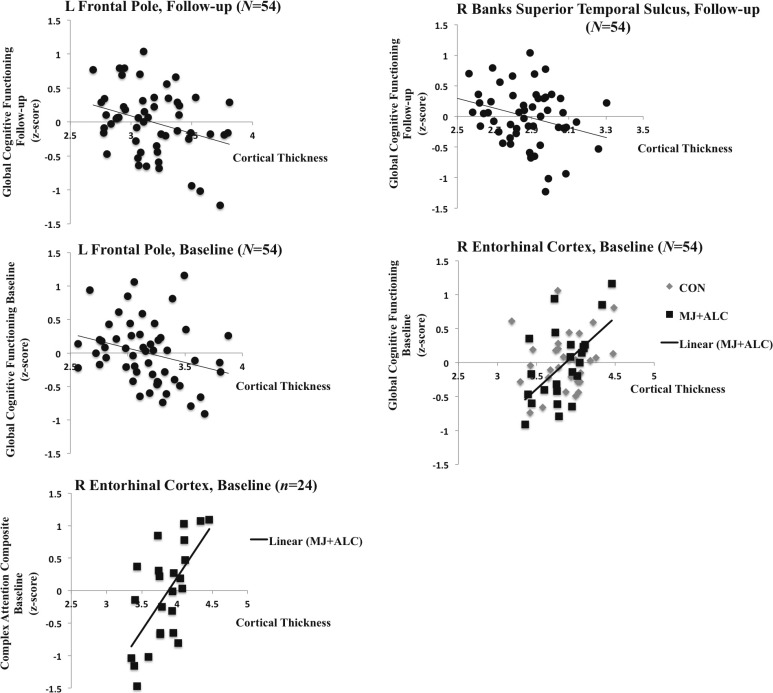
To better understand if cortical thickness is associated with cognitive functioning in this sample, and to limit the number of comparisons, we examined associations between cortical thickness estimates and global neurocognitive functioning at corresponding time points across the whole sample. We found negative associations between cortical thickness in the left frontal pole and global cognitive performance at baseline (r = -.28, p = .04) and follow-up (r = -.31, p = .02) such that increased cortical thickness was associated with poorer global cognitive performance in the larger sample (Figure 5). Likewise, we observed a negative relationship between the right banks of the superior temporal sulcus and global functioning at follow-up (r = -.28, p = .03). These negative associations did not appear to be driven by group or a particular domain (ps > .05).
Figure 5.
Scatter plots depicting associations between global cognitive functioning and cortical thickness
We observed a positive relationship between cortical thickness and global cognitive functioning at baseline (r = .38, p < .01) in only the right entorhinal cortex. These findings appear to be driven by associations between cortical thickness and global functioning in the user group (r = .57, p < .01; Figure 5). Specifically, increased cortical thickness in the right entorhinal cortex was related to better performance in the domain of attention (r = .65, p < .01) in the user group only (Figure 5).
We did not identify any associations between age of onset of regular marijuana or alcohol use and global cognitive functioning in the user group (ps > 10).
Discussion
We prospectively examined cortical thickness estimates and neurocognition in a sample of adolescent heavy marijuana and alcohol users and matched controls before and after 28 days of monitored abstinence. Few group differences in cortical thickness were observed; the majority of areas (e.g., frontal and occipital cortices) were no longer significant after controlling for lifetime alcohol use. Thicker medial temporal lobe estimates (i.e., entorhinal cortex) were found in the user group at baseline and follow-up when ICV and lifetime alcohol use were controlled for. We observed subtle decreasing thickness estimates from baseline to follow-up in seven regions, but no interactions were identified. We found quite divergent relationships between cumulative lifetime alcohol and marijuana use and cortical thickness in the user group. More reported marijuana use (and initiation of regular use at an earlier age) was related to thinner cortices in temporal and frontal regions, and more lifetime alcohol use was related to thicker cortices in all four lobes of the cortex bilaterally.
Improvement in cognitive functioning over time in both the users and controls was observed, given the short retest interval and anticipated gains in performance. We did not see greater improvement in cognition in the user group following abstinence or, surprisingly, consistent group differences in cognition across the five domains as suggested in the literature and previous studies in our laboratory (Hanson et al., 2011; Medina et al., 2007; Meier et al., 2012; Solowij et al., 2011; Tait et al., 2011). MJ + ALC did not improve in their performance on the Complex Figure Copy subtest; however, this pattern of performance was not found across the other tests. In general, thinner cortices were related to better global cognitive performance in the larger sample with the exception of the right entorhinal cortex and cognitive functioning in the user group at baseline, in which thicker cortices were associated with better attentional processing.
Lopez-Larson and colleagues (2011) cross-sectionally examined cortical thickness in teens, ages 16–19 years, with heavy marijuana use histories. They found decreased thickness in frontal regions and the insula, along with increased thickness in lingual, temporal, and parietal regions (Lopez-Larson et al., 2011). Our findings are similar given that we found increased thickness in temporal and posterior regions, such as the entorhinal (temporal) cortex (and lingual and pericalcarine cortex before controlling for alcohol), and relationships showing thinner cortices associated with increased severity of use (younger age of initiation and increased quantity) in frontal regions. The authors discuss multiple pathways for tissue disruption, including altered neurodevelopmental trajectories (interference with synaptic refinement) and/or tissue loss or remodeling. Similarly, Mata and colleagues (2010) found flattening and thinning of the sulci in frontal regions in adolescent marijuana users, suggesting chronic cannabinoid exposure may link to atypical trajectories of the gyral folding process.
The mechanism by which marijuana may alter brain tissue (and cortical thickness) during development remains unclear. Marijuana may interfere with the cannabinoid system by altering patterning, plasticity, and connectivity during neurodevelopment, and trigger neurochemical and protein activity in response to neural injury (Iversen, 2003; Rubino and Parolaro, 2008; Stella, 2013). Macrostructural findings typically focus on structures with a high density of cannabinoid type 1 (CB1) receptors (e.g., hippocampus, neocortex), and findings show larger structural volume in areas such as the anterior cerebellum and amygdala (Cousijn et al., 2012; McQueeny et al., 2011; Medina et al., 2010), whereas others show decreased gray matter volume and density (Ashtari et al., 2011; Demirakca et al., 2011; Matochik et al., 2005; Yücel et al., 2008). Cousijn and colleagues (2012) found that amygdala and hippocampal volume negatively correlated with weekly cannabis use, as more use was related to smaller limbic structures. In our study, cortical thickness differences persisted after controlling for alcohol in the entorhinal cortex. Given the high concentration of CB1 receptors in temporal lobe structures (i.e., hippocampus), marijuana use may be particularly influencing developmental events in this anatomical region.
Positive associations between thickness in the entorhinal cortex and cognitive functioning (i.e., thinner cortices related to poorer cognitive functioning for our marijuana users) is in contrast to the finding that thinner cortices are related to better global cognitive performance in the larger sample. However, greater reported marijuana use (at baseline and follow-up) and earlier age of initiation (at follow-up) were also associated with thinner cortices in frontal and temporal brain regions. We suggest that endocannabinoid system alterations or marijuana-related toxicity may trigger developmental consequences such as premature cortical thinning and subsequent declines in cognitive functioning. It is unclear why associations between age of initiation and thickness estimates were observed at follow-up only. Subtle neural architectural changes may be occurring over the abstinence period, and the acute impact on neural development may not be fully captured at baseline for this predictor. Neural recovery is likely to extend past 28 days and well into the year following cessation of use, as residual effects of marijuana use have been reported in cognitive and neuroimaging markers from days to months following cessation of use (Ashtari et al., 2011; Bava et al., 2009; Matochik et al., 2005; Medina et al., 2007; Meier et al., 2012; Pope et al., 2001; Schweinsburg et al., 2008; Tapert et al., 2007). Pre-existing structural differences are also likely to contribute, as smaller orbitofrontal cortex volume predicted initiation of cannabis use by age 16 (Cheetham et al., 2012).
The positive dose-dependent relationships with lifetime alcohol use are particularly notable given the subclinical heavy episodic drinking patterns reported by the sample. Thickness estimates in these regions were related to number of heavy drinking episodes reported, which is particularly concerning given the consistently high rates of heavy episodic drinking reported by adolescents in the United States (Johnston et al., 2014). The cerebral cortex is highly vulnerable to the effects of alcohol (Jacobus and Tapert, 2013). Squeglia and colleagues (2012) found that female heavy episodic drinkers had thicker cortices in frontal brain regions compared to female controls, and thicker cortices were associated with worse cognitive functioning for both males and females. However, thinner cortices were identified for male heavy episodic drinkers compared to controls (Squeglia et al., 2012), similar to recent prospective findings showing decreased thickness estimates in adolescents who transitioned into subclinical heavy episodic drinking (Luciana et al., 2013). Our findings suggest widespread (frontal, parietal, temporal, and occipital) increases in cortical thickness with increased lifetime alcohol use and heaving drinking episodes. The present study, combined with Squeglia et al. (2012), suggests that more often thicker cortices are associated with worse neurobehavioral performance. Despite the quantity of reported alcohol use in our users being more modest compared to that of treatment seeking individuals, differences in these studies of adolescents reporting similar alcohol use patterns may be attributed to methodological design (e.g., cross sectional, pre/post initiation, pre/post abstinence) or an interaction between co-occurring alcohol and marijuana use.
Neuroprotective properties of marijuana may modulate neurotransmission and mitigate ethanol-induced neural injury; however, marijuana may trigger neurotoxic chemical cascades leading to changes in endocannabinoid signaling, altered developmental trajectories, increased alcohol administration, and worse psychosocial outcomes in the developing brain (Ceccarini et al., 2013; Hamelink et al., 2005; Hansen et al., 2008; Pope et al., 2010; Rezayof et al., 2012; Swartzwelder et al., 2012). Although initial cross-sectional studies in our laboratory suggested evidence for white matter neuroprotection in those using marijuana and alcohol (Jacobus et al., 2009), poorer outcomes for co-occurring use from adolescence to young adulthood were found after a 3-year follow-up (Jacobus et al., 2013a). The mechanism of alcohol-related toxicity on the cerebral cortex remains unclear. Alcohol may interfere with temporal sequences of neurodevelopment (e.g., thinning), myelination, and/or generation and survival of cortical cells (Crews and Nixon, 2009); overall, the unanticipated associations with alcohol found in this study underscore the deleterious impact adolescent alcohol use likely has on neurodevelopment when used independently or concomitantly with marijuana.
Our sample was predominantly male (>70%). Therefore, it is unlikely that excluding our female participants would have changed the observed relationships. However, gender may moderate these findings (Bramen et al., 2012; Crane et al., 2013; Squeglia et al., 2012). Studies have found gender to play a significant role in gray and white matter neural architecture and neurocognition in both healthy adolescents/young adults and those engaging in substance use (Lisdahl and Price, 2012; Medina et al., 2009; Simmonds et al., 2014; Squeglia et al., 2012). We suspect that findings represent a “longer-term” impact of marijuana and alcohol use, given the monitored abstinence period. The majority of findings present at baseline were present at follow-up, but this was not the case for all regions (e.g., left paracentral cortex and lifetime marijuana use). The prospect of neural recovery after cessation of use is understudied in the adolescent literature, although there is some suggestion that brain structural changes can occur within the initial 24 months of abstinence from alcohol in an adult sample of former heavy alcohol users reporting 1 month to 26 years of abstinence (Fortier et al., 2011). Our preliminary findings need to be replicated and expanded upon. Longer follow-up periods are necessary to understand changes in marijuana use trajectories over time and differences in residual versus acute effects. Given the preliminary and exploratory nature of this work, large number of analyses conducted, and modest effect sizes, replication of findings is crucial.
Subtle alterations in neurodevelopmental trajectories may have long-term consequences for cognition and daily functioning. To identify how alcohol and marijuana use leads to brain changes, disentangling pre-existing, substance-related, and acute versus residual effects is important. The present findings raise concern for adolescent alcohol and marijuana users and provide more evidence for a longer-term effect on neural tissue development. Our future work will integrate longer follow-up periods with pre- and post-initiation data to understand how such commonly used substances affect the developing brain.
Acknowledgments
The authors extend appreciation to Anthony Scarlett, Diane Goldenberg, and Rachel Thayer for their assistance with data collection and to participating families and schools.
Footnotes
This study was supported by National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism Grants P20 DA024194, R01 DA021182, F32 DA032188, R01 AA013419, T32 AA013525, and U01 AA021692.
References
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. Journal of Psychiatric Research. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision. 4th ed. Washington, DC: Author; 2000. [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) San Antonio, TX: Psychological Corporation; 1978. [Google Scholar]
- Blakemore SJ. Imaging brain development: The adolescent brain. NeuroImage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Sowell ER. Sex matters during adolescence: Testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE. 2012;7(3):e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics, 121, Supplement. 2008;4:S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Casteels C, Koole M, Bormans G, Van Laere K. Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: A combined PET and microdialysis study. European Journal of Nuclear Medicine and Molecular Imaging. 2013;40:1582–1594. doi: 10.1007/s00259-013-2456-1. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biological Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Corwin J, Bylsma FW. Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy,” and P. A. Osterrieth’s “The Complex Figure Copy Test.”. The Clinical Neuropsychologist. 1993;7:3–21. [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Gonzalez R. Preliminary evidence for a sex-specific relationship between amount of cannabis use and neurocognitive performance in young adult cannabis users. Journal of the International Neuropsychological Society. 2013;19:1009–1015. doi: 10.1017/S135561771300088X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E. Delis-Kaplan Executive Functioning Scale Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Second Edition. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, Hermann D. Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug and Alcohol Dependence. 2011;114:242–245. doi: 10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, Acheson A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. 2013;226:307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Lacerda ALT. Cannabis use before age 15 and subsequent executive functioning. British Journal of Psychiatry. 2011;198:442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, Williams V, McGlinchey RE. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcoholism: Clinical and Experimental Research. 2011;35:2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber AJ, Peterson BS, Giedd JN, Lalonde FM, Celano MJ, White SL, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. 2012;34:962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neuroscience Letters. 2012a;511:89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012b;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Krutz B, Sifringer M, Stefovska V, Bittigau P, Pragst F, Ikonomidou C. Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Annals of Neurology. 2008;64:42–52. doi: 10.1002/ana.21287. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of Addictive Behaviors. 2011;25:127–142. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubley AM, Tremblay D. Comparability of total score performance on the Rey–Osterrieth Complex Figure and a modified Taylor Complex Figure. Journal of Clinical and Experimental Neuropsychology. 2002;24:370–382. doi: 10.1076/jcen.24.3.370.984. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology. 2012;222:675–684. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: A 3-year investigation. Psychiatry Research. 2013a;214:374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sciences. 2013b;3:396–414. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Current Pharmaceutical Design. 2014;20:2186–2193. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2013. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society. 2012;18:678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, Yurgelun-Todd DA. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Research. 2012;202:224–232. doi: 10.1016/j.pscychresns.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Friman P. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. American Journal of Drug and Alcohol Abuse. 2013;39:345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santiañez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, Crespo-Facorro B. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Research. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural Brain Research. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addiction Biology. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, Weisner C. Concurrent and simultaneous drug and alcohol use: Results of the 2000 National Alcohol Survey. Drug and Alcohol Dependence. 2007;90:72–80. doi: 10.1016/j.drugalcdep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pope C, Mechoulam R, Parsons L. Endocannabinoid signaling in neurotoxicity and neuroprotection. Neurotoxicology. 2010;31:562–571. doi: 10.1016/j.neuro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: A robust approach. NeuroImage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Ghandipour M, Nazari-Serenjeh F. Effect of co-injection of arachydonilcyclopropylamide and ethanol on conditioned place preference in rats. Physiology & Behavior. 2012;107:301–308. doi: 10.1016/j.physbeh.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Molecular and Cellular Endocrinology, 286, Supplement. 2008;1:S108–S113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Regier DA. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Simmonds D, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of Analytical Toxicology. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow back. A technique for assessing self-reported alcohol consumption. New York, NY: Humana Press; 1992. [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PCL, Yücel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011;216:131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N. Chronic THC intake modifies fundamental cerebellar functions. Journal of Clinical Investigation. 2013;123:3208–3210. doi: 10.1172/JCI70226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychology Review. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings (NSDUH Series H-46, HHS Publication No. SMA 13–4795) Rockville, MD: Author; 2013. [Google Scholar]
- Swartzwelder NA, Risher ML, Abdelwahab SH, D’Abo A, Rezvani AH, Levin ED, Acheson SK. Effects of ethanol, Δ(9)-tetrahydrocannabinol, or their combination on object recognition memory and object preference in adolescent and adult male rats. Neuroscience Letters. 2012;527:11–15. doi: 10.1016/j.neulet.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106:2195–2203. doi: 10.1111/j.1360-0443.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM, Johnston LD. Simultaneous alcohol and marijuana use among U.S. high school seniors from 1976 to 2011: Trends, reasons, and situations. Drug and Alcohol Dependence. 2013;133:71–79. doi: 10.1016/j.drugalcdep.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, Yeo RA. Adolescent substance abuse: The effects of alcohol and marijuana on neuropsychological performance. Alcoholism: Clinical and Experimental Research. 2011;35:39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale, 3rd Edition. New York, NY: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. WAIS-III Manual. New York, NY: Psychological Corporation; 1997b. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]