Abstract
Background
Discordance between small aortic valve area (AVA; < 1.0 cm2) and low mean pressure gradient (MPG; < 40 mm Hg) affects a third of patients with moderate or severe aortic stenosis (AS). We hypothesized that this is largely due to inaccurate echocardiographic measurements of the left ventricular outflow tract area (LVOTarea) and stroke volume alongside inconsistencies in recommended thresholds.
Methods
One hundred thirty-three patients with mild to severe AS and 33 control individuals underwent comprehensive echocardiography and cardiovascular magnetic resonance imaging (MRI). Stroke volume and LVOTarea were calculated using echocardiography and MRI, and the effects on AVA estimation were assessed. The relationship between AVA and MPG measurements was then modelled with nonlinear regression and consistent thresholds for these parameters calculated. Finally the effect of these modified AVA measurements and novel thresholds on the number of patients with small-area low-gradient AS was investigated.
Results
Compared with MRI, echocardiography underestimated LVOTarea (n = 40; −0.7 cm2; 95% confidence interval [CI], −2.6 to 1.3), stroke volumes (−6.5 mL/m2; 95% CI, −28.9 to 16.0) and consequently, AVA (−0.23 cm2; 95% CI, −1.01 to 0.59). Moreover, an AVA of 1.0 cm2 corresponded to MPG of 24 mm Hg based on echocardiographic measurements and 37 mm Hg after correction with MRI-derived stroke volumes. Based on conventional measures, 56 patients had discordant small-area low-gradient AS. Using MRI-derived stroke volumes and the revised thresholds, a 48% reduction in discordance was observed (n = 29).
Conclusions
Echocardiography underestimated LVOTarea, stroke volume, and therefore AVA, compared with MRI. The thresholds based on current guidelines were also inconsistent. In combination, these factors explain > 40% of patients with discordant small-area low-gradient AS.
Résumé
Introduction
La discordance entre une surface valvulaire aortique rétrécie (SVA; < 1,0 cm2) et un faible gradient de pression moyen (GPM; < 40 mm Hg) touche un tiers des patients souffrant d’une sténose aortique (SA) modérée ou grave. Nous avons posé l’hypothèse que ceci est grandement dû aux mesures échocardiographiques inexactes de la surface de la chambre de chasse du ventricule gauche (surface de la CCVG) et au volume systolique de même qu’à l’incohérence des seuils recommandés.
Méthodes
Cent trente-trois (133) patients souffrant de SA légère à grave et 33 témoins ont subi une échocardiographie complète et une imagerie cardiovasculaire par résonance magnétique (ICRM). Le volume systolique et la surface de la CCVG ont été calculés à l’aide de l’échocardiographie et de l’ICRM, et les effets sur l’estimation de la SVA et les mesures du GPM ont alors été modelés à l’aide de la régression non linéaire et les seuils cohérents de ces paramètres ont été calculés. Finalement, l’effet de ces mesures modifiées de la SVG et des nouveaux seuils sur le nombre de patients ayant une SA à surface rétrécie et à faible gradient a été examiné.
Résultats
Comparativement à l’ICRM, l’échocardiographie a sous-estimé la surface de la CCVG (n = 40; −0,7 cm2; intervalle de confiance [IC] à 95 %, −2,6 à 1,3), les volumes systoliques (−6,5 ml/m2; IC à 95 %, −28,9 à 16,0) et, conséquemment, la SVA (−0,23 cm2; IC à 95 %, −1,01 à 0,59). De plus, une SVA de 1,0 cm2 correspondait à un GPM de 24 mm Hg selon les mesures échocardiographiques et de 37 mm Hg après la correction des volumes systoliques issus de l’ICRM. Selon les mesures traditionnelles, 56 patients avaient une SA à surface rétrécie et à faible gradient. À partir des volumes systoliques issus de l’ICRM et des seuils révisés, une réduction de la discordance de 48 % a été observée (n = 29).
Conclusions
L’échocardiographie a sous-estimé la surface de la CCVG, le volume systolique et, par conséquent, la SVA comparativement à l’ICRM. Les seuils des lignes directrices actuelles étaient également incohérents. Combinés, ces facteurs expliquent la raison pour laquelle > 40 % des patients souffrent d’une SA dont la surface rétrécie et le faible gradient sont discordants.
Discordant small aortic valve area (AVA; < 1.0 cm2), low mean pressure gradient (MPG; < 40 mm Hg) aortic stenosis occurs in approximately 30% of patients with aortic stenosis evaluated using echocardiography.1, 2 This has classically been attributed to patients with low flow states, such as those with reduced left ventricular (LV) ejection fractions.3 However, in recent years, it has been recognized that small-area low-gradient aortic stenosis can also be observed in the presence of a preserved ejection fraction: so-called “paradoxical low-flow, low-gradient severe aortic stenosis.” The outcomes associated with such patients have been variable in different studies,4, 5, 6, 7 presumably reflecting a heterogeneous population and highlighting the uncertainty with regard to the actual severity of aortic stenosis in this subgroup.
Using the continuity equation, the AVA is calculated based on the ratio between the Doppler stroke volume and the post-aortic valve flow. Doppler stroke volume relies crucially on accurate estimation of the LV outflow tract (LVOT) area (LVOTarea) according to the formula: Doppler stroke volume = LVOTarea × LVOT flow. On 2-dimensional echocardiography, the LVOTarea is derived from LVOT diameter measurements made on the parasternal long-axis view and the assumption that the LVOT is circular. However, recent experience from transcatheter aortic valve replacement sizing has demonstrated that the LVOT is frequently elliptical and not circular, and as a consequence, measurements made using echocardiography underestimate the true LVOTarea.8, 9 The implication is therefore that echocardiography might also underestimate the true LV stroke volume and AVA.
In addition, it is widely acknowledged that the severity thresholds for AVA and MPG recommended in current guidelines are inherently inconsistent,1, 10 with theoretical models suggesting an AVA of 1.0 cm2 corresponds more closely to a MPG of 30-35 mm Hg than the recommended threshold of 40 mm Hg.10, 11
We hypothesized that the combination of LVOTarea underestimation and inconsistent thresholds might influence the classification of aortic stenosis severity, and contribute to the number of patients with discordant small-area low-gradient aortic stenosis. The aims of the study were first to compare stroke volume estimation using echocardiography with the gold standard noninvasive cardiovascular magnetic resonance imaging (MRI) assessment and to establish the optimal thresholds for severe aortic stenosis. Subsequently we then sought to investigate whether correcting for these 2 factors might affect the number of patients with discordant small-area low-gradient aortic stenosis.
Methods
Study participants
Patients with mild to severe aortic stenosis were prospectively recruited from the Edinburgh Heart Centre, and control individuals without aortic stenosis were recruited from the local community. We excluded patients with other significant valvular heart disease (moderate to severe), contraindications to MRI, and cardiomyopathies (inherited or acquired).
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local ethics committee. Written informed consent was obtained from all subjects.
Echocardiography
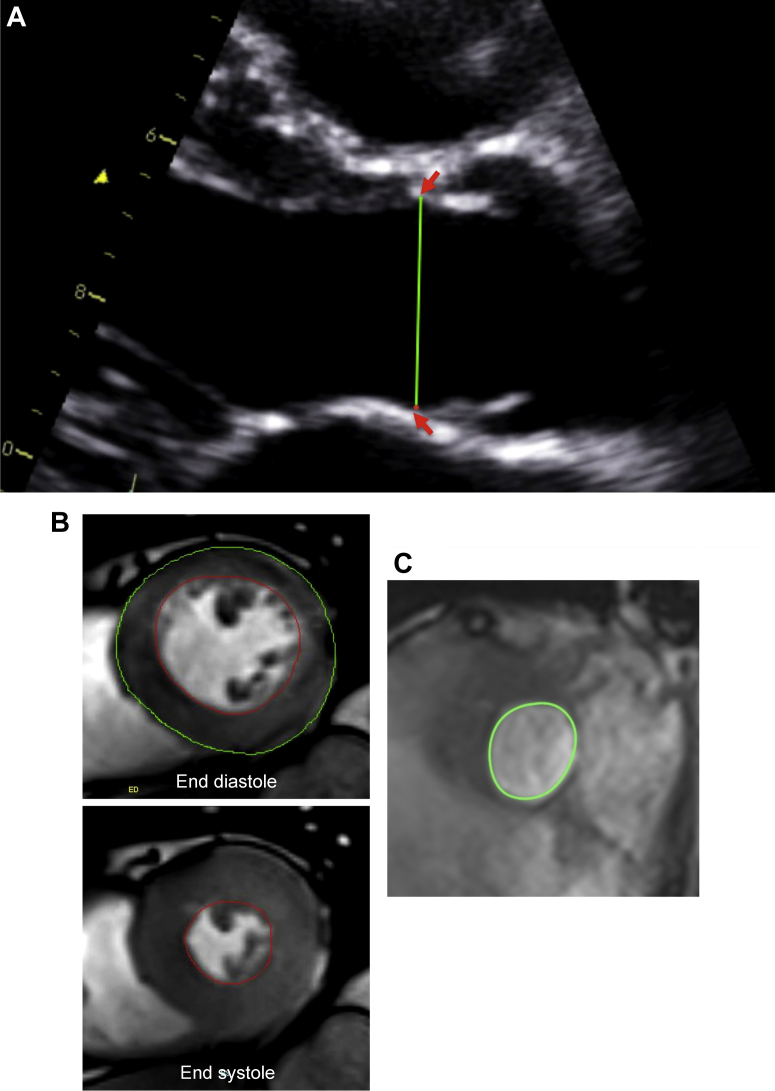
Transthoracic echocardiography was performed in all patients (iE33, Philips Medical Systems, Best, The Netherlands) by a research ultrasonographer (A.C.W.), and a cardiologist trained in echocardiography (C.W.L.C.). The severity of aortic stenosis was assessed according to the American College of Cardiology/American Heart Association guidelines. Specifically, severe aortic stenosis was defined as an AVA of < 1 cm2 and MPG > 40 mm Hg.12 In the parasternal long-axis view, the LVOT diameter was measured at the insertion of the aortic cusps, from the inner edge of the septal endocardium to the inner edge of the anterior mitral leaflet in midsystole (Fig. 1A), because the cross-sectional shape is believed to be more circular at this level.3 LVOT velocity-time integral was measured in the apical 5-chamber view using pulsed-wave Doppler just proximal to the aortic valve. We were careful to obtain a laminar spectral tracing, avoiding contamination from flow across the aortic valve.
Figure 1.
Estimation of left ventricular outflow tract (LVOT) area using echocardiography and magnetic resonance imaging. (A) The LVOT diameter was measured at the aortic cusp insertion points (red arrows) in the parasternal long axis view. The LVOT area was estimated from the diameter measured. (B) The stroke volume was calculated as the difference between end-diastolic and end-systolic volumes. Planimetry of the endocardial borders (red contours in end-diastolic and end-systolic frames) was performed including the papillary muscles and minor trabeculations in volume measurements during both phases of the cardiac cycle. Left ventricular mass was calculated by multiplying the total end-diastolic myocardial volume (green and red contours in the end-diastolic frame) by the specific gravity of the myocardium (1.05 g/mL). Papillary muscles and minor trabeculations were excluded in mass measurements, with care taken to avoid right ventricular trabeculations. (C) Planimetry of the LVOT area in the coaxial short axis view on cardiovascular magnetic resonance imaging at mid-systole.
The peak aortic jet velocity and MPG were derived from the aortic valve velocity-time integral, using continuous-wave Doppler. The highest aortic jet velocity and MPG was determined in multiple acoustic windows using standard S51 and D2cwc probes (Philips Medical Systems), and corroborated by the 2 operators. The mean of 3 readings (5 if the patient had atrial fibrillation) was recorded. Doppler stroke volume was estimated (LVOTarea × LVOT velocity-time integral) and used to calculate the AVA with the continuity equation (stroke volume/aortic valve velocity-time integral). Normal stroke volume using echocardiography was defined as ≥ 35 mL/m2.13 In a further analysis, we had also estimated stroke volume according to the Teichholz method14 and the effects on aortic stenosis classification.
The severity of aortic valve calcification was assessed in the short-axis view of the aortic valve using a score of 1-4,15 and corroborated between the 2 operators. Valvuloarterial impedance, a measure of global afterload, was calculated as (systolic blood pressure + MPG)/MRI stroke volume.
MRI
All participants underwent MRI at 3T (Magnetom Verio, Siemens AG, Healthcare Sector, Erlangen, Germany). Cine images were acquired using a balanced steady-state free precision sequence in the short-axis of the left ventricle extending from the atrioventricular ring to the apex (8-mm parallel slices with 2-mm spacing). The endocardial borders were planimetered in end-diastole and end-systole to quantify ventricular volumes and function (Argus, Siemens AG, Healthcare Sector). Papillary muscles and minor trabeculations were included in the volume measurements during both phases of the cardiac cycle as previously described (Fig. 1B).16, 17 Stroke volume was measured as the difference between the end-diastolic and end-systolic LV volumes (in the absence of significant mitral regurgitation), and indexed to body surface area. Normal indexed LV volumes, stroke volumes, and ejection function were defined using sex- and age-specific ranges.18 LV mass was calculated from the total end-diastolic myocardial volume multiplied by the specific gravity of the myocardium (1.05 g/mL).
In 40 patients, additional coaxial short-axis cine slices were acquired from the level of the aortic valve. The LVOTarea was planimetered at the base of the aortic valve (the slice at which all 3 cusps were first observed to disappear) in midsystole and comparisons were made with the LVOTarea estimated from the LVOT diameter on 2-dimensional echocardiography (Fig. 1C).
Curve-fitting and statistical analysis
In patients with normal stroke volumes, the relationship between AVA and MPG was modelled according to the Gorlin equation: AVA = c/√MPG (GraphPad Prism 5, GraphPad Software Inc, San Diego, CA). No rules were set for the initial value for the modelling parameter, c. We generated 2 curve-fitting models with AVA derived using Doppler stroke volume and MRI stroke volume.
The distribution of all continuous variables was assessed for normality using the Shapiro-Wilk test, and presented as either mean ± SD or median (interquartile range). Comparison was performed using the Student t test or analysis of variance with post hoc Bonferroni adjustment. The Mann-Whitney U and Kruskal-Wallis with post hoc Dunn tests were used for nonparametric data. Categorical variables were expressed in percentages and compared using the χ2 test. The correlation between continuous data was assessed with the Pearson correlation and presented as r2 values. Comparison between echocardiographic and MRI indices of stroke volume, LVOTarea, and AVA was assessed using the Bland-Altman analysis. Fixed and proportional biases with 95% limits of agreement were reported. A 2-sided P < 0.05 was considered statistically significant.
Results
A total of 133 patients with mild to severe aortic stenosis (AVA, 0.98 ± 0.40 cm2; MPG, 33 ± 20 mm Hg; peak aortic velocity, 3.8 ± 0.9 m/s) and 33 control individuals were recruited. The median interval between echocardiography and MRI was 9 (interquartile range, 5-29) days. Compared with control individuals, patients with aortic stenosis had greater ejection fraction rates (64 ± 4% and 67 ± 7%, respectively; P = 0.02) despite similar LV end-diastolic volumes (75 ± 13 mL/m2 and 72 ± 16 mL/m2, respectively; P = 0.34) and stroke volumes (47 ± 8 mL/m2 and 48 ± 10 mL/m2, respectively; P = 0.59) (Table 1 and Supplemental Table S1).
Table 1.
Baseline characteristics of patients with aortic stenosis and control individuals∗
| Control individuals (n = 33) | Aortic stenosis (n = 133) | P | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, mean years ± SD | 54 ± 23 | 68 ± 12 | < 0.01 |
| Male sex, n (%) | 18 (55) | 89 (67) | 0.40 |
| Hypertension, n (%) | 9 (27) | 85 (64) | < 0.01 |
| Diabetes mellitus, n (%) | 0 | 18 (14) | — |
| Coronary artery disease, n (%) | 3 (9) | 44 (33) | 0.01 |
| Atrial fibrillation, n (%) | 0 | 3 (2) | — |
| Echocardiography | |||
| LVOT diameter, cm | 2.05 ± 0.17 | 2.07 ± 0.24 | 0.66 |
| LVOT cross-sectional area, cm2 | 3.30 ± 0.55 | 3.39 ± 0.85 | 0.60 |
| LVOT velocity time integral, cm | 20.9 ± 3.7 | 23.5 ± 4.4 | 0.01 |
| Doppler stroke volume, mL | 70 ± 19 | 79 ± 19 | < 0.01 |
| Doppler stroke volume (indexed), mL/m2 | 38 ± 8 | 42 ± 10 | < 0.01 |
| Aortic valve area, cm2 | 2.36 ± 0.59 | 0.98 ± 0.40 | < 0.01 |
| Aortic valve area (indexed), cm2/m2 | 1.26 ± 0.26 | 0.52 ± 0.21 | < 0.01 |
| Mean pressure gradient, mm Hg | 4 ± 1 | 33 ± 20 | < 0.01 |
| Peak aortic velocity, m/s | 1.4 ± 0.2 | 3.8 ± 0.9 | < 0.01 |
| Dimensionless index | 0.72 ± 0.10 | 0.28 ± 0.09 | < 0.01 |
| Aortic valve calcium score, median (IQR) | 1 (1, 1) | 3 (3, 4) | < 0.01 |
| Valvuloarterial impedance, mm Hg/mL/m2 | 3.2 ± 0.7 | 4.0 ± 1.0 | 0.34 |
| End-diastolic volume, mL† | 93 ± 25 | 87 ± 26 | 0.17 |
| End-diastolic volume (indexed), mL/m2† | 50 ± 13 | 46 ± 13 | 0.12 |
| End-systolic volume, mL† | 41 ± 14 | 38 ± 14 | 0.29 |
| End-systolic volume (indexed), mL/m2† | 22 ± 7 | 20 ± 7 | 0.16 |
| Stroke volume, mL† | 51 ± 16 | 49 ± 14 | 0.48 |
| Stroke volume (indexed), mL/m2† | 28 ± 8 | 26 ± 7 | 0.16 |
| Ejection fraction, %† | 56 ± 9 | 57 ± 7 | 0.49 |
| Mild mitral regurgitation, n (%) | 2 (6) | 19 (14) | 0.37 |
| Mild aortic regurgitation, n (%) | 2 (6) | 57 (43) | < 0.01 |
| Cardiovascular Magnetic Resonance Imaging | |||
| End-diastolic volume, mL | 140 ± 32 | 135 ± 35 | 0.47 |
| End-diastolic volume (indexed) (EDVi), mL/m2 | 75 ± 13 | 72 ± 16 | 0.34 |
| End-systolic volume, mL | 51 ± 15 | 46 ± 18 | 0.14 |
| End-systolic volume (indexed), mL/m2 | 27 ± 7 | 24 ± 9 | 0.08 |
| Stroke volume, mL | 89 ± 19 | 90 ± 22 | 0.81 |
| Stroke volume (indexed), mL/m2 | 47 ± 8 | 48 ± 10 | 0.59 |
| Ejection fraction, % | 64 ± 4 | 67 ± 7 | 0.02 |
| Left ventricular mass (indexed) (LVMi), g/m2 | 67 ± 15 | 89 ± 22 | < 0.01 |
| LVMi/EDVi, g/mL | 0.90 ± 0.13 | 1.25 ± 0.26 | < 0.01 |
EDVi, indexed end diastolic volume; IQR, interquartile range; LVMi, indexed left ventricular mass; LVOT, left ventricular outflow tract.
Characteristics of patients with aortic stenosis were classified based on aortic valve area estimated using Doppler-derived stroke volume presented in Supplemental Table S1.
Estimated using the Teichholz formula.
Doppler and cardiac MRI stroke volume
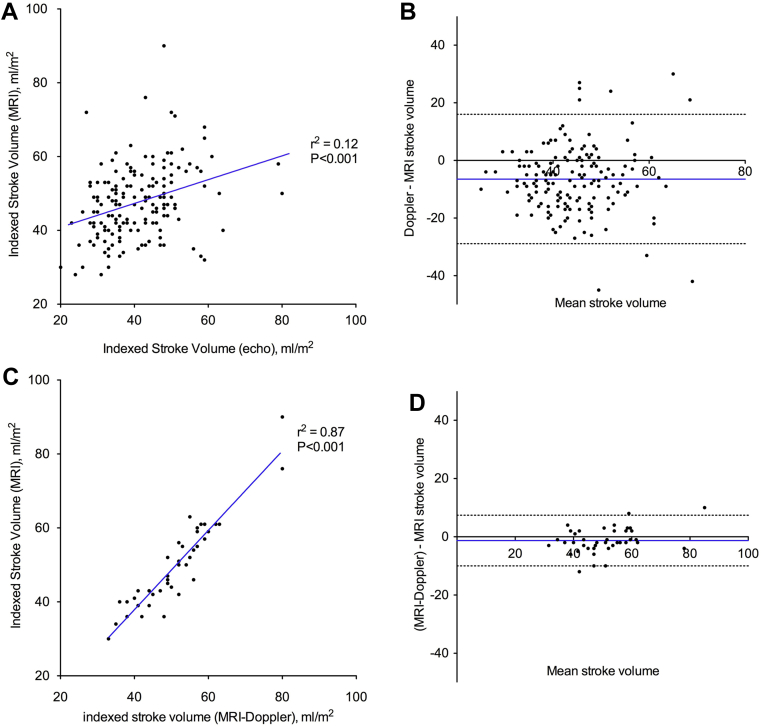
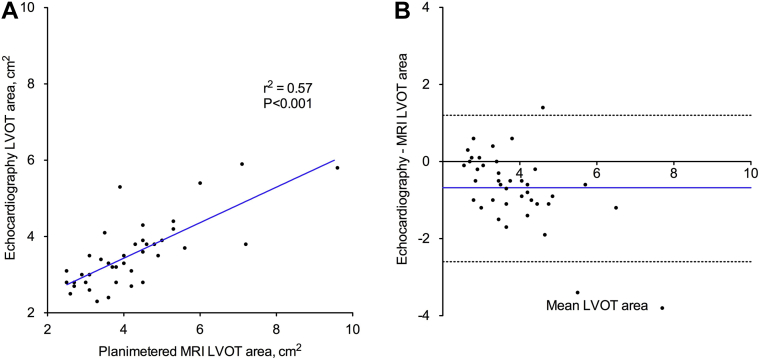
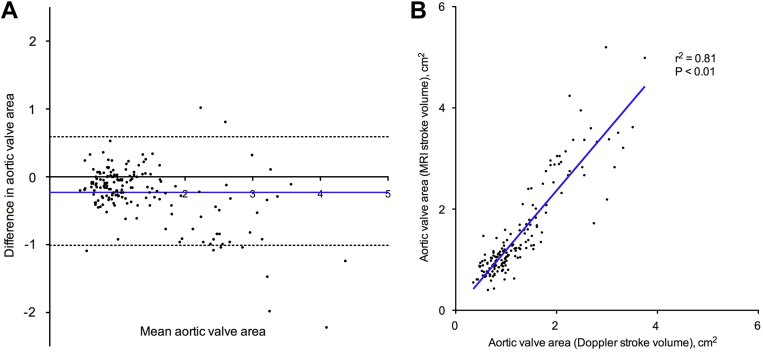
Doppler stroke volume correlated only weakly with MRI stroke volume measurements (r2 = 0.12; P < 0.001; Fig. 2A) and underestimated the stroke volume by > 6 mL/m2 compared with MRI (−6.5 mL/m2; 95% confidence interval [CI], −28.9 to 16.0 mL/m2; Fig. 2B). Similar results were observed after excluding the 19 patients in the cohort with mild mitral regurgitation (r2 = 0.14; P < 0.001; mean difference, −6.1 mL/m2; 95% CI, −28.2 to 16.0 mL/m2). This in part appears to be due to underestimation of the LVOTarea using echocardiography compared with planimetered LVOTarea measurements (−0.7 cm2; 95% CI, −2.6 to 1.3 cm2; Fig. 3). Indeed, when we subsequently recalculated stroke volume using the planimetered LVOTarea, an excellent correlation with MRI stroke volumes was observed (r2 = 0.87; P < 0.001; Fig. 2C) without significant fixed or proportional biases (−1.3 mL/m2; 95% CI, −9.9 to 7.3 mL/m2; Fig. 2D). Moreover, this effect translated into an underestimation of the AVA calculated using echocardiography-derived stroke volumes compared with MRI-measured stroke volumes (−0.23 cm2; 95% CI, −1.01 to 0.59 cm2; Fig. 4). As previously described, the explanation for echocardiographic underestimation of the LVOTarea appears related to its elliptic shape. Indeed, the mean ellipticity ratio (ratio of the maximum to minimum LVOT diameter) was 1.2 ± 0.1, with only 28% of these patients having a circular LVOT (defined as ellipticity ratio of 1.0). Of note, we achieved excellent intraobserver (r2 = 1.00; P < 0.001; mean difference 0.5 ± 2.7%) and interobserver (r2 = 0.98; P < 0.001; mean difference 1.1 ± 5.4%) agreement in the planimetered LVOT measurements using MRI.
Figure 2.
Stroke volume correlation and Bland-Altman analysis. Doppler stroke volume correlated weakly with magnetic resonance imaging (MRI) stroke volume (A), with a fixed bias and wide limits of agreement (B). In 40 patients, stroke volume was calculated using planimetered left ventricular outflow tract area on MRI and Doppler left ventricular outflow tract flow (MRI-Doppler). This approach demonstrated excellent correlation with MRI stroke volume (C), without significant bias (D).
Figure 3.
Left ventricular outflow tract (LVOT) area correlation and Bland-Altman analysis. Although LVOT area estimated using echocardiography demonstrated a moderate correlation with planimetered LVOT area on magnetic resonance imaging (A), the echocardiographic LVOT area underestimated the planimetered area with wide limits of agreement (B).
Figure 4.
Aortic valve area corrleation and Bland-Altman analysis. Aortic valve area estimated using Doppler stroke volume and magnetic resonance imaging-derived stroke volume demonstrated poor agreement and significant underestimation (A), despite excellent correlation (B).
Consistent AVA and MPG cutoffs
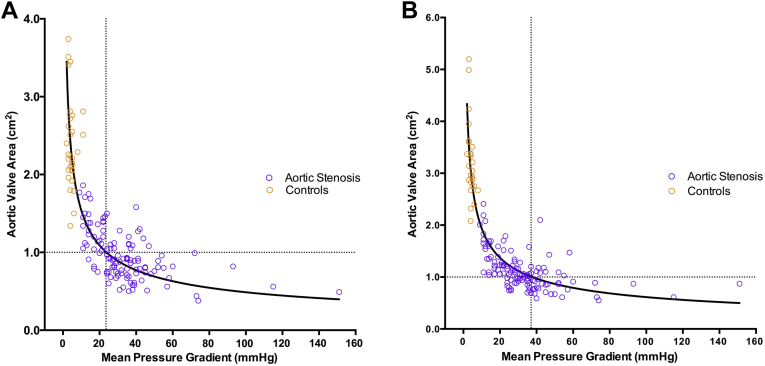
Based on measurements derived from Doppler stroke volume, an MPG of 40 mm Hg corresponded to an AVA of 0.77 cm2, and an AVA of 1.0 cm2 corresponded to an MPG of only 24 mm Hg (AVA = 4.85/√MPG; r2 = 0.73; Fig. 5A). When MRI stroke volume measurements were used to calculate the AVA, an MPG of 40 mm Hg corresponded to an AVA of 0.97 cm2 and an AVA of 1.0 cm2 corresponded to an MPG of 37 mm Hg (AVA = 6.13/√MPG; r2 = 0.81; Fig. 5B).
Figure 5.
Relationship between aortic valve area and mean pressure gradient. The aortic valve area was calculated from the continuity equation using Doppler stroke volume. An aortic valve area of 1.0 cm2 corresponded to a mean pressure gradient of 24 mm Hg (A). Correcting these values using the magnetic resonance imaging stroke volume, an aortic valve area of 1.0 cm2 corresponded to a mean pressure gradient of 37 mm Hg (B).
Discordant small-area low-gradient aortic stenosis
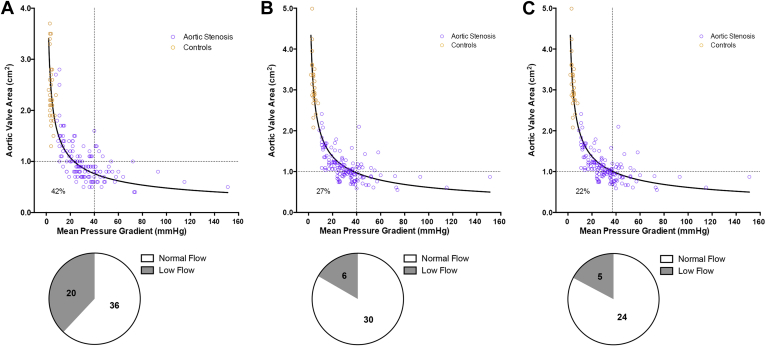
Using the conventional echocardiographic estimation of MPG and AVA, and the thresholds for severe disease based on current guidelines (AVA, 1.0 cm2 and MPG, 40 mm Hg),12, 19 56 patients with aortic stenosis (42%) had discordant small-area low-gradient aortic stenosis (Fig. 6A).
Figure 6.
Reclassification of aortic stenosis severity. Using traditional echocardiographic measurements and the recommended severity cutoffs established in current guidelines (A), 56 patients had discordant small-area low-gradient aortic stenosis. Twenty patients were reclassified to concordant nonsevere aortic stenosis when cardiovascular magnetic resonance imaging stroke volume was used to estimate aortic valve area (B). A further 7 patients were reclassified as having concordant severe disease using the revised thresholds of 1.0 cm2 and 37 mm Hg (C). The corresponding pie charts show the flow states in patients with discordant small-area low-gradient aortic stenosis (stroke volume estimated using cardiovascular magnetic resonance imaging).
Using a stepwise approach, we first assessed the effect of using AVA measurements derived from MRI stroke volumes on this proportion of patients with discordant small-area low-gradient aortic stenosis. This resulted in 20 patients being reclassified as having nonsevere aortic stenosis (median aortic valve calcium score of 3; valvuloarterial impedance, 3.7 ± 0.7 mm Hg/mL/m2), leaving 36 with small-area low-gradient aortic stenosis (Fig. 6B). Subsequently, when we used the revised thresholds already described herein (AVA of 1.0 cm2 and MPG of 37 mm Hg), a further 7 patients were reclassified with severe disease (all had aortic valve calcium score of 4 and valvuloarterial impedance of 4.5 ± 1.2 mm Hg/mL/m2). This left only 29 patients with discordant small-area low-gradient aortic stenosis, a reduction of 48% compared with the original classification (Fig. 6C). Of these, 3 patients had impaired systolic function and 2 had a low stroke volume due to small LV cavity volumes. The remainder appeared to consist of patients with moderate to severe disease with values for a wide range of parameters that were intermediate between concordant moderate and severe disease (Supplemental Table S2). This included the aortic valve calcium score, which was 3 in 48% and 4 in 52% of patients.
Stroke volume estimation and aortic stenosis classification using the Teichholz formula
In a further analysis, we assessed an alternate echocardiographic method for estimating stroke volumes using the Teichholz formula.14 Results were similar, with the correlation between echocardiography-estimated and MRI-derived stroke volumes remaining weak (r2 = 0.16; P < 0.001), and 51% of patients classified as having discordant small-area low-gradient aortic stenosis.
Discussion
In this study, we have systematically demonstrated that echocardiography underestimates the LVOTarea, the LV stroke volume, and as a consequence, the AVA. Moreover we have demonstrated that there are inconsistencies in the guideline thresholds of severity with an AVA of 1.0 cm2 corresponding to an MPG of 24 mm Hg based on standard echocardiographic measures and 37 mm Hg when MRI-derived stroke volumes are used. Finally we have shown that if we correct for these 2 factors using the more accurate MRI estimation of the stroke volume to calculate AVA and revised thresholds, more than 40% of the patients with small-area low-gradient aortic stenosis were reclassified as having concordant measurements.
Two-dimensional and Doppler echocardiography assessments are the predominant methods used worldwide to assess the severity of aortic stenosis. However, the echocardiographic estimation of the AVA relies on accurate measurement of stroke volume. Unfortunately as demonstrated in this study, echocardiography frequently underestimated the stroke volume compared with the noninvasive gold-standard measurements made using MRI. As a consequence, echocardiography would also appear to underestimate the AVA. Our data provide explanations for these observations. A subgroup of 40 patients had coaxial short-axis cine images of their LVOT. This allowed accurate and reproducible planimetered measurements of the LVOTarea to be compared with the derived measurements made using 2-dimensional echocardiographic diameter measurements. Similar to previous studies,8, 9 we have demonstrated that such echocardiographic measures underestimate the true LVOTarea in part because of the fact that the LVOT is frequently elliptical not circular. Indeed, when Doppler stroke volumes were corrected using the more accurate planimetered measurements of the LVOTarea, a good correlation with MRI-derived stroke volumes was subsequently observed. Further to our analyses, we have also explored using other echocardiographic indices such as indexed AVA and the dimensionless index. Unfortunately, these techniques were also associated with inherent limitations related to the LVOTarea measurements (see the Evaluation of Aortic Stenosis Classification Using Indexed Aortic Valve Area and the Dimensionless Index section of the Supplementary Material).
Inconsistencies in the MPG and AVA thresholds recommended in the current guidelines are well described.1, 10, 20 Consistent with previous reports,1, 10 our echocardiography data confirmed an AVA of 1.0 cm2 corresponded to a MPG of only 24 mm Hg, significantly lower than the threshold of 40 mm Hg stated in current guidelines. Interestingly, this improved to 37 mm Hg when MRI stroke volume measurements were used to calculate AVA, much closer to the recommended threshold although still discrepant.
Multiple previous studies have shown that a third of patients with moderate and severe aortic stenosis have discordant disease severity according to their AVA and MPG values. Interest has surrounded this group because of its ubiquity and the uncertainty in the outcome associated with these patients. Although some studies have suggested that patients with small-area low-gradient aortic stenosis have a prognosis similar to those with moderate disease, others have indicated the exact opposite and that their outcomes are more akin to those with severe disease.4, 6, 7, 21
In the final part of the study, we investigated whether the underestimation of the AVA using echocardiography and inconsistencies in the guideline thresholds might explain the ubiquity of patients with small-area low-gradient aortic stenosis and help resolve the true severity of their disease. We demonstrated that correcting for these 2 factors reduced the number of patients with a small-area low-gradient by > 40%. Of the remaining 29 subjects, 3 had low flow due to an impaired ejection fraction and 2 had low flow due to small LV cavity size. The remainder appeared to genuinely sit on the borderline between moderate and severe disease with parameters that were intermediate between those observed in concordant severe and nonsevere groups. Our data would therefore indicate that discordance in the assessment of aortic stenosis severity can be reduced by correcting for AVA underestimation and inconsistent thresholds, but further studies are now needed to investigate the long-term outcomes of patients reclassified using this approach.
Limitations
In this study, assessment of the planimetered LVOTarea using MRI was only available in 40 patients. However, this was believed to be a large enough sample size to assess the inaccuracies associated with LVOT diameter measurements and the data are consistent with the large and expanding literature investigating LVOTarea measurements for the sizing of transcatheter aortic valve bioprostheses.9 Moreover, the baseline characteristics were similar between these 40 patients and the entire cohort of patients with aortic stenosis (see the Baseline Characteristics of the 40 Patients With Planimetered Left Ventricular Outflow Tract Area on Cardiovascular Magnetic Resonance section of the Supplementary Material). We also used echocardiography to assess aortic valve calcification. Although this provides important prognostic information,15 computed tomography provides a more sensitive quantification of aortic valve calcification and has recently been shown to provide differentiation as to the true severity of patients with small-area low-gradient aortic stenosis.2, 22 Phase contrast MRI is an alternate method to estimate stroke volume, but this technique is associated with its own problems, particularly in patients with aortic stenosis in whom complex aortic flow in the ascending aorta can result in measurement inaccuracy. This is a particular problem at 3T. However, in an exploratory analysis, we demonstrated excellent correlation and agreement between phase contrast and volumetric stroke volume on MRI (see the Comparison of Doppler, MRI Volumetric, and Phase Contrast Stroke Volume Estimation section of the Supplementary Material). Finally, we were not able to perform echocardiography and MRI on the same day because many of our elderly patients could not tolerate both procedures at the same visit. However, no patient experienced any cardiac events or changes in medications between the 2 scans and after correcting for inaccuracies in the LVOTarea, an excellent agreement was observed between MRI and echocardiography-derived stroke volumes. This would argue against any significant variability in stroke volumes between the scans.
Conclusions
Echocardiography underestimated the AVA because of an underestimation of the LVOTarea and stroke volume, compared with MRI. These factors, along with inconsistent AVA and MPG cutoffs in the current guidelines, account for > 40% of patients with discordant small-area low-gradient aortic stenosis.
Acknowledgements
The authors thank the Wellcome Trust Clinical Research Facility, Edinburgh, and the Clinical Research Imaging Centre for their support in the study.
Footnotes
See editorial by Clavel et al.,pages 959-961of this issue.
See page 1072 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at http://dx.doi.org/10.1016/j.cjca.2014.04.021.
Funding Sources
M.R.D. and D.E.N. are supported by the British Heart Foundation. C.W.L.C. is supported by the NRF-MOH Healthcare Research Scholarship (PhD) from the National Research Foundation-Ministry of Health, Singapore. The Wellcome Trust Clinical Research Facility and the Clinical Research Imaging Centre are supported by NHS Research Scotland (NRS) through NHS Lothian.
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1.Minners J., Allgeier M., Gohlke-Baerwolf C. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 2.Clavel M.A., Messika-Zeitoun D., Pibarot P. The complex nature of discordant severe calcified aortic valve disease grading. J Am Coll Cardiol. 2013;62:2329–2338. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner H., Hung J., Bermejo J. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Jander N., Minners J., Holme I. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 5.Lancellotti P. Grading aortic stenosis severity when the flow modifies the gradient valve area correlation. Cardiovasc Diagn Ther. 2012;2:6–9. doi: 10.3978/j.issn.2223-3652.2012.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachicha Z., Dumesnil J.G., Bogaty P., Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 7.Eleid M.F., Sorajja P., Michelena H.I. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. doi: 10.1161/CIRCULATIONAHA.113.003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour A., Ismail T.F., Moat N. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation. J Am Coll Cardiol. 2011;58:2165–2173. doi: 10.1016/j.jacc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Achenbach S., Delgado V., Hausleiter J. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR) J Cardiovasc Comput Tomogr. 2012;6:366–380. doi: 10.1016/j.jcct.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Minners J., Allgeier M., Gohlke-Baerwolf C. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96:1463–1468. doi: 10.1136/hrt.2009.181982. [DOI] [PubMed] [Google Scholar]
- 11.Carabello B.A. Aortic stenosis. N Engl J Med. 2002;346:677–682. doi: 10.1056/NEJMcp010846. [DOI] [PubMed] [Google Scholar]
- 12.Bonow R.O., Carabello B.A., Chatterjee K. 2008 Focused Update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 13.Dumesnil J.G., Pibarot P., Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. doi: 10.1093/eurheartj/ehp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teichholz L.E., Kreulen T., Herman M.V., Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 15.Rosenhek R., Binder T., Porenta G. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 16.Schulz-Menger J., Bluemke D.A., Bremerich J. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:1–19. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natori S., Lai S., Finn J.P. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 18.Maceira A., Prasad S., Khan M., Pennell D. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 19.Vahanian A., Alfieri O., Andreotti F. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 20.Michelena H.I., Margaryan E., Miller F.A. Inconsistent echocardiographic grading of aortic stenosis: is the left ventricular outflow tract important? Heart. 2013;99:921–931. doi: 10.1136/heartjnl-2012-302881. [DOI] [PubMed] [Google Scholar]
- 21.Lancellotti P., Magne J., Donal E. Clinical outcome in asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2012;59:235–243. doi: 10.1016/j.jacc.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 22.Dweck M.R., Chin C., Newby D.E. Small valve area with low-gradient aortic stenosis. Beware the hard hearted. J Am Coll Cardiol. 2013;62:2339–2340. doi: 10.1016/j.jacc.2013.08.1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.