Abstract
Objective:
Given the evidence that several cognitive and emotional functions are impaired in adult alcohol-dependent patients and the possibility that some of these deficits are transmitted to their children, the objective of the present study was to test the hypothesis that the perception of complex mental states would be reduced in young adults from families with a positive family history of alcohol dependence. It was also anticipated that social-perceptual deficits would confer unique predictive ability beyond that shared with other cognitive risk factors for alcohol dependence and/or substance use risk.
Method:
Data from 301 youth ages 18–21 years, recruited from an ongoing community longitudinal study of alcoholic and matched control families, were analyzed. Family history of alcohol dependence as well as alcohol-dependence diagnosis in the youth was based on diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. A substance use risk factor measured early problem alcohol/other drug use. The perception of mental states was measured with the computerized version of the Reading the Mind in the Eyes Test (RMET).
Results:
Children of alcohol-dependent parents did not show impairment in the mental states perception task, nor did social perception skills predict alcohol dependence in the youth. Correlational analysis performed between RMET and the substance use risk factor showed no significant association between the variables.
Conclusions:
The study results do not confirm the hypothesis that behaviorally measured social perception impairment is more prevalent in the children of alcohol-dependent parents. In addition, social-perceptual deficits were not a unique marker of either alcohol dependence or high risk for alcohol dependence in this young adult sample.
Studies have consistently reported cognitive-function deficits in alcohol-dependent individuals (Knight and Longmore, 1994), with considerable impairment in specific cognitive domains including visuospatial (Beatty et al., 1996; Parsons and Nixon, 1993) and executive function (Crews and Boettiger, 2009; Dao-Castellana et al., 1998). Some of these neuropsychological deficits—for example, verbal ability (Sher et al., 1991), IQ (Poon et al., 2000; Puttler et al., 1998), and executive function (Nigg et al., 2004)—may be transmitted to children of alcohol-dependent parents, leading to theories of risk for substance use disorders that are transmitted via poor cognitive function.
Studies also have shown evidence for impaired cognitive processing of simple emotions in alcohol-dependent patients (Clark et al., 2007; Kornreich et al., 2001a, 2001b, 2002, 2003; Philippot et al., 1999; Townshend and Duka, 2003). Because poor neuropsychological function may be a risk factor for substance use disorders that are transmitted from parent to child, we hypothesized that poor perception of social information may likewise be a risk factor for substance use disorders and may be transmitted from parent to child, whether biologically or environmentally. Alcohol-dependent patients are confronted with severe interpersonal problems in their daily functioning (Nixon et al., 1992). Both biological and environmental factors have potential impact on social functioning (Brüne and Brüne-Cohrs, 2006); thus, within alcoholic families, specific biological predisposition as well as an adverse family environment may create a disadvantageous background for development of social cognitive skills. Indeed, some studies have found impaired emotional processing in children of alcohol-dependent parents (Glahn et al., 2007; Heitzeg et al., 2008; Miranda et al., 2002).
Of special interest is the ability to ascribe complex mental states (desires, beliefs, feelings, intentions) to oneself and to other people to make predictions about other peoples’ behavior in social situations (e.g., Premack and Woodruff, 1978; Wellman, 1990), called theory of mind. It is generally acknowledged that theory of mind comprises two component processes. The social-perceptual component is the ability to decode others’ mental states based on observable social information (e.g., a photograph of the eye region). The social-cognitive component is the ability to predict or explain others’ actions on the basis of the perceived knowledge about others’ intentions, emotions, beliefs, or desires (Tager-Flusberg and Sullivan, 2000). Adequate decoding of complex mental states is a precondition for good social and interpersonal adaptation and could be impaired in alcohol dependence, yet data on theory of mind in alcohol dependence are scarce and inconsistent. Three studies have assessed the social-perceptual component in abstinent alcohol-dependent patients and healthy controls using the Reading the Mind in the Eyes Test (RMET; Baron-Cohen et al., 2001). One study found no significant between-group difference in social perception (Mátyássy et al., 2006), whereas in the other two, deficits were reported in recognition of mental states in alcohol dependent subjects compared with healthy controls (Maurage et al., 2011; Thoma et al., 2013). In another study testing the social-cognitive component of theory of mind, Uekermann et al. (2007) found impairment in the ability to understand others’ mental states among alcohol-dependent patients compared with healthy controls.
Even less is known about the relationship between theory of mind and risk for developing alcohol dependence or alcohol-related problems. Research with normally developing children indicates that individual differences in theory of mind are influenced by intergenerational transmission (e.g., Hughes and Cutting, 1999; Sabbagh and Seamans, 2008). To our knowledge, there has been only one study in which the theory of mind social-perceptual component was measured with the RMET in young adults at high genetic risk for developing alcohol dependence (Hill et al., 2007). In this study, adolescents/young adults from alcoholic families were not different in RMET performance in comparison with controls but presented reduced blood oxygen level–dependent response in brain regions previously found to be active during theory of mind task performance.
Given the discrepancies in findings among previous studies but the importance of the issue in terms of emotional and social impairments in alcohol dependence, examination of whether such differences could be detected precursively in a young, high-risk sample is of considerable importance. The present investigation was designed with this in mind, using a sample of young adults whose emotional and social processing had not yet been distorted by the long history of heavy drinking and conflict that is present in adult treatment samples. To the extent that differences in social perception could be detected between young adults from families with versus those without a positive family history of alcohol dependence would suggest such impairments were precursive to the disorder, not a result of it. If such differences are not found in such an unconfounded sample, it would raise significant questions about the validity of the emotional/social information processing hypothesis. These differences should be present above and beyond those deficits created by other impairments to cognitive capability (e.g., IQ, substance use).
Method
Participants
The present work is part of the Michigan Longitudinal Study, an ongoing multiwave prospective study (Zucker et al., 1996, 2000) following a community sample of families with high levels of alcohol use disorders and other drug use, along with a community contrast sample of families drawn from the same neighborhoods but without the high substance use profile. Although not drawn from a treatment population, the sample is designed to overrepresent youth at high risk for substance misuse and substance use disorders by targeting offspring of alcoholic fathers. For a more detailed description, see Zucker et al. (2000). Children and both parents were assessed extensively at home following the initial recruitment (Wave 1, original target child ages 3–5 years), with the assessment repeated every 3 years using parallel measures. Data presented here are from the offspring at Wave 6 (i.e., 15 years into the study; the first wave with available relevant data) when the youth were ages 18–21 years. The data are from 301 youth who had completed the RMET. Exclusion criteria at study recruitment included the presence of fetal alcohol syndrome and autism. None of the subjects was diagnosed with schizophrenia, schizophreniform disorder, or schizoaffective disorder.
Measures
The social-perceptual component of theory of mind—the focus of this study—was measured with a computerized version of the RMET. Photographs of the eye region of 28 different faces (male and female) expressing a complex mental state—all standardized to one size (15 cm × 10 cm), all black and white, with the same region of the face selected for each photo (from midway along the nose to just above the eyebrow)—were presented with four adjectives displayed around the photograph. Subjects had to decide which adjective best described what the person in the photograph was feeling. Decision time was unlimited; however, participants were instructed to answer as fast as possible (reaction times were not recorded). A glossary presenting a brief definition of each adjective was available if needed. The first item was used as an example. Because prior work suggested that variance in mental states recognition in the REMT task depends on the emotional valence of the items, except for the global score (RMETtotal), three additional valence scores were calculated (following a validated RMET item valence classification; Harkness et al., 2005). Positive emotions score (RMETpositive) was calculated as a percentage of correct responses for positive emotion items, negative emotions score (RMETnegative) was percentage correct for negative emotion items, and nonemotional mental states (RMETneutral) was percentage correct for neutral items. The version of the RMET used was slightly modified from the original: 8 of the original 36 photos were omitted to decrease subject burden (a large number of assessment measures are completed at each wave). The photos deleted were items that showed little discriminability across several different general population groups and/or were repetitious in terms of the feelings/thoughts they portrayed (Baron-Cohen et al., 2001).
Psychosocial and clinical measures
Family history of alcohol dependence was based on the Wave 5 lifetime diagnosis of alcohol dependence of either the mother or father, according to criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994). A positive family history of alcohol dependence was found for 202 subjects, and 99 had a negative family history of alcohol dependence. Current psychiatric diagnosis, including the diagnosis of alcohol dependence of the youth, was based on Wave 6 DSM-IV criteria and assessed with the Diagnostic Interview Schedule–Child (Costello et al., 1984). A total of 25 subjects fulfilled the criteria for alcohol dependence (ADs); in 276 subjects, the alcohol-dependence diagnosis was negative (non-ADs). To assess whether early problem alcohol/other drug use that may be present before dependence has developed, a substance use risk factor (SRF) was used. SRF was calculated as a composite score that includes the following five components: onset of drinking by age 14, onset of drunkenness by age 15, onset of marijuana use by age 14, onset of illicit drug use other than marijuana by age 16, high number (above the 70th percentile) of drinking-related problems by age 17. The value range of SRF was 0–5. Scores were prorated when one or more factors were missing.
Subjects were divided based on the median split of the SRF score into low-risk (score: 0 and 1) and high-risk groups (score: 2–5). Number of drinking-related problems was assessed using the self-report Drinking and Drug History Form for Children/Youth, a child/adolescent version of the Drinking and Drug History Form for Adults (Zucker and Fitzgerald, 1994; Zucker et al., 1990). The Beck Depression Inventory II (Beck et al., 1996) was used to measure current depressive symptoms. Intelligence was measured as an average of WAIS-IQ from Waves 2, 3, and 4. Internalizing and externalizing behavior problems were assessed with the Youth Self-Report form of the Child Behavior Checklist (Achenbach, 1991).
Analytic approach
Between-group differences were assessed with Student’s t test or analysis of variance (ANOVA). Pearson linear correlation analyses were performed to identify associations between normally distributed continuous variables. Impact of potentially confounding variables including IQ, demographic (e.g., age, gender) and clinical variables (externalizing and internalizing behavior problems, severity of depressive symptoms), and psychiatric comorbidity (major depressive episode, major depressive disorder, bipolar disorder, social phobia, antisocial personality disorder, nicotine dependence, marijuana and other cannabis dependence) on RMET performance was assessed by correlation or ANOVA. All significant variables in the primary correlation analyses were included in analysis of covariance (ANCOVA) comparing RMET performance in high and low substance use risk groups. The data from alcohol-dependent subjects were excluded when high versus low SRF groups were compared.
The criterion for statistical significance in all tests was p < .05. The data were analyzed using SPSS Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL).
Results
There was no difference between males and females in RMETtotal, t(299) = 1.62, p = .11, RMETneutral, t(299) = 0.59, p = .55, RMETnegative, t(299) = 1.15, p = .25, and RMETpositive, t(299) = 2.03, p = .06, performance. Positive correlations between IQ and RMETtotal (r = .44, p < .0005), RMETneutral (r = .32, p < 00005), RMETnegative (r = .31, p < .0005), and RMETpositive (r = .26, p < .0005) performance were observed. Correlation and ANOVA analyses showed no effect of psychiatric comorbidity, externalizing behavior problems, internalizing behavior problems, age, or severity of depressive symptoms on RMET performance.
There were no differences in RMET (total, neutral, negative, positive) performance between those with a positive family history of alcohol dependence and those with a negative family history of alcohol dependence; however, significant between-group differences were revealed for IQ, education, externalizing problems, and internalizing problems (Table 1). One-way ANOVAs showed no differences between ADs and non-ADs for age, education, and IQ, as well as in RMET (total, neutral, negative, positive) performance; however there was a between-group difference in severity of depressive symptoms (Table 1). Correlational analysis performed between RMET (total, neutral, negative, positive) and SRF showed no significant association between the variables. There was a negative correlation between IQ and SRF (r = -.21, p <.0005).
Table 1.
Demographic, clinical characteristics and Reading the Mind in the Eyes Test (RMET) results of the family history–positive (FH+), family history–negative (FH−), alcohol-dependent (AD), and non–alcohol-dependent (non-AD) subjects
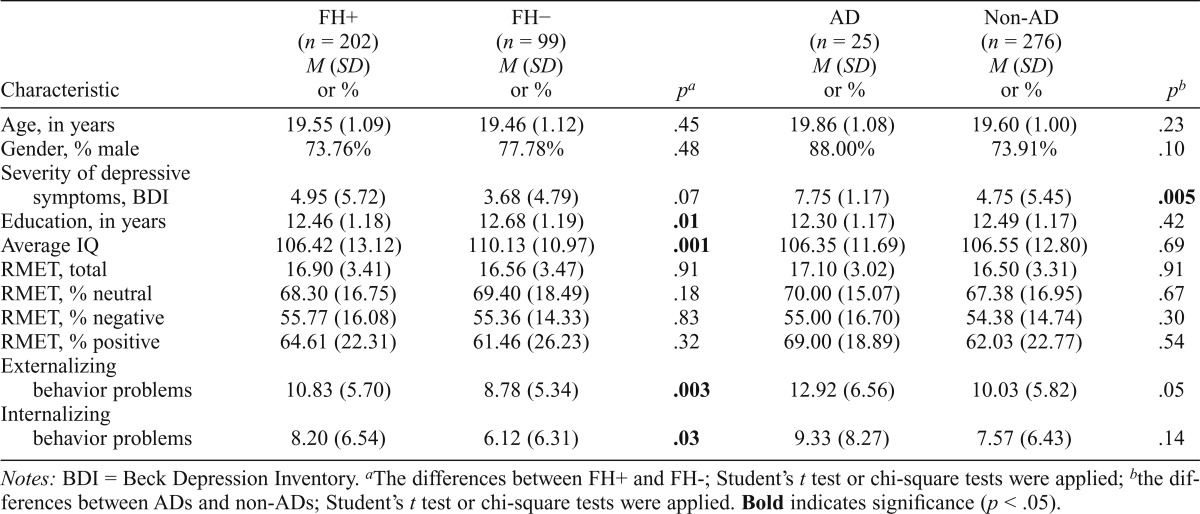
| Characteristic | FH+ (n = 202) M (SD) or % | FH− (n = 99) M (SD) or % | Pa | AD (n = 25) M (SD) or % | Non-AD (n = 276) M (SD) or % | pb |
| Age, in years | 19.55 (1.09) | 19.46 (1.12) | .45 | 19.86 (1.08) | 19.60 (1.00) | .23 |
| Gender, % male | 73.76% | 77.78% | .48 | 88.00% | 73.91% | .10 |
| Severity of depressive symptoms, BDI | 4.95 (5.72) | 3.68 (4.79) | .07 | 7.75 (1.17) | 4.75 (5.45) | .005 |
| Education, in years | 12.46 (1.18) | 12.68 (1.19) | .01 | 12.30 (1.17) | 12.49 (1.17) | .42 |
| Average IQ | 106.42 (13.12) | 110.13 (10.97) | .001 | 106.35 (11.69) | 106.55 (12.80) | .69 |
| RMET, total | 16.90 (3.41) | 16.56 (3.47) | .91 | 17.10 (3.02) | 16.50 (3.31) | .91 |
| RMET, % neutral | 68.30 (16.75) | 69.40 (18.49) | .18 | 70.00 (15.07) | 67.38 (16.95) | .67 |
| RMET, % negative | 55.77 (16.08) | 55.36 (14.33) | .83 | 55.00 (16.70) | 54.38 (14.74) | .30 |
| RMET, % positive | 64.61 (22.31) | 61.46 (26.23) | .32 | 69.00 (18.89) | 62.03 (22.77) | .54 |
| Externalizing behavior problems | 10.83 (5.70) | 8.78 (5.34) | .003 | 12.92 (6.56) | 10.03 (5.82) | .05 |
| Internalizing behavior problems | 8.20 (6.54) | 6.12 (6.31) | .03 | 9.33 (8.27) | 7.57 (6.43) | .14 |
Notes: BDI = Beck Depression Inventory.
The differences between FH+ and FH-; Student’s t test or chi-square tests were applied;
the differences between ADs and non-ADs; Student’s t test or chi-square tests were applied. Bold indicates significance (p < .05).
Additional analysis was performed to compare the high and low substance use risk groups. ANOVA revealed significant between-group differences in RMETtotal, F(1, 274) = 4.04, p = .04; RMETneutral, F(1, 274) = 5.67, p = .02 performance, as well as in IQ, F(1, 246) = 7.53, p = .007. The RMETtotal and RMETneutral between-group differences were no longer significant when IQ was accounted for in the ANCOVA analyses.
Discussion
We did not find any evidence that the perception of complex mental states is impaired in children of parents with alcohol dependence over and above what could be explained by differences in IQ. Consistent with previous data from our sample, the primary cognitive risks appear to be in the intelligence (Noll et al., 1992; Poon et al., 2000; Puttler et al., 1998) and executive function domains (Nigg et al., 2004). As found by Hill et al. (2007), our large sample of high-risk adolescents/young adults from alcohol-dependent families was not different in RMET performance in comparison with controls. At the same time, based on the brain-behavior interaction findings reported by Hill et al. (2007), the unimpaired behavioral performance might be achieved through different processing strategies. Additional or alternative brain networks might be involved in the processing of social stimuli in children of alcoholics compared with healthy controls, giving enough resources to perform the task properly. Studies using functional imaging methods are necessary to test this hypothesis. Some other neuroimaging studies have found impaired emotional processing in children of alcohol-dependent parents. Looking at the small sample of young adults with no diagnosis, Glahn and colleagues (2007) observed reduced amygdala response in reaction to fearful faces in participants with positive family history of alcoholism who were also disinhibited. A comparison between children of alcoholics who were showing signs of risky alcohol use and children of alcoholics who were not revealed a dissociable neural activation pattern in response to stimuli with emotional valence, suggesting involvement of different functional structures responsible for processing of emotions in the two groups (Heitzeg et al., 2008).
It was further anticipated that, irrespective of the family history of alcoholism, social-perceptual skills would uniquely mark a predisposition to develop alcohol dependence. Among those at risk, social-perceptual deficits might be revealed when they are exposed to alcohol, exposed to other drugs, or develop alcohol dependence. Contrary to our expectations, we did not find differences in mental states perception among youth who had already developed alcohol dependence or those who were at high risk compared with those who were not. Neither positive family history of alcoholism nor other significant comorbidities influenced this relationship. There have been studies showing that alcoholic patients demonstrate deficits in the accurate labeling of the simple emotions displayed on faces and overestimate the emotional intensity of emotional stimuli compared with normal controls (e.g., Kornreich et al., 2001a, 2001b; Philippot et al., 1999). The processing of more complex social information as measured by RMET has been tested in only three studies, with conflicting results. Mátyássy et al. (2006) did not find RMET performance impairment in a sample of alcohol-dependent patients with at least 6 months of abstinence. In contrast, Maurage and colleagues (2011) found RMET impairment in the recognition of positive and negative emotions but not neutral mental states in early abstinent alcohol-dependent patients when compared with healthy controls. Thoma et al. (2013) found RMET performance deficit in alcohol-dependent subjects undergoing inpatient detoxification between the fourth and fourteenth day of treatment. These differences in results could be explained by the variability of alcohol-dependent sample characteristics. Our data are unique in that they come from a nonclinical sample of young adults. As the previous studies were of clinical groups of adult alcohol-dependent individuals, it seems plausible that the impairment seen on the applied social perception measure may have occurred as a consequence of prolonged alcohol drinking. It can be speculated that RMET performance would still predict more severe alcoholism that has not yet had time to emerge. This is most probably because, even though the young adults have begun to drink and become dependent, they still have not yet engaged in years of heavy drinking. Our results also provide evidence against the hypothesis that the differences in social perception are the consequence of the inherent vulnerability of subjects that may be manifested as they develop alcohol dependence. Further longitudinal data are needed to reveal the dynamic changes that may occur in the alcohol-dependence group as they drink heavily and get older.
There is one significant limitation to the present study. In particular, the RMET test captures only the social-perceptual component of theory of mind. As other social processing abilities may be compromised in alcohol-dependence risk populations, additional tests that cover the social-cognitive component of theory of mind would allow a better examination of the potential social behavioral differences in this group. The present work does not address this issue.
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants R37 AA-07065 (to Robert A. Zucker) and R01 AA12217 (to Robert A. Zucker and Mary M. Heitzeg), and by Fogarty International Center/National Institute on Drug Abuse Grant D43 TW05818 (to Robert A. Zucker).
References
- Achenbach TM. Manual for the Youth Self-Report Form and 1991 Profile. Burlington, VT: University Associates in Psychiatry; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:241–251. [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. Journal of Studies on Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8683962&dopt=Abstract. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brüne M, Brüne-Cohrs U. Theory of mind—evolution, ontogeny, brain mechanisms and psychopathology. Neuroscience and Biobehavioral Reviews. 2006;30:437–455. doi: 10.1016/j.neubiorev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A, Edelbrook C, Dulcan M, Kalas R, Klanc S. Development and Testing of the NIMH Diagnostic Interview Schedule for Children in a Clinic Population. Rockville, MD: Center for Epidemiological Studies, National Institute of Mental Health; 1984. [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry, and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychological Medicine. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: Studies from the Oklahoma family health patterns project. Biological Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Sabbagh MA, Jacobson JA, Chowdrey NK, Chen T. Enhanced accuracy of mental state decoding in dysphoric college students. Cognition and Emotion. 2005;19:999–1025. [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WYW, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: Differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism: Clinical and Experimental Research. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcoholism: Clinical and Experimental Research. 2007;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Cutting AL. Nature, nurture, and individual differences in early understanding of mind. Psychological Science. 1999;10:429–432. [Google Scholar]
- Knight RG, Longmore BE. Cognitive impairment in alcoholics. In: Knight RG, Longmore BE, editors. Clinical neuropsychology of alcoholism. London, England: Lawrence Erlbaum Associates; 1994. pp. 225–265. [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Dan B, Foisy ML, Hess U, Verbanck P. Impaired emotional facial expression recognition in alcoholism compared with obsessive-compulsive disorder and normal controls. Psychiatry Research. 2001a;102:235–248. doi: 10.1016/s0165-1781(01)00261-x. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noël X, Streel E, Verbanck P. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. Journal of Studies on Alcohol. 2001b;62:533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Foisy ML, Philippot P, Dan B, Tecco J, Noël X, Verbanck P. Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Research. 2003;119:251–260. doi: 10.1016/s0165-1781(03)00130-6. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Verbanck P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol and Alcoholism. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Mátyássy A, Kelemen O, Sárközi Z, Janka Z, Kéri S. Recognition of complex mental states in patients with alcoholism after long-term abstinence. Alcohol and Alcoholism. 2006;41:512–514. doi: 10.1093/alcalc/agl045. [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noël X, Joassin F, Hanak C, Verbanck P, Philippot P. The “Reading the Mind in the Eyes” test as a new way to explore complex emotions decoding in alcohol dependence. Psychiatry Research. 2011;190:375–378. doi: 10.1016/j.psychres.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Buchanan TW, Lovallo WR. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2002;26:441–448. [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. Journal of Abnormal Psychology. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Parsons OA. Interpersonal problem-solving in male and female alcoholics. Alcoholism: Clinical and Experimental Research. 1992;16:684–687. doi: 10.1111/j.1530-0277.1992.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Noll RB, Zucker RA, Fitzgerald HE, Curtis WJ. Cognitive and motoric functioning of sons of alcoholic fathers and controls: The early childhood years. Developmental Psychology. 1992;28:665–675. [Google Scholar]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurologic Clinics. 1993;11:205–218. [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Verbanck P. Alcoholics’ deficits in the decoding of emotional facial expression. Alcoholism: Clinical and Experimental Research. 1999;23:1031–1038. [PubMed] [Google Scholar]
- Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: Differences related to subtypes of familial alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1020–1027. [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences. 1978;1:515–526. [Google Scholar]
- Puttler LI, Zucker RA, Fitzgerald HE, Bingham CR. Behavioral outcomes among children of alcoholics during the early and middle childhood years: Familial subtype variations. Alcoholism: Clinical and Experimental Research. 1998;22:1962–1972. [PubMed] [Google Scholar]
- Sabbagh MA, Seamans EL. Intergenerational transmission of theory-of-mind. Developmental Science. 2008;11:354–360. doi: 10.1111/j.1467-7687.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Sullivan K. A componential view of theory of mind: Evidence from Williams syndrome. Cognition. 2000;76:59–90. doi: 10.1016/s0010-0277(00)00069-x. [DOI] [PubMed] [Google Scholar]
- Thoma P, Winter N, Juckel G, Roser P. Mental state decoding and mental state reasoning in recently detoxified alcohol-dependent individuals. Psychiatry Research. 2013;205:232–240. doi: 10.1016/j.psychres.2012.08.042. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: Alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Channon S, Winkel K, Schlebusch P, Daum I. Theory of mind, humour processing and executive functioning in alcoholism. Addiction. 2007;102:232–240. doi: 10.1111/j.1360-0443.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Wellman HM. The child’s theory of mind. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, Sanford K. Other evidence for at least two alcoholisms II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology. 1996;8:831–848. [Google Scholar]
- Zucker RA, Fitzgerald HE. Drinking and Drug History Form for Children/Youth. Ann Arbor, MI: University of Michigan Department of Psychiatry, Addiction Research Center; 1994. [Google Scholar]
- Zucker RA, Fitzgerald HE, Noll RB. Drinking and Drug History (Revised Edition, Version 4) Ann Arbor, MI: University of Michigan Department of Psychiatry, Addiction Research Center; 1990. [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zuckerman BS, editors. Children of addiction: Research, health, and public policy issues. New York, NY: RoutledgeFalmer; 2000. [Google Scholar]


