Abstract
Objective:
Despite the commonly observed comorbidity of alcohol and tobacco use disorders and years of research, the mechanism underlying concurrent use of alcohol and tobacco is not yet clear. In this study, we used functional magnetic resonance imaging (fMRI) to investigate the relationship between brain responses to alcohol and smoking cues in 45 subjects with episodic drinking and regular smoking.
Method:
fMRI data were collected from two studies performing an alcohol-craving task and a smoking-craving task. First, we identified brain voxels significantly activated for both substance cues and then associated the activation of these voxels with various alcohol- and nicotine-dependence measures. Significant clusters (cluster-wise p < .05) correlated with behavioral assessments were extracted, and clusters identified from both cues were compared.
Results:
The association tests with various dependence scores showed that the loss of behavioral control subcategory in the Alcohol Dependence Scale was significantly correlated with brain activation of the posterior cingulate cortex (PCC) and right posterior insula regardless of cue types.
Conclusions:
Our findings suggest that the PCC and right posterior insula, each playing a role in the salience network, are affected significantly by impaired control for alcohol and in turn influence brain responses to not only alcohol but also smoking cues, providing insight to neuronal mechanisms for concurrent use or comorbidity of alcohol and nicotine dependence.
Arecent national survey on drug use and health taken in 2011 showed that 54.9% of heavy alcohol users ages 12 years or older also smoked cigarettes, whereas only 18.1% of non–heavy episodic drinkers and 15.3% of non–alcohol users were current smokers (Substance Abuse and Mental Health Services Administration, 2012). Concurrent use or misuse of both substances has been of great interest to clinicians and scientists. There are potentially many reasons for this comorbidity, including similar genetic factors that may increase the vulnerability to abuse and dependence of both substances (Davis and de Fiebre, 2006; Grucza and Bierut, 2006). Studies of brain function have suggested that both substances may involve similar neuronal mechanisms underlying psychological dysfunctions associated with reward, emotion, and cognitive control processes common to many drugs of misuse (Funk et al., 2006; Pierce and Kumaresan, 2006). More specifically, several theories have been put forth attempting to explain the concurrent heavy use of cigarettes and alcohol (Durazzo et al., 2007), including conditioned cue reactivity leading to cravings for both substances (Drobes, 2002). Several studies have examined this notion by testing self-reported cravings after exposure to alcohol and smoking cues (Epstein et al., 2007; Erblich et al., 2009; King et al., 2009; Sayette et al., 2005), and it was found that moderate oral doses of alcohol increase nicotine craving in tobacco “chippers” or light smokers. To our knowledge, only one study used a functional magnetic resonance imaging (fMRI) approach to examine brain responses to an alcohol-induced smoking urge in heavy drinking nondaily smokers, where the ventral striatum demonstrated increased activation after alcohol intake (King et al., 2010).
Neuroimaging techniques in conjunction with paradigms designed to engage specific brain regions have been essential to investigate various dysfunctions associated with substance use disorders. In particular, craving responses have been widely studied via fMRI with alcohol or smoking cue reactivity tasks. Repeated findings have shown significant activation in the striatum, medial frontal, insula, and anterior cingulate regions (Filbey et al., 2008; George et al., 2001; Tapert et al., 2004; Wrase et al., 2002) during exposure to alcohol cues. These types of cue exposure studies have also characterized the alcohol-craving responses in connection with alcohol-dependence severity (Claus et al., 2011a; Filbey et al., 2008). Similarly, there have been many investigations of brain responses to smoking cues, showing increased activation in the orbitofrontal cortex, anterior cingulate, striatum, nucleus accumbens, and ventral tegmental area (David et al., 2005; Franklin et al., 2011; Lee et al., 2005; Lim et al., 2005), in which responses were also analyzed for associations with various clinical assessments. Although such studies have provided crucial information about brain circuits underlying cravings for each type of substance, including many common regions, combined studies may reveal additional information about the relation, such as an overlap in brain activation elicited by both types of cues or common modulation of brain responses. Given existing reports, it is reasonable to hypothesize that not only are significant brain regions elicited during both alcohol and smoking cues, but also certain brain regions may be partially responsible for the concurrent use of substances in such a way that craving for (or dependence on) one substance may introduce a craving for the other.
In this study, we combined two separate cue reactivity studies, one designed for brain responses to alcohol cues (Claus et al., 2011a) and the other for brain responses to smoking cues (Claus et al., 2013). The goal was to directly characterize the relation between brain responses to alcohol and smoking cues and their association with various aspects of dependence on each substance. We hypothesized that brain regions within the reward and salience networks are recruited by both cues and also are part of the cognitive control network. The reward network, which is involved in anticipation of reward and regulation of related emotions, typically includes the ventral tegmental area, nucleus accumbens, caudate, putamen, and anterior cingulate as a part of the dopamine projection pathway. The salience network as defined in Sutherland et al. (2012) is composed of insula and anterior and posterior cingulate and plays a crucial role in the initiation, maintenance, and adjustment of attentional control. The cognitive control network consists of mainly prefrontally distributed areas such as the orbital frontal cortex, dorsolateral prefrontal cortex, inferior frontal, medial prefrontal, and parietal areas, and its function includes monitoring, cognitive reasoning, and decision making. The activities within these regions, or some key regions, are affected by the level of dependence on either one or both substances. We expected that the overall dependence on alcohol or nicotine would specifically affect brain response to the substance in certain regions (e.g., caudate for alcohol, orbitofrontal for nicotine), but some aspects of dependence may affect brain response to both substances. The cross effect of substance dependence (i.e., the dependence on alcohol affects brain responses to not only alcohol cues but also smoking cues) will provide insight into the neural mechanism underlying concurrent use of substances.
Method
Subjects and tasks
Subjects studied here are from two studies. One investigates brain response to alcohol stimuli. Treatment-naive subjects with a minimum of five heavy drinking episodes in the past month were recruited through advertisements (Claus et al., 2011a). Heavy episodic drinking was defined as five or more drinks per episode for men and four or more drinks for women. The second study investigates brain response to cigarette smoking stimuli. Similarly, subjects who reported regularly smoking cigarettes in the past 90 days were recruited through advertisements (Claus et al., 2013). Both studies were approved by the Institutional Review Board of the University of New Mexico. Potential participants were excluded if they reported receiving treatment for or diagnosis of a psychiatric illness; prior head injury; use of medications that affect the central nervous system; regular use of drugs including methamphetamine, cocaine, Ecstasy, prescription pain medication, prescription sedatives, or stimulants; or any contraindications for participating in an MRI study (e.g., pregnancy, nonremovable metallic implants). Marijuana use information was also collected, but subjects were not excluded based on marijuana use. All subjects provided written informed consent and were given a breath alcohol analysis before scanning to avoid intoxication. There were 45 subjects enrolled in both studies, and we focused on the investigation of these 45 subjects hereafter.
The age and gender of the participants are summarized in Table 1, with significantly more men than women and no age difference between men and women. Multiple assessments were administered to gauge overall alcohol and nicotine use for all participants, including the Alcohol Dependence Scale (ADS; Skinner and Allen, 1982), Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993), Fagerström Test of Nicotine Dependence (FTND; Heatherton et al., 1991), the Impaired Control Scale (ICS) for alcohol (Heather et al., 1993), and the average number of drinking or smoking days in the 90-day period preceding the interview. AUDIT and FTND scores are listed in Table 1. For these 45 subjects, the mean AUDIT scores are 17.3 for men and 15.1 for women. Based on suggested criteria (AUDIT ≥ 15 for men or ≥13 for women) (Johnson et al., 2013), 62% of these subjects are likely to be alcohol dependent. Similarly, the mean FTND score was 7.2 for men and 6.6 for women. Sixty percent of these subjects are likely to be nicotine dependent (FTND ≥ 6) (de Leon et al., 2003). These 45 subjects had an average of 6.8 drinks per day (SD = 3.15) and drank an average of 16.4 times per month. They also smoked an average of 14.0 cigarettes per day. A total of 33% of the subjects would likely exhibit dependence on both substances. This makes it more relevant to study their brain activation in response to both alcohol and smoking cues.
Table 1.
Demographic information about participants
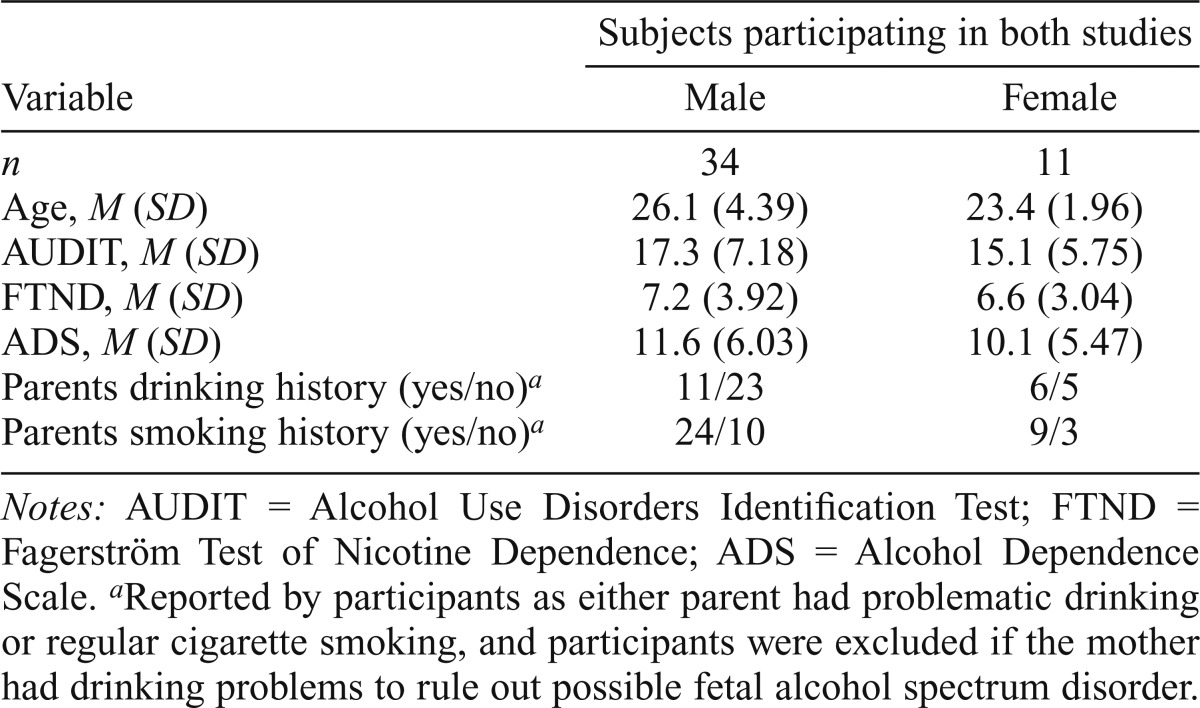
| Subjects participating in both studies |
||
| Variable | Male | Female |
| n | 34 | 11 |
| Age, M (SD) | 26.1 (4.39) | 23.4 (1.96) |
| AUDIT, M (SD) | 17.3 (7.18) | 15.1 (5.75) |
| FTND, M (SD) | 7.2 (3.92) | 6.6 (3.04) |
| ADS, M (SD) | 11.6 (6.03) | 10.1 (5.47) |
| Parents drinking history (yes/no)a | 11/23 | 6/5 |
| Parents smoking history (yes/no)a | 24/10 | 9/3 |
Notes: AUDIT = Alcohol Use Disorders Identification Test; FTND = Fagerström Test of Nicotine Dependence; ADS = Alcohol Dependence Scale.
Reported by participants as either parent had problematic drinking or regular cigarette smoking, and participants were excluded if the mother had drinking problems to rule out possible fetal alcohol spectrum disorder.
Image collection and processing
Before the scanning session, participants in the alcohol study were asked to refrain from drinking for 24 hours. The alcohol study used an alcohol-taste-cue paradigm. Participants were given 1 ml of either their preferred alcoholic beverage or a control (lychee juice) stimuli via Teflon tubing using a computer-controlled system (Frank et al., 2003). Twelve pseudo-randomized trials, six alcohol and six control, were presented to participants. Each trial delivered liquid for 24 seconds and allowed 16 seconds of rest time for the liquid taste to dissipate. However, because early piloting of the task suggested that participants could not differentiate the two beverages before swallowing, our model of the task treated the first 10 seconds as baseline and the next 30 seconds as active taste. Details of the alcohol-taste-cue design can be found in a previous study (Filbey et al., 2008).
Participants in the nicotine study refrained from smoking for 3 hours. The nicotine study used a visual smoking cue paradigm. Participants were presented with a pseudo-random series of 14 smoking-related or food-related videos, 7 of each type. Smoking videos included scenes of cigarettes being lit, inhaled, and exhaled; food videos depicted the preparation and consumption of food. Each video ranged in length from 7 to 14 seconds. Between the videos, participants viewed a fixation cross for variable times. More details of the smoking cue task can be found in a recent study (Claus et al., 2013).
FMRI data were collected on a 3-tesla Siemens Trio scanner (Siemens AG, Munich, Germany) using an echo planar gradient–echo pulse sequence (TR = 2,000 ms, TE = 29 ms, flip angle = 75°). Each volume consisted of 33 axial slices (64 × 64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap). In addition, a high-resolution T1-weighted MP-RAGE image was acquired (TR = 2,530 ms, TE = 1.64 ms, flip angle = 7°, slice thickness = 1 mm, field of view = 256 mm, resolution = 256 × 256) for each participant. Initial image processing for both studies was conducted using the FMRIB software library (FSL; FMRIB Analysis Group, Oxford, England). The first seven volumes from each functional run were discarded to allow the magnet to reach steady state. Motion correction and realignment to the first volume in each run was done with FSL’s linear image realignment tool (Jenkinson et al., 2002), followed by skull stripping and spatial smoothing with an 8-mm full-width half-maximum Gaussian kernel. A general linear model with multiple regressors was implemented. Regressors for the alcohol study included conditions of alcohol taste cue, alcohol pre-swallow baseline, juice taste cue, and juice pre-swallow baseline. Similarly, the regressors for the nicotine study represented conditions of smoking cue, food cue, and fixation baseline.
The contrasts of interest reported here used the alcohol taste cue versus alcohol pre-swallow baseline and the smoking cue versus fixation baseline. We tested the contrasts of the alcohol taste cue versus the juice taste cue and the smoking cue versus the food cue. Although similar brain regions with activation correlated with substance-dependence scores, strong brain activation elicited by food complicates the interpretation of contrasts, whether to smoking or food. Thus, we report here the analyses using contrasts of the alcohol taste cue versus baseline and the smoking cue versus baseline.
Data analyses
First, from the contrast maps of each study, brain voxels significantly activated to the alcohol or smoking cues were extracted separately using a voxel-wise one-sample t test across subjects, controlling for a false discovery rate of 0.01 (Benjamini and Hochberg, 1995). The activated voxels derived from each study were then intersected to find common areas for both substance cues, following the conjunction inference principle recently described (Nichols et al., 2005). Within the common activated voxels, we conducted voxel-wise association tests with substance-dependence assessment scores. Given the possibility that brain activation elicited by one type of substance cue might be affected by the dependence on other substances, we associated brain function during both cues with dependence measures on both substances. A linear model was applied with contrast of either alcohol versus baseline or smoking versus baseline as a dependent variable, and the independent variables included age, gender, and one behavioral assessment score from AUDIT, FTND, ADS, or ICS (or subcategories). Because the assessments were not independent of each other, we tested them separately rather than putting them into one model. Specifically, we first tested the total scores from the ADS, AUDIT, ICS, and FTND. When the total scores showed an association with brain activation, we further dissected them into subcategories, including ADS–loss of behavioral control (ADS–lbc), ADS–obsessive-compulsive drinking style, ADS–psychoperceptual withdrawal, ADS–psychophysical withdrawal, AUDIT–alcohol consumption, AUDIT–alcohol dependence, AUDIT–alcohol problems, ICS–failed control, ICS–attempted control, and ICS–perceived control. We also tested cigarette consumption using FTND Question 4 (how many cigarettes per day do you smoke currently?).
In our sample, age was highly correlated with years of drinking and smoking (r = .90 and .73, respectively). When regressing out the age effect, we most likely also removed the effects of years of drinking and smoking. Thus, we do not discuss the years of drinking/smoking effect. We also screened the potential outliers in assessment scores. Although there were two subjects with FTND scores of zero, they were not outliers in the FTND distribution, and we kept them in the analyses. From the voxel-wise correlation values after we regressed out age and gender, we identified clusters (brain regions with activation) significantly correlated with behavioral assessments using FSL cluster tools with a cluster-wise p < .05 and input voxels thresholded by absolute correlation > .29 (corresponding to an uncorrected p < .01). The spatially overlapping clusters identified during alcohol and smoking cues, which related to the same behavioral assessment, were further investigated using the scatter plots between voxel’s activation and behavioral scores.
Results
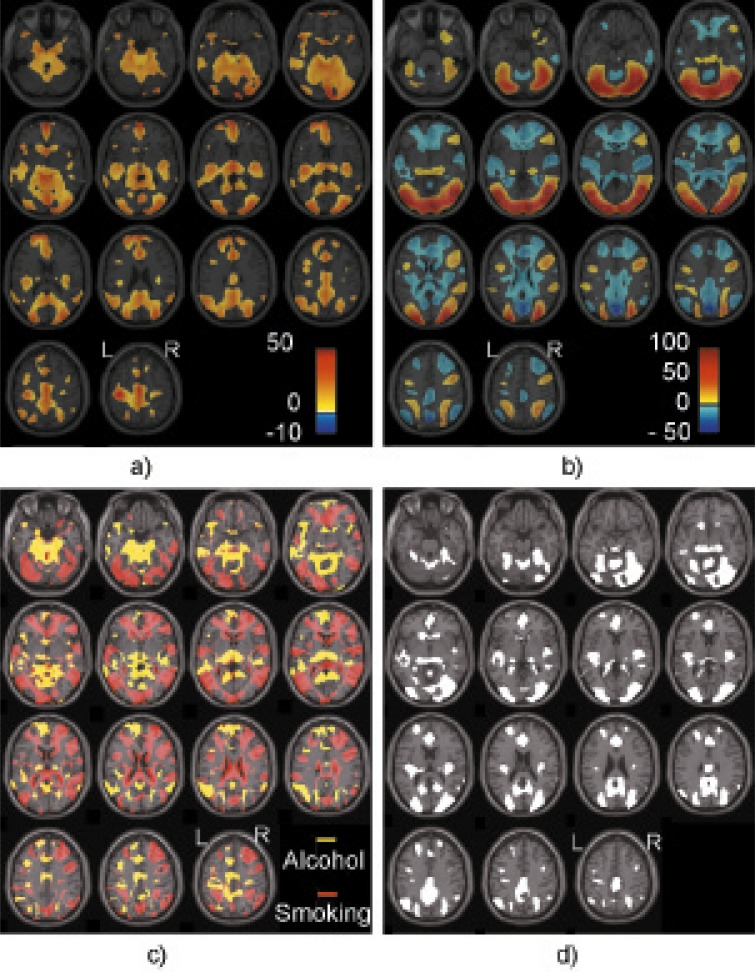
Using the 45 individuals who participated in both studies, we identified significantly activated voxels for the contrast of alcohol versus baseline and smoking versus baseline separately, which corresponds to the brain response to alcohol stimuli (Figure 1a) and the brain response to smoking stimuli (Figure 1b). In Figures 1a and 1b, the colors yellow to red show regions with positive activations, and the color blue shows regions with negative activations. Figure 1c plots the distinct regions activated by either the alcohol or smoking cue, with yellow showing alcohol-activated regions and red showing smoking-activated regions. Figure 1d plots the common regions activated by both alcohol and smoking cues regardless of the sign. Regions activated in both contrasts mainly include the precuneus, cuneus, medial frontal gyrus, anterior cingulate, cingulate gyrus (middle and posterior cingulate), superior frontal gyrus, precentral gyrus, caudate, insula, thalamus, parahippocampal gyrus, superior/middle temporal gyri, middle/inferior occipital gyri, lingual gyrus, and fusiform gyrus.
Figure 1.
Voxels significantly activated during the (a) alcohol versus baseline cue, (b) smoking versus baseline cue, (c) distinctly activated by either alcohol or smoking cue, with yellow showing alcohol-activated voxels and red showing smoking-activated voxels, and (d) both alcohol and smoking cues. In Figures 1a and 1b, color indicates the contrast values: yellow to red shows voxels with the positive activations, and blue shows voxels with negative activations. Laterality convention is neurological convention, indicated in the figure.
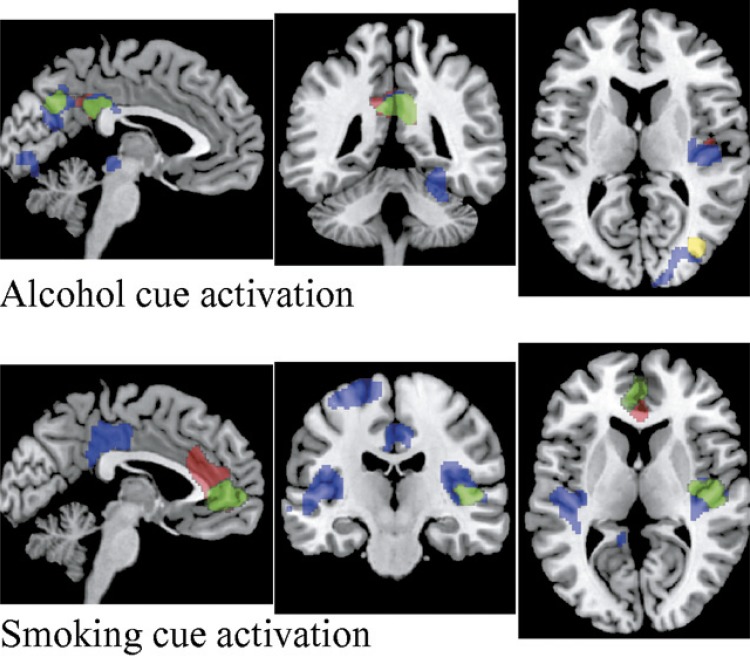
Association tests of brain activation with ADS, AUDIT, ICS, and FTND scores revealed several clusters of brain regions significantly related to behavioral assessments. From alcohol cue reactivity, the AUDIT, ADS, and ICS scores, but not FTND scores, all showed an association with brain activation, and the associations were positive. As plotted in the top panel of Figure 2, regions (in red) of the posterior cingulate cortex (PCC), precuneus (cluster-wise p = .0003), and right posterior insula (rp-insula, cluster-wise p = .04) were significantly correlated with the AUDIT total score. Although the AUDIT subscore of drinking problems showed similar results, the AUDIT–alcohol consumption score was correlated with the right precuneus and middle occipital gyrus (in yellow, cluster-wise p = .01). Both the ADS total score and the subscore of ADS-lbc related to alcohol cue activation in a very similar pattern. We plotted the regions (in blue) associated with ADS-lbc because it showed broader regions and more significance. The largest cluster was in the right fusiform gyrus (cluster-wise p = 1.86 × 10-6), then the middle/posterior cingulate and precuneus (cluster-wise p = 1.02 × 10-5), followed by the rp-insula (cluster-wise p = .02) and the superior/middle occipital gyrus (cluster-wise p = .03). In green we plotted the regions associated with ICS score, including the PCC and precuneus (cluster-wise p = .0001). The subscore ICS–failed control related to alcohol cue reactivation in a similar pattern to ICS total score.
Figure 2.
Clusters with brain activation associated with behavioral assessments. Top panel: Clusters during the alcohol cues. Red regions are correlated with the Alcohol Use Disorders Identification Test (AUDIT) total score, including the posterior cingulate cortex (PCC), precuneus, and right posterior insula (rp-insula). Yellow regions are correlated with the AUDIT–alcohol consumption, including the right precuneus and middle occipital gyrus. Blue regions are correlated with the Alcohol Dependence Scale–loss of behavioral control (ADS-lbc) score, including the right fusiform gyrus, middle/posterior cingulate, precuneus, rp-insula, and superior/middle occipital gyrus. Green regions are correlated with the ICS score, including the PCC and precuneus. Bottom panel: Clusters during the smoking cues. Red regions are correlated with the Fagerström Test of Nicotine Dependence (FTND) score, including the anterior cingulate and medial frontal gyrus. Blue regions are correlated with the ADS-lbc score, including the left and right posterior insula, PCC, and left superior frontal gyrus. Green regions are correlated with the Impaired Control Scale (ICS) score, including the anterior cingulate, medial frontal gyrus, and rp-insula. When the regions overlap, only the top color is shown.
Smoking cue reactivity in clusters of brain regions significantly, positively correlated with FTND, ADS, and ICS total scores but not AUDIT. In the bottom panel of Figure 2, we plotted the regions associated with FTND in red, regions associated with ADS-lbc in blue (although ADS total score was associated with similar regions, regions associated with ADS-lbc were broader and more significant), and regions associated with ICS in green. The anterior cingulate and medial frontal gyrus were associated with the FTND score (cluster-wise p = 9.77 × 10-5) and ICS total score (cluster-wise p = .007). The ICS total score also related to the rp-insula (cluster-wise p = .04). The ICS subscores of attempted control and perceived control were associated with similar brain regions as the ICS total score. Both the left and right posterior insula were associated with the ADS-lbc (cluster-wise p = .001 [left], .0001 [right]), followed by the PCC (cluster-wise p = .0004) and the left superior frontal gyrus (cluster-wise p = .05).
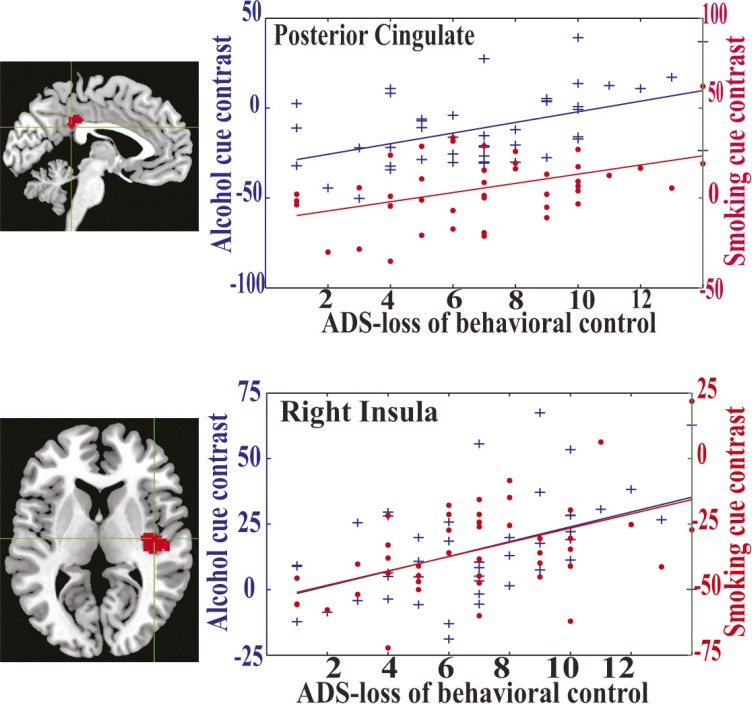
Among the clusters, only the PCC (Figure 3, top panel) and rp-insula (Figure 3, bottom panel) were associated with behavioral assessments while responding to both alcohol and smoking cues. These two regions were significantly associated with the ADS score, in particular the ADS-lbc subscore. All voxels in these two regions were positively correlated with the ADS-lbc while responding to both substance cues. To illustrate clearly, we selected one peak voxel from each region and plotted their activation (after regressing out age and gender effects) against the ADS-lbc score. In Figure 3, blue markers and lines present activation in alcohol cues and the slopes associated with the ADS-lbc, whereas red markers and lines are for smoking cues. In details, the activation of the peak voxel in the PCC was significantly correlated with the ADS-lbc, with r = .48 (p = .0009 uncorrected for voxel-wise tests) in alcohol cues and r = .44 (p = .002 uncorrected) in smoking cues, but the correlation between them was not significant, with r = .18. Similarly, activation of the rp-insula was significantly correlated with the ADS-lbc, with r = .48 (p = .0009 uncorrected) in alcohol cues and r = .51 (p = .0004 uncorrected) in smoking cues, but not significantly cross-correlated between them (r = .26).
Figure 3.
Brain activation of the peak voxels in the posterior cingulate cortex (PCC) and right posterior insula during both alcohol and smoking cues. Top panel presents the location of the PCC and its activation. Bottom panel presents the location of the right posterior insula and its activation. The x-axis shows the Alcohol Dependence Scale–loss of behavioral control (ADS-lbc) scores. The y-axes are contrast values of either alcohol versus baseline or smoking versus baseline. Blue markers and lines present activation with alcohol cues and slopes associated with the ADS-lbc, whereas red markers and lines are for smoking cues.
Discussion
Both alcohol and smoking cues elicited activation from large numbers of brain regions in these 45 subjects. Figure 1a presents the alcohol cue–induced significant, most positive, activations compared with the baseline, mainly from the medial, superior, and middle frontal gyri; anterior, posterior, and middle cingulate; precuneus, cuneus, and superior parietal gyrus; insula; thalamus; parahippocampal gyrus; superior and middle temporal gyri; and middle and inferior occipital gyri. Smoking cues elicited both positive and negative activations as shown in Figure 1b. Positive activation was mainly located in the superior, middle, and inferior occipital gyri; fusiform and lingual gyri; inferior and middle frontal gyri; superior and middle temporal gyri; precuneus, cuneus, and superior parietal gyrus; and parahippocampal gyrus. The negative activation regions mainly include the anterior, posterior and middle cingulate; medial, superior, and middle frontal gyri; insula; caudate; thalamus; claustrum; and precuneus and inferior parietal lobule. Clearly, there are differences between brain responses to alcohol and smoking cues. Part of the differences may come from the different presentation of cues, such as large positive activation in the occipital lobe because of the visual presentation of smoking videos. The large negative activation to the smoking cues partially reflects the large default mode network responses. The reason we did not observe such large default mode network responses in the alcohol cues may be because the baseline for the alcohol cue is the liquid delivery phase (vs. the swallow and taste phase) instead of fixation in the smoking cues. Other differences, for instance, distinct active regions to alcohol or smoking cues in Figure 1c, may result from the specific brain response to smoking or alcohol. Further investigation is necessary to confirm this. Here, we focus on the regions responding to both cues as plotted in Figure 1d, aiming at a better understanding of the neural mechanism underlying concurrent use of substances.
Our results confirmed that a significant number of brain regions respond to both alcohol and smoking cues. All the regions have been reported in previous studies on alcohol (Drobes, 2002; Filbey et al., 2008; George et al., 2001; Park et al., 2007; Tapert et al., 2004) and smoking cue reactivity (David et al., 2005; Lee et al., 2005; Lim et al., 2005; Zhao et al., 2012). Not surprisingly, the most reliable cue-reactivity regions as stated in recent meta-analyses (Engelmann et al., 2012; Schacht et al., 2013) were identified, including the striatum, medial prefrontal cortex, and anterior cingulate, with each playing a major role in the dopaminergic reward network. The dopamine mesocorticolimbic pathway has been widely recognized as contributing to the motivation and reinforcing processes, thus leading to the development of dependence common to many substances (Franken et al., 2005; Volkow et al., 2011; Wise, 2009). In addition, the cognitive control network is another major player in substance dependence (Hutchison, 2010; Volkow et al., 2011) and influences the choice to drink/smoke or not to drink/smoke. Regions in the frontal lobe participate in the cognitive control network, including those most commonly observed, the medial prefrontal, orbitofrontal, and dorsal lateral prefrontal. The precuneus and cuneus are involved in many functions and are also part of the default mode network that anti-correlates with active tasks. In particular, the precuneus increases activation when individuals with alcohol use problems make impulsive decisions (Claus et al., 2011b). A third network recently hypothesized to be involved in substance dependence is the “salience network.” This network regulates attention allocation and strikes a balance between the incentive reward network and control network (Seeley et al., 2007; Sutherland et al., 2012; Vollstädt-Klein et al., 2012). Key areas in this network include, but are not limited to, the anterior and posterior cingulate, insula, and thalamus (Filbey et al., 2008; George et al., 2001; Park et al., 2007; Seeley et al., 2007; Sutherland et al., 2012; Vollstädt-Klein et al., 2012). These three networks are interconnected and appear to respond together to both substance cues in our data.
The association tests further narrow activated voxels to the clusters with activation specifically related to certain behavioral assessments. Results indicate the specificity of the AUDIT score to alcohol cue reactivity and the FTND score to smoking cue reactivity, as the AUDIT score only relates to brain responses to alcohol cues, not smoking cues, and the FTND score only relates to brain responses to smoking cues, not alcohol cues. In contrast, some behavior scores more likely affect brain responses to both substance cues. For instance, the ICS score is related to brain activation of the PCC responding to alcohol cues and activation of the anterior cingulate and rp-insula responding to smoking cues. The ADS-lbc score is related to brain activation of much broader areas to both cues. The broader area and stronger correlation of the ADS-lbc versus the ADS total score with brain activation suggest that the subscore of the ADS-lbc may be the true contributing (underlying) factor instead of the ADS total score. Therefore, we focused on the ADS-lbc. In particular, the PCC and rp-insula while responding to both cues are related to the ADS-lbc. In other words, the ADS-lbc is associated with brain response in the PCC and rp-insula regardless of alcohol or smoking cues.
We have to note that the PCC is associated with not only the ADS-lbc but also the AUDIT and ICS in alcohol cue reactivity. Similarly, the rp-insula is correlated with the AUDIT in alcohol cue reactivity and ICS in smoking cue reactivity, in addition to the ADS-lbc. But the connection with the ADS-lbc for both the PCC and rp-insula is most significant while responding to both cues. Given that the ADS-lbc, ICS, and AUDIT scores are all highly correlated in our sample, the relative strength of the ADS-lbc for the PCC and rp-insula indicates that the ADS-lbc is most likely a more accurate factor associated with brain responses in the PCC and rp-insula. The ADS-lbc score reflects impaired control over drinking, specifically impaired physical control, including pass out, black out, stumble, stagger, and weave. Our findings suggest that the level of impaired physical control influences or is reflected in brain activation of the PCC and rp-insula regardless of substance cues.
This implication is further confirmed by checking the relation of individual voxel activation with ADS-lbc scores. Figure 3 shows that the ADS-lbc is significantly associated with the PCC and rp-insula when responding to both smoking and alcohol cues. More interestingly, activation in the PCC or rp-insula is not directly cross-correlated between the alcohol and smoking cues, even though they all relate to the ADS-lbc score. It suggests that a loss of behavioral control may act as a common modulator to these regions (i.e., the responses to both alcohol and smoking cues are affected by the level of impaired control) while at the same time they exhibit substance-specific properties. Previous studies have reported that alcohol can cause a craving for nicotine (Epstein et al., 2007; Erblich et al., 2009; Sayette et al., 2005), in line with our findings about the impaired control effect on the brain, in particular on brain responses to smoking cues.
When we articulate the function of the PCC and insula within the context of the dependence model, we found evidences suggesting PCC and insula participating in the salience network. These two regions, despite consistent findings showing their activation in the cue reactivity studies (Filbey et al., 2008; George et al., 2001; Park et al., 2007), have been historically overlooked within the drug-dependence literature, largely because they are not known to be a direct target of the dopamine system. This bias has begun to change given the increasing evidence of the control network and salience network in addition to the dopamine reward network. Also, the PCC is a major node in the default mode network of the brain interacting with awareness, emotion, and memory (Kang et al., 2012; Leech et al., 2012; Maddock et al., 2003). As Kang et al. (2012) reported in their smoking cue fMRI study, the PCC and other regions are associated with the attentional bias to smoking-related cues rather than cue-induced craving and smoking urges. A recent impulsivity study indicates that the PCC is related to the ability to resist cigarette craving, and this highlights a need for more emphasis on the PCC in drug-dependence research. The insula, a key region for emotion, homeostasis, and self-awareness (Craig, 2003, 2009; Pollatos et al., 2007; Stein et al., 2007), has been hypothesized to have a significant role in interoceptive awareness. In particular, the posterior insula together with the middle-posterior cingulate gyrus is likely a part of the general salience and action network involving environmental monitoring, response selection, and skeletomotor body orientation (Craig, 2003; Taylor et al., 2009). The functional impact of the insula on dependence has been clearly demonstrated in studies of individuals with lesions to the insula, who show much higher rates of successful smoking cessation relative to individuals with lesions in other regions (Naqvi et al., 2007). Thus, the insula may have a casual role in maintaining dependence through its representation of bodily experiences associated with withdrawal and craving (Naqvi and Bechara, 2009). Furthermore, a rodent study showed that the anterior and posterior regions of the insula play a crucial role in the long-term memory associated with drug use and confirmed the previously found role of the posterior insula in the perception of drug craving (Contreras et al., 2012). Together, the PCC and posterior insula may influence attention and salience functions and regulation of incentive reward and cognitive control, which in turn may influence the concurrent use and comorbidity of alcohol and nicotine dependence.
Several limitations must be considered when interpreting the results presented here. Clearly, design differences between our alcohol and smoking cue paradigms have an impact on the data, as one involves the taste of alcohol and the other involves watching a smoking video. Such differences may trigger different brain responses, such as a large visual region activated in the smoking cue. Even with such a limitation, the common regions between both contrasts unquestionably carry the potential to be related to the addictive mechanism for both substances, in particular given that the association between brain activation and the ADS-lbc exists independent of designs. A future study using similar task designs for both substances will be able to verify and refine our results. In addition, the small sample size and no control samples also limit our ability to identify the associated brain regions. Yet, a direct comparison of the ADS, AUDIT, FTND, and ICS scores still provides information about the relative strength and spatial location of the impact of impaired control on brain function. In summary, our results confirmed a significant amount of regional intersection of the fMRI responses to alcohol and smoking cues. This large intersection suggests that the craving responses for both alcohol and smoking are coupled. In particular, impaired control for alcohol may have a substantial effect on the craving for both substances by modulating brain responses to cues in the PCC and posterior insula, likely affecting the function of salience networks.
Acknowledgments
The authors thank Dr. David Boutte for his initial data organization and preprocessing.
Footnotes
This work was supported by National Institutes of Health Grants R33DA027626 (to Jingyu Liu), R01EB005846 (to Vince D. Calhoun), and R01AA012238 (to Kent E. Hutchison).
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, bold response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011a;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcoholism: Clinical and Experimental Research. 2011b;35:1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Billeke P, Vicencio S, Madrid C, Perdomo G, Gonzalez M, Torrealba F. A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology. 2012;37:2101–2108. doi: 10.1038/npp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD., (Bud) Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD., (Bud) How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Research & Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Becoña E, Gurpegui M, Jurado D, Gonzalez-Pinto A. Exploring brief measures of nicotine dependence for epidemiological surveys. Addictive Behaviors. 2003;28:1481–1486. doi: 10.1016/s0306-4603(02)00264-2. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcoholism: Clinical and Experimental Research. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol and Alcoholism. 2007;42:174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Cinciripini PM. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Erblich J, Montgomery GH, Bovbjerg DH. Script-guided imagery of social drinking induces both alcohol and cigarette craving in a sample of nicotine-dependent smokers. Addictive Behaviors. 2009;34:164–170. doi: 10.1016/j.addbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Carter CS, Brooks S, May C, Fissell K, Stenger VA. The evaluation of brain activity in response to taste stimuli—A pilot study and method for central taste activation as assessed by event-related fMRI. Journal of Neuroscience Methods. 2003;131:99–105. doi: 10.1016/s0165-0270(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Franken IH, Booij J, van den Brink W. The role of dopamine in human addiction: From reward to motivated attention. European Journal of Pharmacology. 2005;526:199–206. doi: 10.1016/j.ejphar.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Childress AR. Effects of varenicline on smoking cue–triggered neural and craving responses. Archives of General Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Lê AD. Biological processes underlying co-use of alcohol and nicotine: Neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Research & Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Bierut LJ. Co-occurring risk factors for alcohol dependence and habitual smoking: Update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Research & Health. 2006;29:172–178. [PMC free article] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, Zamir R. Development of a scale for measuring impaired control over alcohol consumption: A preliminary report. Journal of Studies on Alcohol. 1993, November;54:700–709. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: A validation study. Alcoholism: Clinical and Experimental Research, 37, Supplement. 2013;1:E253–E259. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- Kang OS, Chang DS, Jahng GH, Kim SY, Kim H, Kim JW, Chae Y. Individual differences in smoking-related cue reactivity in smokers: An eye-tracking and fMRI study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;38:285–293. doi: 10.1016/j.pnpbp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Angstadt M, Phan KL. Neural substrates of alcohol-induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacology. 2010;35:692–701. doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology. 2009;207:107–117. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Applied Psychophysiology and Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. Journal of Neuroscience. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Pae CU, Joo RH, Yoo SS, Choi BG, Kim DJ, Lee CU. fMRI investigation on cue-induced smoking craving. Journal of Psychiatric Research. 2005;39:333–335. doi: 10.1016/j.jpsychires.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: The insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol and Alcoholism. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and Biobehavioral Reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychology of Addictive Behaviors. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addiction Biology. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-44. Rockville, MD: HHS Publication No. (SMA) 12–4713; 2012. Author. Retrieved from: http://www.samhsa.gov/data/NSDUH/2k11Results/NSDUHresults2011.htm. [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addictive Behaviors. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Kiefer F. Validating incentive salience with functional magnetic resonance imaging: Association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction Biology. 2012;17:807–816. doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends in Neurosciences. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grüsser SM, Klein S, Diener C, Hermann D, Flor H, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. European Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Tian J, Wang W, Qin W, Shi J, Li Q, Lu L. The role of dorsal anterior cingulate cortex in the regulation of craving by reappraisal in smokers. PLoS ONE. 2012;7(8):e43598. doi: 10.1371/journal.pone.0043598. [DOI] [PMC free article] [PubMed] [Google Scholar]