The authors found that oncologists respond rapidly to new evidence and professional guidelines, and readily incorporate predictive biomarkers into clinical practice.
Abstract
Purpose:
Although initially approved for metastatic colorectal cancer (mCRC) tumors with epidermal growth factor receptor (EGFR) overexpression, the use of anti-EGFR antibodies is now restricted to wild-type KRAS tumors. Little is known about prescribers' response to new clinical data, practice guidelines, and US Food and Drug Administration (FDA) label change with regard to the use of anti-EGFR antibodies in clinical practice.
Methods:
Commercially insured patients with mCRC who received second-line therapy between 2004 and 2010 were identified by dusing the LifeLink Health Plan Claims Database. We calculated the fraction of patients receiving anti-EGFR antibody in 2-month intervals. χ2 tests were used to compare treatment rates at four time points: time 1: June 2008, ASCO presentation of clinical data; time 2: February 2009, ASCO guidelines publication; time 3: August 2009, FDA label change; time 4: April 2010 to 8 months after FDA label change.
Results:
Five thousand eighty-nine patients received second-line therapy; of these, 2,599 patients received an anti-EGFR antibody. Median age was 60 years (range, 20 to 97), with 57% male sex. The majority of patients (59.4%) received an anti-EGFR antibody at time 1, with significant decrease at each of the subsequent time points (time 2: 46.2% [P = .019]; time 3: 35.2% [P < .001]; Time 4: 16.2% [P < .001]). Multivariable logistic regression did not show any affect of age, sex, comorbidities, or region of the country on this pattern.
Conclusions:
The use of anti-EGFR antibodies for mCRC decreased after the presentation of clinical trial data, ASCO guidelines publication, and FDA label change. These data suggest that oncologists respond rapidly to new evidence and professional guidelines, and readily incorporate predictive biomarkers into clinical practice.
Introduction
The treatment of metastatic colorectal cancer (mCRC) has changed dramatically in the last two decades with introduction of new targeted therapy, including two new inhibitors of the epidermal growth factor receptor (EGFR). Cetuximab (Eli Lily, Indianapolis, IN) was approved by the US Food and Drug Administration (FDA) in 2004, followed by approval of panitumumab (Amgen, Thousand Oaks, CA) in late 2006.1–3 The initial approval of cetuximab was restricted to mCRC with positive immunohistochemistry (IHC) staining for EGFR. However, in March 2005, the selection of patients based on IHC staining was brought into question, with evidence of response to treatment among patients who did not fit the initial criteria.4,5
In April 2006, Lievere et al6 published the first report identifying KRAS mutation status as a possible predictive marker of response to cetuximab. These results were confirmed by larger studies and subset analyses of phase III clinical trials with these agents, resulting in temporary suspension of National Cancer Institute–sponsored clinical trials using anti-EGFR agents.7–11 These data led to ASCO issuing a Provisional Clinical Opinion in February 2009, recommending tumor KRAS mutation testing for all patients with mCRC before therapy with anti-EGFR antibodies, and avoiding therapy among those patients with documented KRAS mutation12,13 in their tumor. The FDA labels for panitumumab and cetuximab were changed in July 2009 to reflect this recommendation.
The adoption of evidence-based new therapies among oncologists has been studied in various disease sites. A recent study of by Neugut et al14 showed rapid uptake of oxaliplatin, after its approval in 2004, into adjuvant treatment regimens for node-positive early-stage colon cancer, as well as for metastatic disease. A similar pattern was noted for the incorporation of bevacizumab into treatment of patients with mCRC.14 These trends have been reported in other diseases including breast cancer, lung cancer, and prostate cancer.15–19 However, the decrease in use of approved drugs or interventions by oncologists based on emerging evidence is less well studied. In this analysis, we aimed to describe the patterns of anti-EGFR therapy use and understand the impact of practice guidelines and changes to the FDA label on the de-adoption of previously approved cancer therapy.
Methods
Data Source
This retrospective study analyzed pharmaceutical insurance claims contained in the LifeLink Health Plan Claims Database (formerly the PharMetrics Patient-Centric Database), which contains data on 82.5 million lives. This database has been used widely in studies evaluating health care economics in oncology and other disciplines.20–22 This is an administrative claims database, which encompasses medical and pharmacy claims from various commercial health plans, including Medicare Managed Care plans in four U.S. geographical regions. The claims database contains details such as date of service, International Classification of Diseases Ninth Revisions, Clinical Modifications (ICD-9-CM) codes, procedure codes, and national drug codes. It does not include any tumor-related features such as stage or histologic findings. De-identified data representing the national commercially insured population were obtained through a license agreement.
Time Periods of Evaluation
We reviewed claims between the years 2004 and 2010 in order to include the relevant events that resulted in recommendations to change use of anti-EGFR antibodies. Within this timeline, the following time points were used for the analysis: time 1: June 2008, ASCO presentation of clinical data; time 2: February 2009, publication of ASCO Provisional Clinical Opinion recommending KRAS testing; time 3: August 2009, FDA drug label change; time 4: April 2010 to 8 months after FDA drug label change.
Patient Selection
We identified patients 18 years of age or older at the onset of metastatic disease, with medical claims for the diagnosis of mCRC. Patients were identified as having CRC using the diagnosis codes of metastatic colon or rectal cancer (ICD9 codes of 153.x or 154.x). Patients with anal cancer (ICD 154.3) were excluded. We further identified patients with a diagnosis ICD9 code of metastatic disease site (in organs: 197.0-197.7 and 198.0-199.0; or lymph nodes: 196.0, 196.1, 196.3-196.5, 196.7-196.9). Healthcare Common Procedure Coding System (HCPCS; J codes) were used for identification of drug therapy (J9055, cetuximab; C9235 and J9303, panitumumab; J9206, irinotecan; C9257, J9035, Q2024, S0116, bevacizumab; J9263, oxaliplatin; J9190, fluorouracil therapy; J8520, J8521, capecitabine). We excluded adjuvant chemotherapy by including only claims submitted after the first date of metastatic diagnosis code documentation. Comorbidity burden was assessed using the Charlson Comorbidity Index calculated from claims available up to 1 year before the onset of mCRC.23 Geographic regions were defined by the database as East, West, Midwest, and South.
Identification of Lines of Therapy
Because this claims database did not identify specific lines of therapy, we developed an algorithm to identify treatment lines based on the drugs used in each case. National patterns of care studies found that anti-EGFR antibodies treatment was most frequently used after progression in patients receiving combination chemotherapy with or without bevacizumab.24 Therefore, we focused our analysis on patients who received second-line therapy and beyond. Patients treated with anti-EGFR agents (cetuximab or panitumumab) in the first-line setting were excluded from the analysis. To identify a cohort of patients who received second-line therapy or beyond, we defined first line as any chemotherapy (fluorouracil, capecitabine, irinotecan or oxaliplatin) given with or without bevacizumab after submission of a claim for mCRC. We subsequently defined the beginning of second-line therapy by the initiation of a new chemotherapy agent (irinotecan or oxaliplatin) after first-line therapy, with or without anti-EGFR agent. For patients treated with fluorouracil and bevacizumab in the first-line setting, second-line therapy was defined as the first day of treatment with irinotecan, oxaliplatin, and/or an anti-EGFR agent. We examined patients based on when they began second line therapy, grouping the cohort into in 2-month time intervals.
Statistical Analyses
Categorical variables were tabulated, and means were calculated for continuous variables. The patients' characteristics were summarized on the basis of whether they received anti-EGFR directed therapy. χ2 test (for categorical variables) and Wilcoxon's test (for continuous variables) were used to compare the groups in the univariable analysis. As for the multivariable analysis (MVA), we used multiple logistic regression models to describe the use of anti-EGFR agent over time, controlling for covariates such as age, sex, region, and comorbidity score. All analyses were done using SAS 9.2. A P value of less than .05 was considered statistically significant.
Analysis
Because the goal of our study was to measure the use of anti-EGFR therapy at any point beyond first-line therapy, our primary analysis evaluated the percentage of patients who received anti-EGFR antibody at any point beyond the date of initiation of second-line therapy. The following equation was used: [number of patients starting anti-EGFR therapy in second-line or beyond] ÷ [number of patients starting second-line therapy in a given 2 month period]. We estimated the percentage of patients treated with anti-EGFR agent at each time point as defined above. χ2 tests were used to compare the percentages between the four time points. We then performed multivariable logistic regression on our primary analysis to determine if patient characteristics were predictive of use of anti-EGFR directed therapy. In these models, treatment with EGFR-directed therapy was the outcome of interest, and time period of treatment, sex, age, comorbidity scores, and geographic region were the independent variables. SAS 9.2 was used for all analyses. A P value of less than .05 was considered statistically significant.
For further confirmation of the identified trends, we conducted a secondary analysis examining the use of anti-EGFR therapy at any point before the patient's last claim. We calculated the percentage of patients who received an anti-EGFR antibody in the second-line setting or beyond among patients who had their last claim submitted in a given 2-month interval. The following equation was used: [number of patients treated with an anti-EGFR agent in second line or beyond] ÷ [number of patients having their last claim filed in a given 2-month period]. Similar to our primary analysis, χ2 tests were used to compare the percentages between the predefined four time points.
Results
Baseline Characteristics of Analysis Group
One hundred seventy thousand nine hundred ninety-eight patients were diagnosed with colorectal cancer, of whom 44,271 had documentation of mCRC. Five thousand eighty-nine patients received second-line therapy, of whom 2,599 received an anti-EGFR antibody. The median number of claims for this population was 979 (range, 10 to 6,872), and the median length of time from first claim for mCRC to last was 20.6 months (range, 0 to 89.7 months). Patients' characteristics are summarized in Table 1. The median age was 60 years (range, 20 to 97 years), 57% were male, and more than half presented with a comorbidity score of 0 to 1. Patients treated with anti-EGFR agent were slightly younger than those who did not receive this therapy (59 v 60 years; P = .003). These patients also more commonly presented with a comorbidity index of 0 (P = .009). There was no significant difference in regional representation in our cohort of patients.
Table 1.
Baseline Characteristics
| Characteristic | Any Second-Line Therapy (N = 5,089) |
With Anti-EGFR Agent (n = 2,599) |
No Anti-EGFR Agent (n = 2,490) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | .0027 | ||||||
| Median | 60 | 59 | 60 | ||||
| SD | 11.5 | 11.56 | 11.46 | ||||
| Sex | |||||||
| Male | 2,898 | 56.9 | 1,505 | 57.91 | 1,393 | 55.94 | .157 |
| Female | 2,191 | 43.1 | 1,094 | 42.09 | 1,097 | 44.06 | |
| Region | |||||||
| East | 1,123 | 22.07 | 574 | 22.09 | 549 | 22.09 | .077 |
| Midwest | 1,575 | 30.95 | 837 | 32.20 | 738 | 29.64 | |
| South | 1,595 | 31.34 | 775 | 29.82 | 820 | 32.93 | |
| West | 796 | 15.64 | 413 | 15.89 | 383 | 15.38 | |
| Charlson comorbidity score* | |||||||
| 0 | 2,833 | 55.83 | 1,505 | 58.00 | 1,328 | 53.57 | .0092 |
| 1 | 1,440 | 28.38 | 714 | 27.5 | 726 | 29.29 | |
| 2 | 486 | 9.58 | 229 | 8.82 | 257 | 10.37 | |
| ≥ 3 | 315 | 6.21 | 147 | 5.66 | 168 | 6.78 | |
| Chemotherapy exposure at any time after mCRC diagnosis† | |||||||
| FU/capecitabine | 4,890 | 96 | 2,485 | 95.6 | 2,405 | 96.6 | |
| Irinotecan | 4,709 | 92.5 | 2,391 | 92 | 2,318 | 93.1 | |
| Oxaliplatin | 4,371 | 85.9 | 2,011 | 77.4 | 2,360 | 94.8 | |
| Bevacizumab | 4,039 | 79.4 | 2,174 | 83.7 | 1,865 | 74.9 | |
| First-line chemotherapy† | |||||||
| FU/capecitabine | 4,563 | 89.7 | 2,350 | 90.4 | 2,213 | 88.9 | |
| Irinotecan | 1,532 | 30.1 | 866 | 33.3 | 666 | 26.8 | |
| Oxaliplatin | 2,834 | 55.7 | 1,415 | 54.4 | 1,419 | 56.9 | |
| Bevacizumab | 3,290 | 64.5 | 1,795 | 69.0 | 1,495 | 60.0 | |
| Second line and beyond† | |||||||
| Anti-EGFR only | 180 | 3.5 | 180 | 6.9 | — | ||
| Any chemotherapy other than anti-EGFR agents | 4,909 | 96.5 | 2,419 | 93.1 | 2,490 | 100 | |
| FU/capecitabine | 3,952 | 77.7 | 1,799 | 69.2 | 2,153 | 86.5 | |
| Irinotecan | 4,024 | 79.1 | 2,194 | 84.4 | 1,830 | 73.5 | |
| Oxaliplatin | 1,992 | 39.1 | 914 | 35.2 | 1,078 | 43.3 | |
| Bevacizumab | 2,800 | 55 | 1,303 | 50.1 | 1,497 | 60 | |
Abbreviations: EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer; FU, fluorouracil.
Missing data on 15 patients in the full cohort.
P value could not be computed for these analyses because the numbers are not mutually independent.
Source: IMS LifeLink Information Assets-Health Plan Claims Database, January 2004-June 2010, Copyright 2014, IMS Health Incorporated. All Rights Reserved.
The most frequently used drugs in the first-line setting were fluorouracil, oxaliplatin, and bevacizumab. In the second-line setting or beyond, only 6.9% of patients received anti-EGFR therapy as a single agent without any other chemotherapy agents used concurrently or sequentially. Of the patients who received anti-EGFR treatment in the second-line setting or beyond, 93.1% were treated with additional chemotherapy agents concurrently or sequentially (fluorouracil/capecitabine, 69.2%; irinotecan, 84.4%; oxaliplatin, 35.2%; and bevacizumab, 50.1%).
Analysis of Adoption and De-adoption of Anti-EGFR Therapy
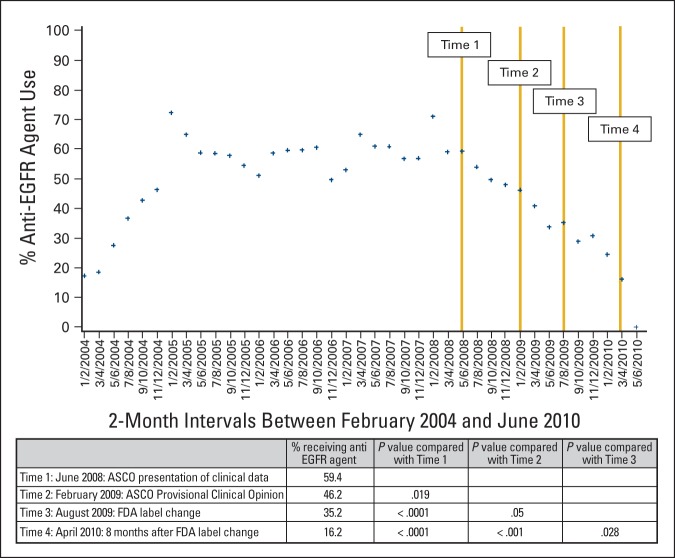
We calculated the percentage of patients who received anti-EGFR agents at any point after initiation of second-line therapy (Figure 1). At time 1, after the ASCO presentation of clinical data, 59.4% of patients in our cohort were treated with an anti-EGFR antibody. We noted a decrease in use rate at the subsequent time points (time 2: publication of ASCO Provisional Clinical Opinion, 46.2%, P = .019; time 3: FDA label change, 35.2%, P < .05 for all comparisons). The lowest percentage was noted at time 4 (8 months after the FDA label change), with only 16.2% of patients in this sample receiving anti-EGFR agents (P < .05 for all comparisons). In our logistic regression, we found declines across all three time periods, but the greatest during the third time period (following the FDA label change). These changes were not affected by age, sex, comorbidity status or region of the country (Table 2).
Figure 1.
Primary analysis of de-adoption of anti–epidermal growth factor receptor (EGFR) agents: percentage of patients starting an anti-EGFR agent at any time after initiation of second-line therapy. The analysis estimated the percentage of patient who would start an anti-EGFR agent in the future on the basis of the number of patient initiating second-line therapy in a given 2-month interval. The following equation was used: [number of patients treated with anti-EGFR therapy in second line or beyond] ÷ [number of patients starting second line therapy in a given 2-month period]. FDA, US Food and Drug Administration.
Table 2.
Logistic Regression Analysis of Rates of Decline of Anti-EGFR Use, and the Effect of Demographic Characteristics on These Rates
| Predictor | Odds Ratio Estimate | P |
|---|---|---|
| Time period | ||
| Before time 1 | Ref | |
| Time 1-time 2 (June 2008-February 2009) | 0.87 | .003 |
| Time 2-time 3 (February 2009-August 2009) | 0.87 | .03 |
| Time 3-time 4 (August 2009-April 2010) | 0.83 | .02 |
| Charlson comorbidity score | ||
| 0 | Ref | |
| 1 | 0.81 | .11 |
| 2 | 1.0 | .97 |
| ≥ 3 | 0.8 | .32 |
| Sex | ||
| Female | Ref | |
| Male | 1.2 | .06 |
| Age (continuous variable) | 0.99 | .1 |
| Region | ||
| West | Ref | |
| East | 1.1 | .5 |
| Midwest | 1.2 | .4 |
| South | 1.3 | .2 |
Abbreviations: EGFR, epidermal growth factor receptor; Ref, reference.
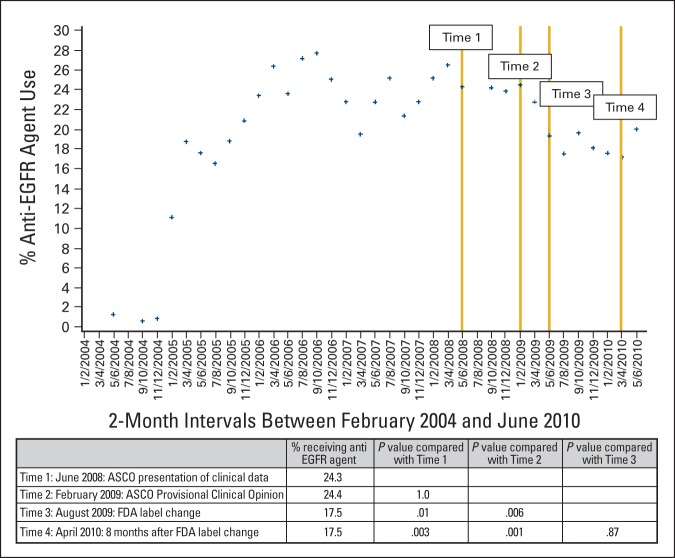
A secondary analysis to confirm this trend calculated the number of patients who were treated with anti-EGFR antibodies at any time before submission of their last claim (Appendix Figure A1, online only). In this analysis, at time 1, the percentage of patients receiving anti-EGFR agents was 24.3%. The decrease in the rates of anti-EGFR antibodies use was more moderate in this analysis, with 24.4%, 17.5%, and 17.1% of patient receiving the drugs at time 2, 3, and 4, respectively (P < .05).
Discussion
Our results demonstrate de-adoption of treatment with anti-EGFR antibodies after the ASCO Provisional Clinical Opinion recommendations and FDA label changes. This analysis evaluated the use of these agents from 2004 to 2010, capturing the initial increase in the use of these agents and the decline after the provisional clinical opinion and FDA recommendations. The percentage of patients who were treated with anti-EGFR antibodies continued to decrease from the time of clinical trial results presentations (June 2008) though publication of the ASCO Provisional Clinical Opinion (February 2009) and FDA label change (July 2009). The most significant decrease was seen 8 months after the FDA label change (16.2% v 59.4% at the time of publication of clinical trial results, P < .001)). Demographic characteristics such as age, gender, comorbidity score, or geographic region did not affect these trends. These results suggest the attentiveness of the oncologic community to clinical presentations at national meetings and ASCO guidance, with rapid incorporation of the recommendations into clinical practice. Furthermore, these results indicate that oncologists are receptive to the adoption of biomarkers that will enhance their ability to deliver effective therapy. It is also evident that oncologists change their practice promptly in the face of highly publicized data, even years after a drug's original approval.
These results are significant in the face of the increasing identification of biomarkers and development of companion diagnostics to guide therapy in oncology. Studies have demonstrated rapid uptake of new therapies by oncologists,14,15 especially after presentation of clinical trial results and publication of treatment guidelines.16,18 However, studies evaluating the de-adoption of previously approved cancer therapies based on new recommendations are rare. A Canadian study found funding policy changes and health warnings were strong factors influencing the use of erythropoiesis-stimulating agents (ESAs) among patients with cancer. After the safety warnings of thromboembolism, tumor progression, and mortality associated with ESAs, use decreased by 81% among patients with cancer.25 The adoption of gene expression profile testing and an associated reduction in the use of adjuvant chemotherapy was seen in a recent analysis of more than 7,000 patients with breast cancer.26 The de-adoption of drugs in other specialties has been studied to a limited extent. One study demonstrated a decrease in the use of intravenous nesiritide for management of acute decompensated heart failure after the reports of increased risk of mortality and renal failure with this agent.27
Understanding how treatments may be adopted or de-adopted is essential given the high cost of novel cancer treatments.17,19 With improved care and new therapies for mCRC, the median survival of patients with mCRC is estimated at 24 months, resulting in high incremental costs per life-year gained.28 The incremental cost of cetuximab over best supportive care in end-stage mCRC was estimated at about $24,000 dollars per patient for 0.12 years improvement in survival.29 Identification of biomarkers predictive for treatment response is essential in order to avoid delivering ineffective and toxic therapies to those who will not benefit from them. A recent study estimated that KRAS testing in mCRC would save more than $400 million per year in the United States.30 To our knowledge, our study is the first to quantify the effect of presentation of results, professional society recommendations, and FDA label change on the de-adoption of expensive anticancer therapies in the United States. Our results provide evidence that oncologists in clinical practice follow new recommendations. Compliance with these guidelines can result in significant cost savings by reducing the use of these drugs in patients who will not benefit.
Observational studies such as ours should be viewed within the context of their limitations. This data set lacked clinical details (ie, disease presentation, stage, pathology, etc), which may have led to misclassification of the patients included in the analysis. We identified our cohort by using ICD9 codes for disease and metastatic site and HCPCS for therapy, with the goal being to optimize subject identification. We excluded adjuvant therapy from this analysis by reviewing claims submitted only after the first date of metastatic diagnosis code documentation. However, inaccurate coding may account for inclusion of patients who do not fit these criteria. The median length of claims in our data set was 20.6 months, which is consistent with the median overall survival of patients with mCRC, suggesting that that we did not have a significant misidentification bias.
In addition, this database does not delineate line of therapy within the metastatic setting. This limitation is common to many retrospective studies of patients with mCRC, for whom a “stop-and-go” approach is often applied whereby treatment breaks are instituted from one or more drugs.31 In order to overcome this challenge, we developed an algorithm to define line of therapy and identify patients receiving second-line treatment or beyond. The strength of the criteria chosen for identification of second-line therapy is supported by the similar trends of de-adoption of anti-EGFR therapy in both the primary and secondary analyses. However, it is possible that our algorithm may have misclassified the true intentions of the treating physicians, particularly if patients with adjuvant therapy were misclassified as receiving treatment for metastatic disease. However, we expect that this bias would be nondifferential and should not significantly affect our results.
Another limitation is that this administrative claims database includes only patients with commercial insurance, and may not be generalizable to patients with other type of insurances, such as Medicare or Veterans Affairs coverage. Although the database does include patients over the age of 65 enrolled in Medicare managed care plans, since this was a commercially insured population, we anticipated seeing a younger group of patients in our cohort. Whereas the median age of CRC patients at diagnosis is 71, the median age of patients in our study was 60.32 Additional differences in race and socioeconomic status may exist between our cohort and the general CRC patient population, which could not be evaluated with the available data set. Finally, there are many factors that contribute to the oncologist's therapeutic decisions, including oncologist's previous experience, perception of benefit, and interaction with other physicians or industry that cannot be examined in this analysis. Although a decrease in the rate of usage of anti-EGFR agents is clearly seen in these analyses, we do not have any data regarding the actual use of KRAS testing before the decision not to prescribe these drugs, or the results of these tests. Although our assumption is that testing led to decreased use, other physician or patient-related factors could have also contributed. Furthermore, we cannot comment on the appropriate use of these medications. KRAS mutations are found in approximately 40% of tumors, with 60% of patients eligible for anti-EGFR therapy. The percentage of patients treated with anti-EGFR antibodies in our data is lower. Factors such as performance status or patient's/physician's preferences may explain the lack of use of this therapy, which we cannot ascertain with the current data set. However, the de-adoption in therapy was consistent and significant across the time points studied, supporting our hypothesis.
In summary, we found a rapid de-adoption of anti-EGFR antibodies for treatment of mCRC after clinical data presentation, ASCO Provisional Clinical Opinion, and FDA label change. These data also suggest that new biomarkers that are predictive of response to therapy are likely to be quickly adopted into clinical oncology practice. This highlight the importance of rapid disclosure and communication of new clinical trials results that are relevant to standard practice.33
Acknowledgment
Supported by a Cancer Center Support Grant No. 3 P30 CA006927-47S4 and a National Cancer Institute Grant No. K07CA136995, an Institutional Research Grant from the American Cancer Society (IRG 92-027-15), and Cancer Center Support Grant No. P30 CA43703. Previously presented at the American Society of Clinical Oncology Quality Care Symposium, San Diego, CA, November 30-December 1, 2012. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): LifeLink Health Plan Claims Database (2004-2010), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Appendix
Figure A1.
Secondary analysis of the percentage of patients with an anti–epidermal growth factor receptor (EGFR) agent use before last claim submission: This analysis calculated the percentage of patients who were treated with an anti-EGFR agent in the past, out of the number of patients who had their last claim submitted in a given 2-month interval. The following equation was used: [number of patients treated with an anti-EGFR agent in second-line or beyond] ÷ [number of patients having their last claim filed in a given 2-month period]. FDA, US Food and Drug Administration.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Neal J. Meropol, American Society of Clinical Oncology (U), Eastern Cooperative Oncology Group (U) Consultant or Advisory Role: Yu-Ning Wong, Bristol Myers Squibb (C), Bristol Myers Squibb (U) Stock Ownership: J. Robert Beck, GlaxoSmithKline Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
Author Contributions
Conception and design: Efrat Dotan, Neal J. Meropol, J. Robert Beck, Yu-Ning Wong
Financial support: Efrat Dotan, Yu-Ning Wong
Collection and assembly of data: Efrat Dotan, Yu-Ning Wong
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 4.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Meropol NJ. Epidermal growth factor receptor inhibitors in colorectal cancer: It's time to get back on target. J Clin Oncol. 2005;23:1791–1793. doi: 10.1200/JCO.2005.10.951. [DOI] [PubMed] [Google Scholar]
- 6.Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Lang I, D'haens G, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. J Clin Oncol. 2008;26(suppl) abstr 2. [Google Scholar]
- 9.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 10.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 11.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 12.Javle M, Hsueh CT. Updates in gastrointestinal oncology: Insights from the 2008 44th annual meeting of the American Society of Clinical Oncology. J Hematol Oncol. 2009;2:9. doi: 10.1186/1756-8722-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 14.Neugut AI, Becker DJ, Insel BJ, et al. Uptake of oxaliplatin and bevacizumab for treatment of node-positive and metastatic colon cancer. J Oncol Pract. 2012;8:156–163. doi: 10.1200/JOP.2011.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershman DL, Wilde ET, Wright JD, et al. Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer. J Clin Oncol. 2012;30:806–812. doi: 10.1200/JCO.2011.37.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dranitsaris G, Evans WK, Milliken D, et al. The impact of practice guidelines and funding policies on the use of new drugs in advanced non-small cell lung cancer. J Eval Clin Pract. 2005;11:350–356. doi: 10.1111/j.1365-2753.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirvani SM, Pan IW, Buchholz TA, et al. Impact of evidence-based clinical guidelines on the adoption of postmastectomy radiation in older women. Cancer. 2011;117:4595–4605. doi: 10.1002/cncr.26081. [DOI] [PubMed] [Google Scholar]
- 19.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 20.Lang K, Sussman M, Friedman M, et al. Incidence and costs of treatment-related complications among patients with advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2009;135:582–588. doi: 10.1001/archoto.2009.46. [DOI] [PubMed] [Google Scholar]
- 21.Crawford ED, Black L, Eaddy M, et al. A retrospective analysis illustrating the substantial clinical and economic burden of prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:162–167. doi: 10.1038/pcan.2009.63. [DOI] [PubMed] [Google Scholar]
- 22.Hatoum HT, Lin SJ, Smith MR, et al. Treatment persistence with monthly zoledronic acid is associated with lower risk and frequency of skeletal complications in patients with breast cancer and bone metastasis. Clin Breast Cancer. 2011;11:177–183. doi: 10.1016/j.clbc.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Zafar SY, Marcello JE, Wheeler JL, et al. Longitudinal patterns of chemotherapy use in metastatic colorectal cancer. J Oncol Pract. 2009;5:228–233. doi: 10.1200/JOP.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir MA, Gomes T, Winquist E, et al. Effects of funding policy changes and health warnings on the use of erythropoiesis-stimulating agents. J Oncol Pract. 2012;8:179–183. doi: 10.1200/JOP.2011.000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauptman PJ, Schnitzler MA, Swindle J, et al. Use of nesiritide before and after publications suggesting drug-related risks in patients with acute decompensated heart failure. JAMA. 2006;296:1877–184. doi: 10.1001/jama.296.15.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong YN, Meropol NJ, Speier W, et al. Cost implications of new treatments for advanced colorectal cancer. Cancer. 2009;115:2081–2091. doi: 10.1002/cncr.24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittmann N, Au HJ, Tu D, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: Evaluation of National Cancer Institute of Canada Clinical Trials Group CO. 17 trial. J Natl Cancer Inst. 2009;101:1182–1192. doi: 10.1093/jnci/djp232. [DOI] [PubMed] [Google Scholar]
- 30.Vijayaraghavan A, Efrusy MB, Göke B, et al. Cost-effectiveness of KRAS testing in metastatic colorectal cancer patients in the United States and Germany. Int J Cancer. 2012;131:438–445. doi: 10.1002/ijc.26400. [DOI] [PubMed] [Google Scholar]
- 31.Maindrault-Goebel F, Lledo G, Chibaudel B, et al. Final results of OPTIMOX2, a large randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC): A GERCOR study. J Clin Oncol. 2007;25(suppl) abstr 4013. [Google Scholar]
- 32.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 33.Blanke CD, Goldberg RM, Grothey A, et al. KRAS and colorectal cancer: Ethical and pragmatic issues in effecting real-time change in oncology clinical trials and practice. Oncologist. 2011;16:1061–1068. doi: 10.1634/theoncologist.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]