Abstract
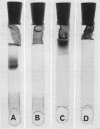
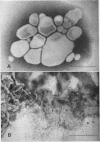
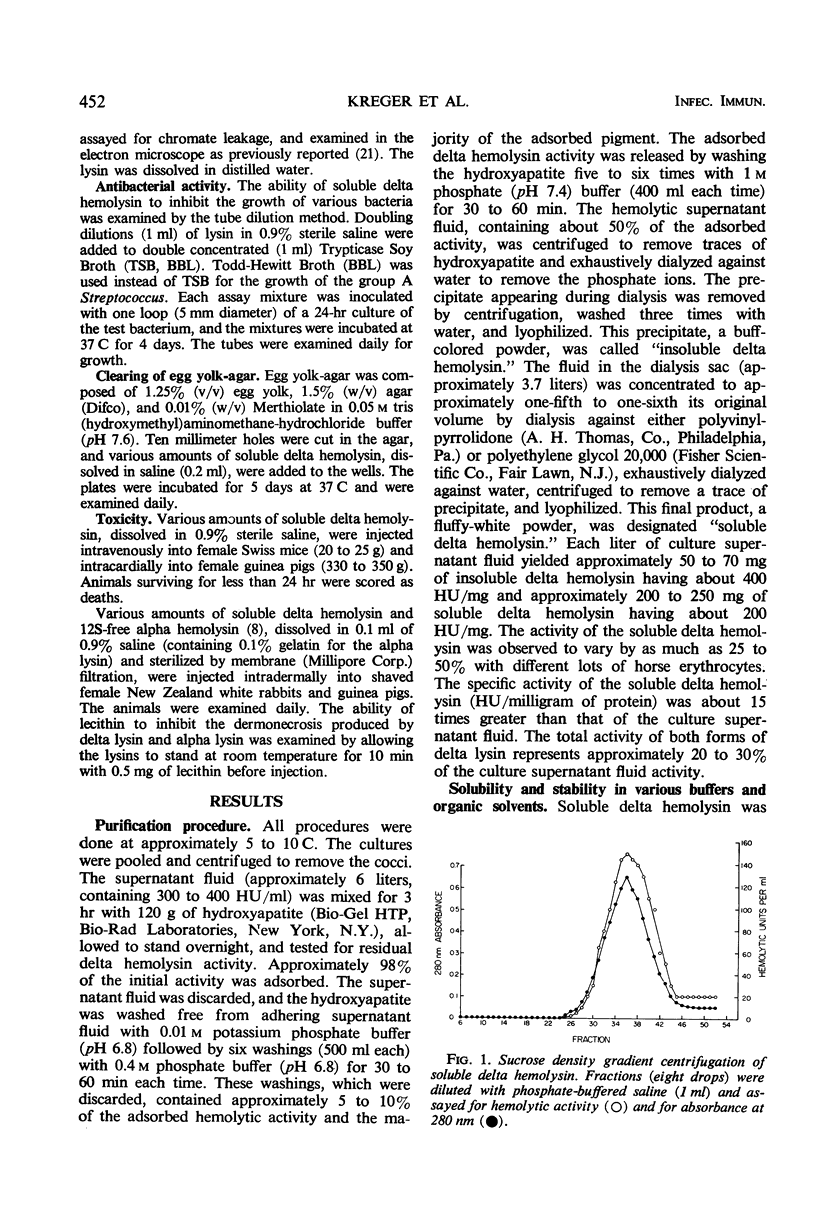
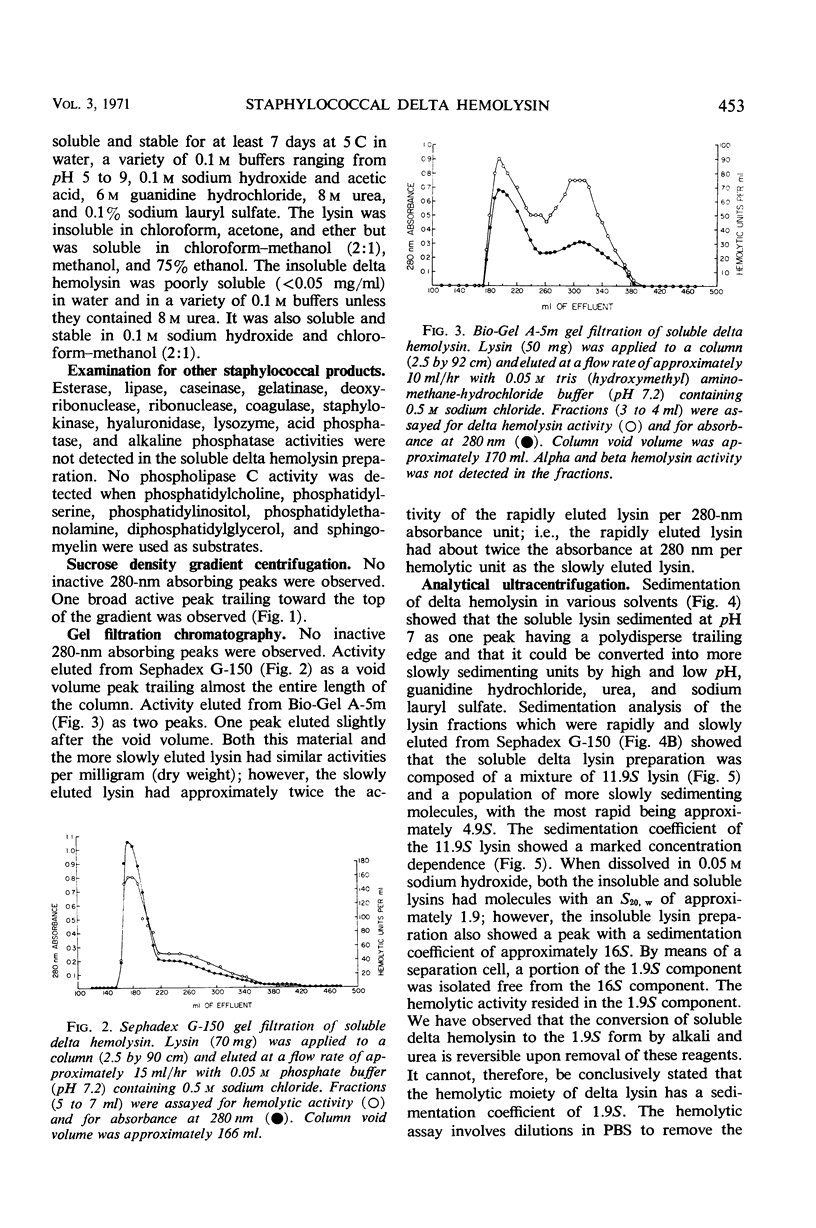
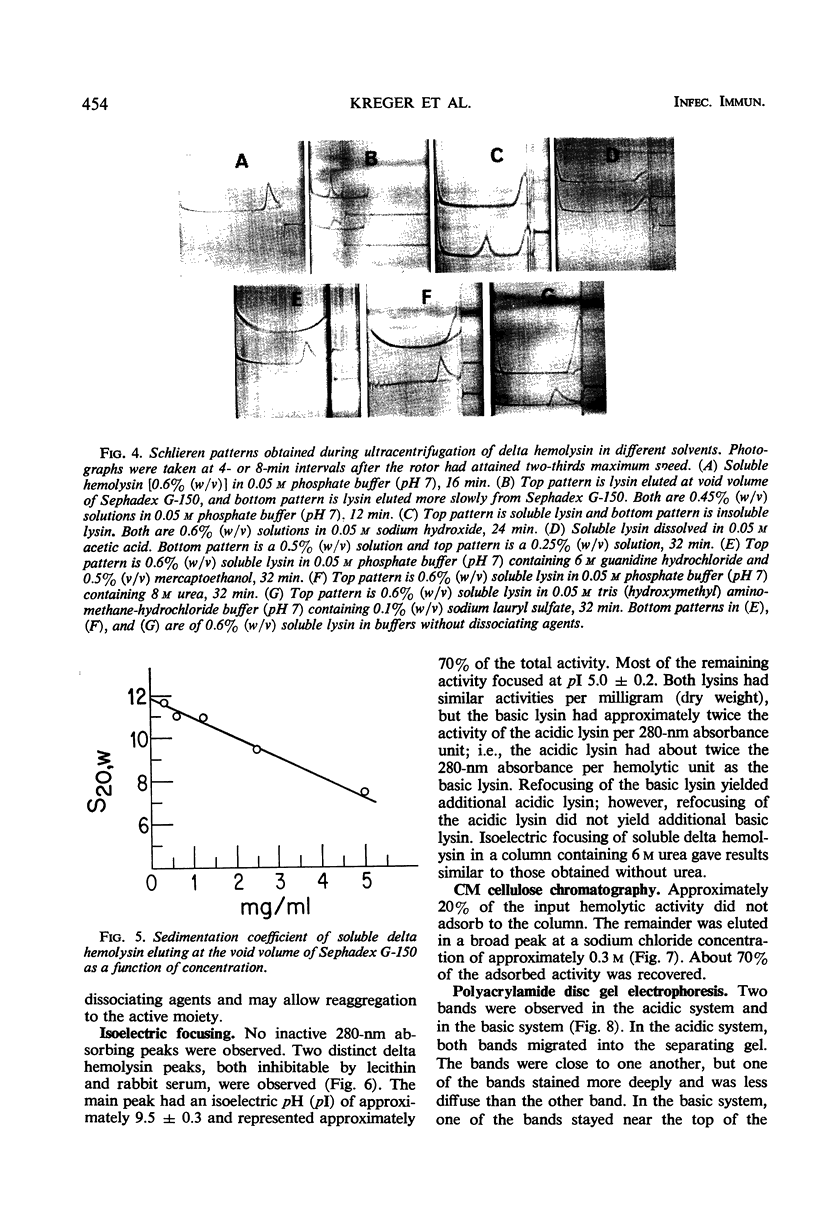
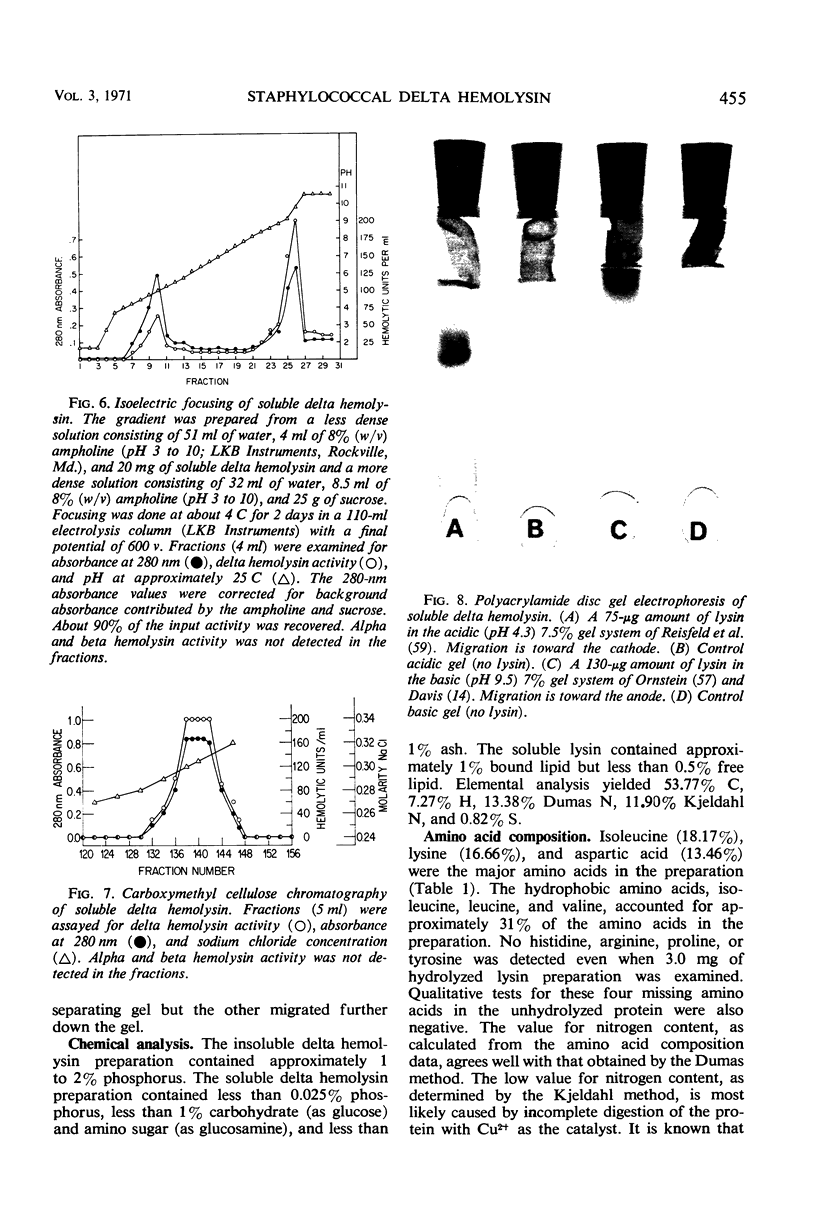
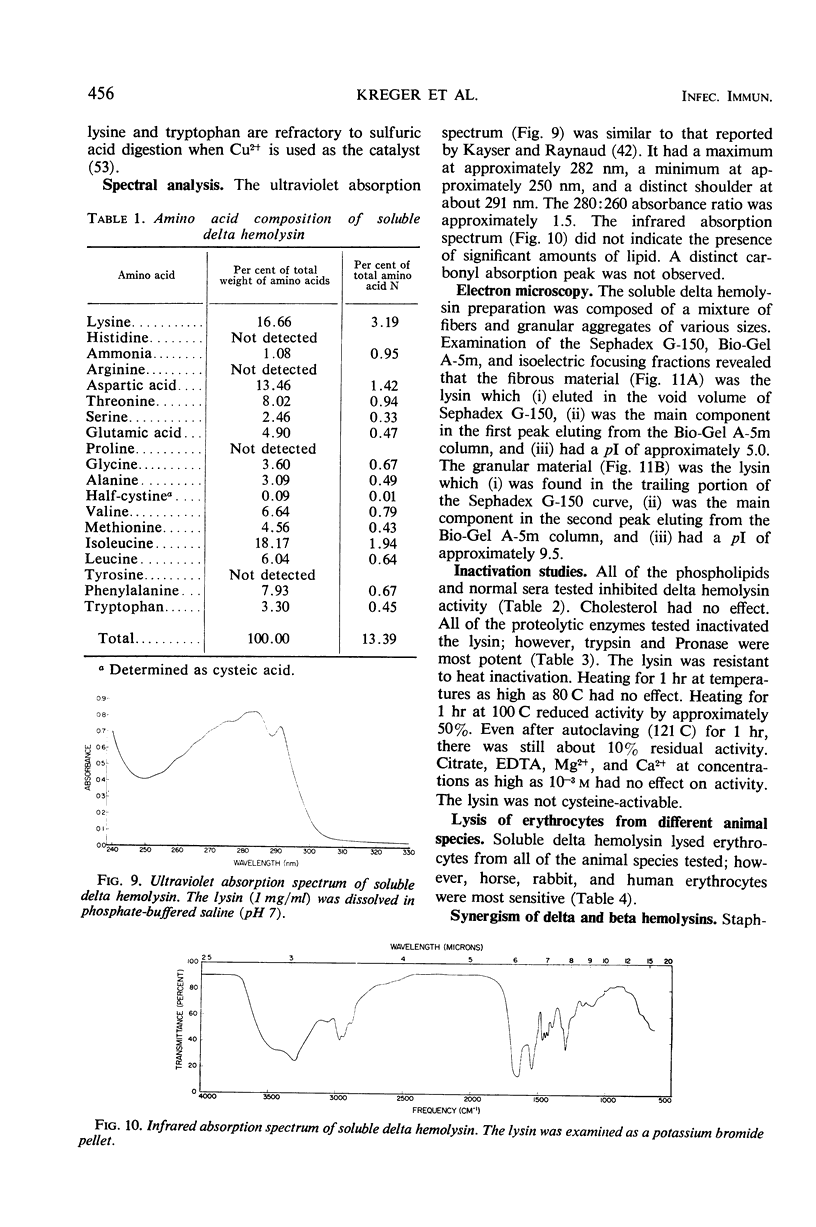
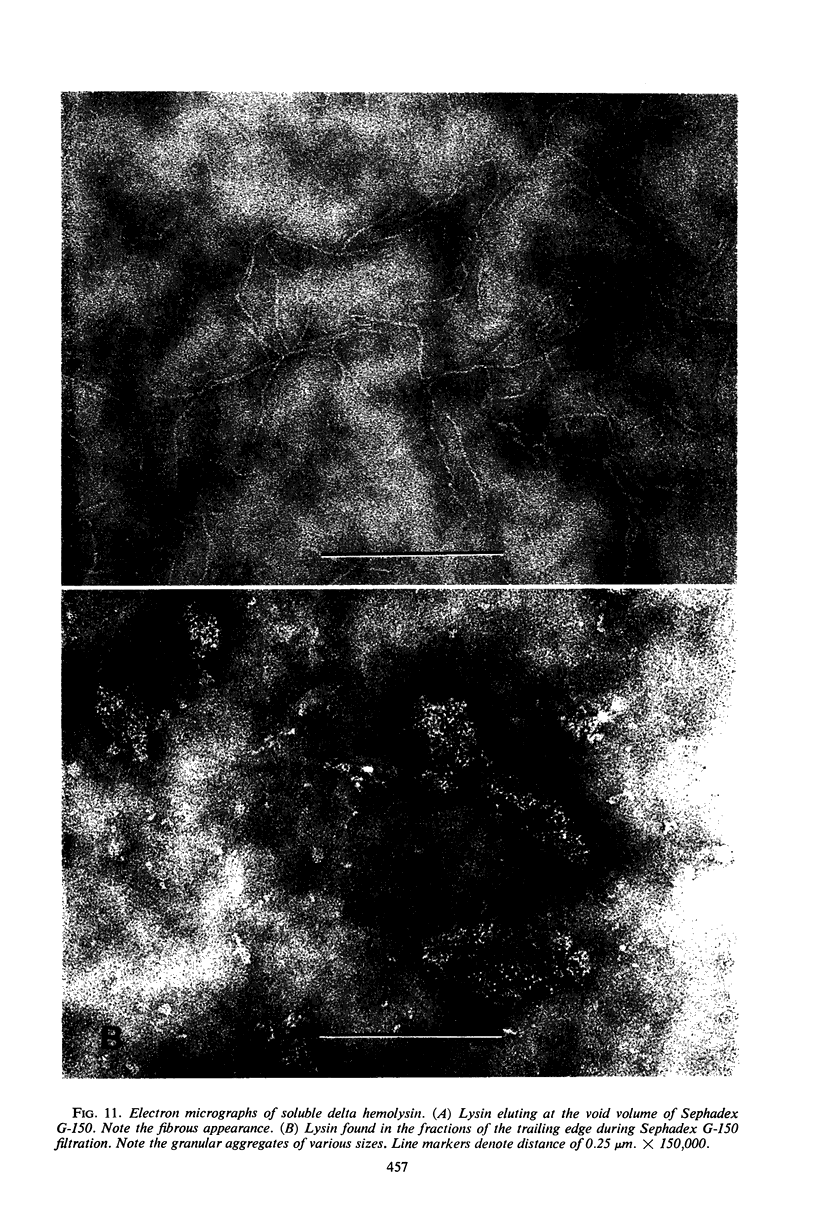
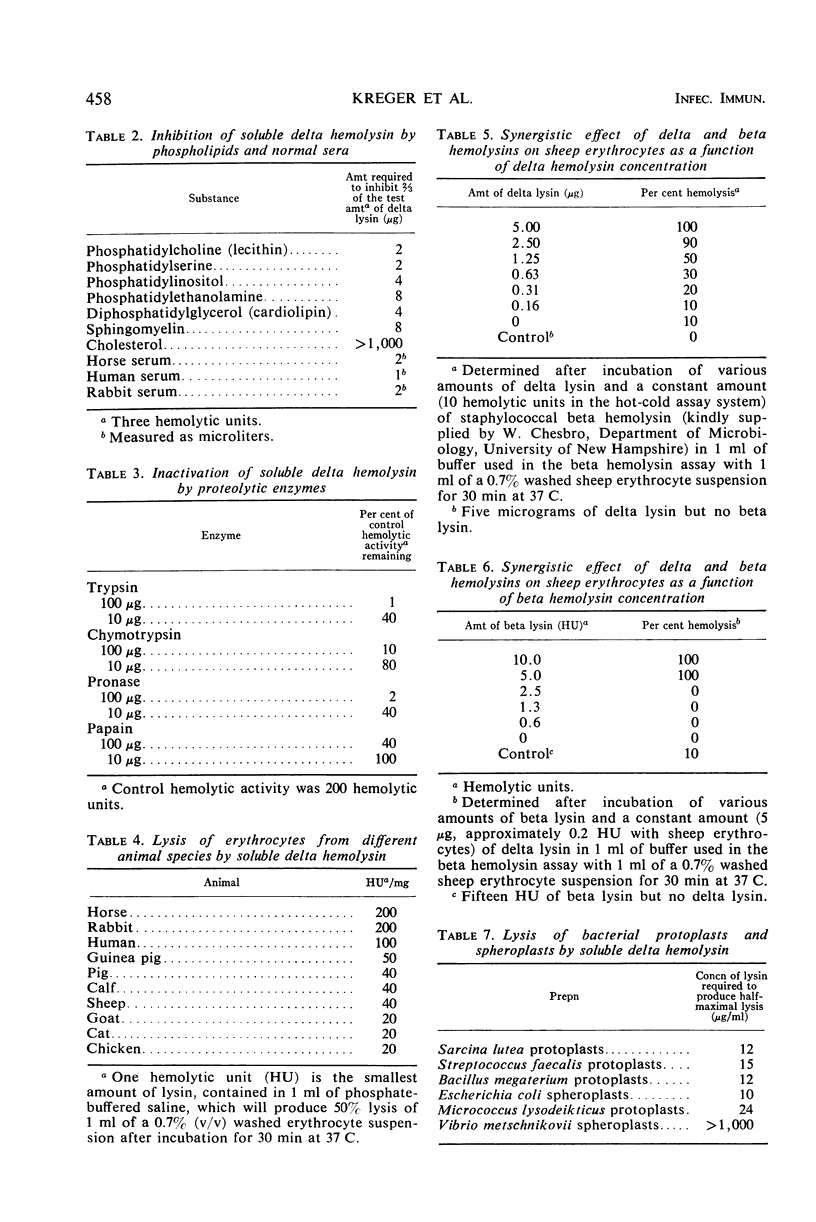
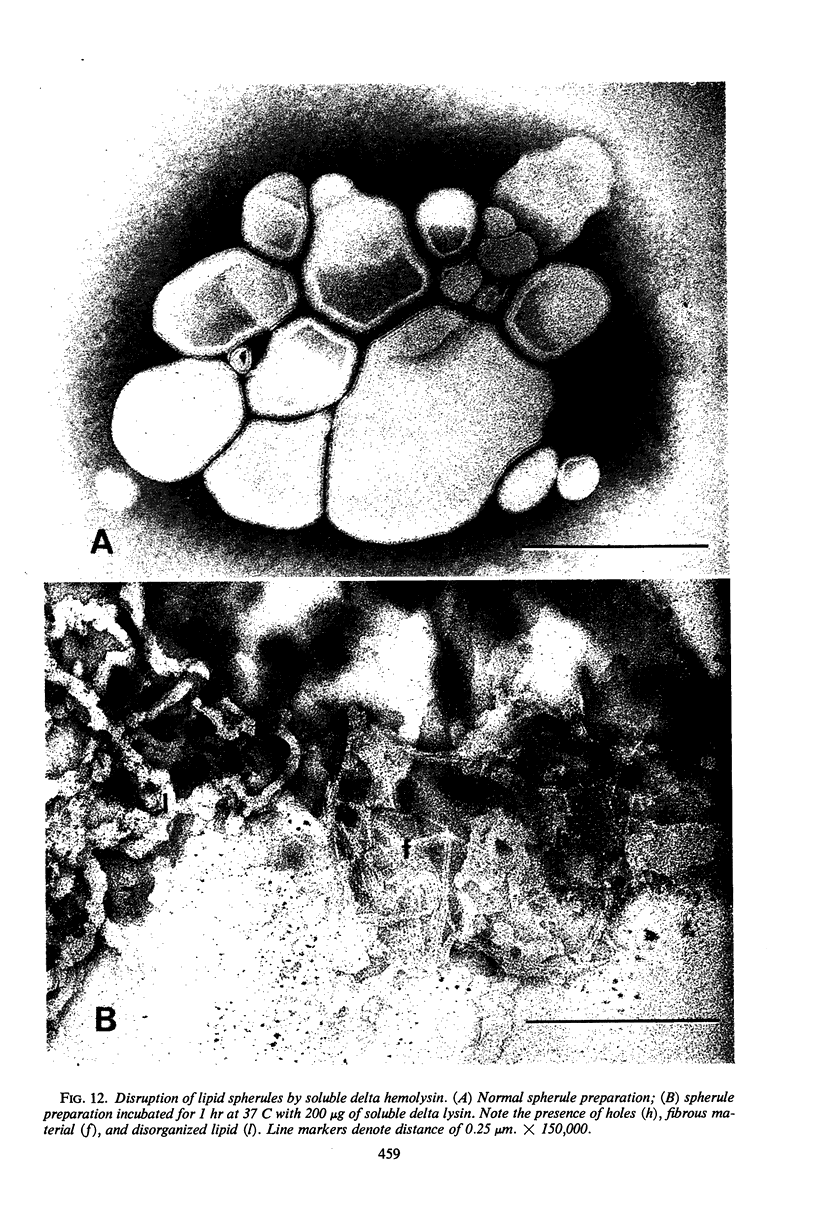
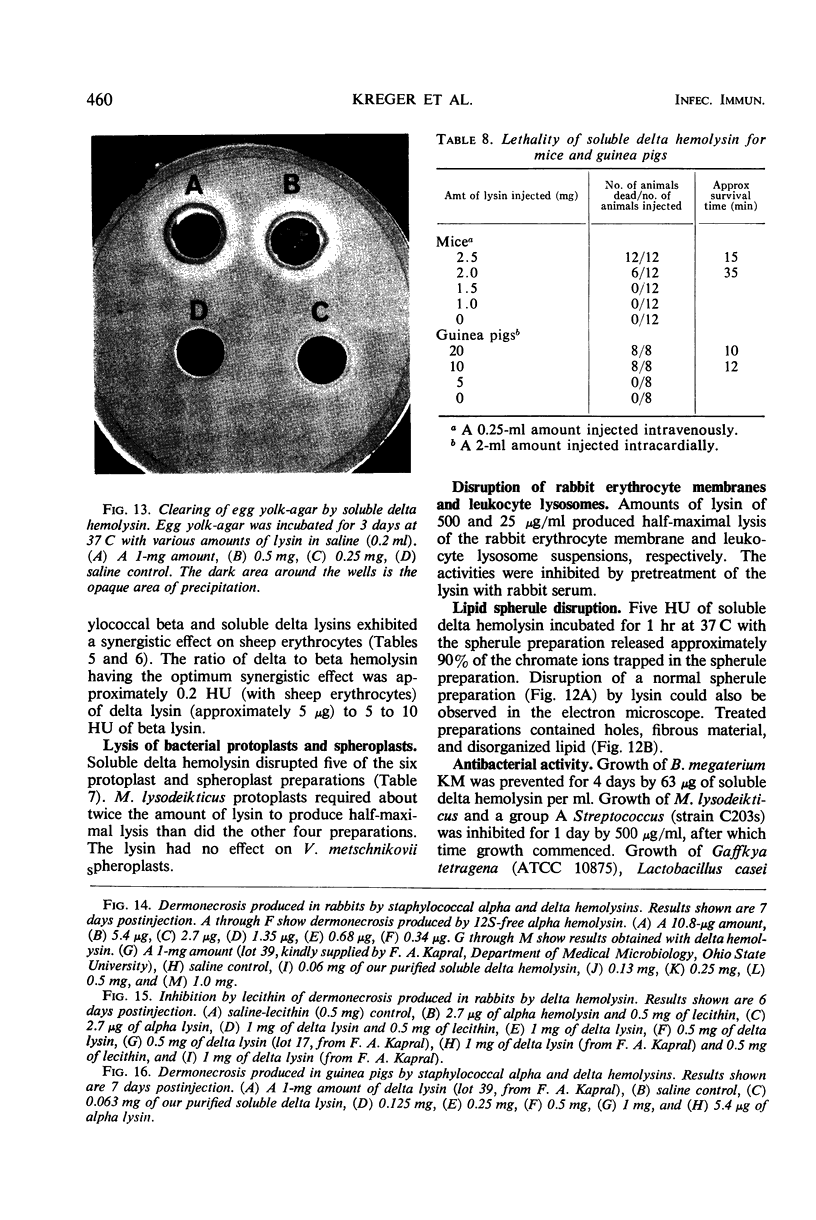
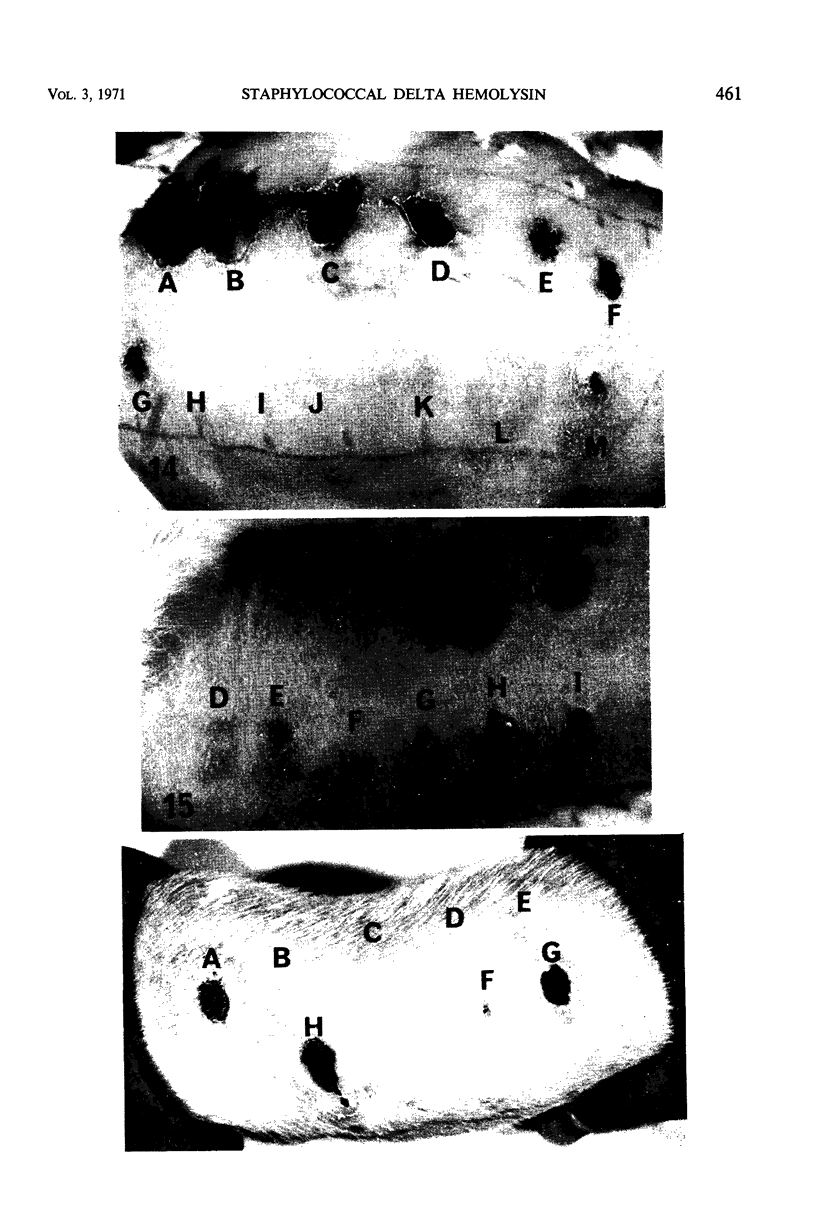
Large amounts (200 mg per liter of culture supernatant fluid) of highly purified staphylococcal soluble delta hemolysin were obtained by adsorption to and selective elution from hydroxyapatite followed by exhaustive dialysis against water, concentration by polyvinylpyrrolidone or polyethylene glycol 20,000 dialysis, and a final water dialysis. No carbohydrate, phosphorus, or inactive 280-nm absorbing material was detected in the preparation; however, analysis by density gradient centrifugation, gel filtration, analytical ultracentrifugation, carboxymethyl cellulose chromatography, polyacrylamide disc gel electrophoresis, isoelectric focusing, and electron microscopy revealed that the lysin was molecularly heterogeneous. The preparation contained an acidic fibrous lysin (S20,w of 11.9) and a basic lysin component composed of a population of granular aggregates of various sizes, with a maximum S20,w of approximately 4.9. No other staphylococcal products were detected in the preparation. The lysin was active against erythrocytes from many animal species and acted synergistically with staphylococcal beta hemolysin against sheep erythrocytes. It was soluble in chloroform-methanol (2:1), was inactivated by various phospholipids, normal sera, and proteolytic enzymes, but was partially resistant to heat inactivation. Activity was not affected by Ca2+, Mg2+, citrate, ethylenediaminetetraacetic acid, or cysteine. The lysin preparation also disrupted bacterial protoplasts and spheroplasts, erythrocyte membranes, lysosomes, and lipid spherules, was growth-inhibitory for certain bacteria, and clarified egg yolk-agar. Large amounts produced dermonecrosis in rabbits and guinea pigs. The minimum lethal intravenous dose for mice and guinea pigs was approximately 110 and 30 mg/kg, respectively.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- Abramson C., Friedman H. Staphylococcal hyaluronate lyase: purification and characterization studies. J Bacteriol. 1968 Oct;96(4):886–892. doi: 10.1128/jb.96.4.886-892.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott J. P., Gemmell C. G., Kent J., Lyell A. Haemolysin and enzyme patterns of coagulase-positive staphylococci isolated from toxic epidermal necrolysis, Ritter's disease and impetigo contagiosa. J Med Microbiol. 1969 Nov 4;2(4):479–487. doi: 10.1099/00222615-2-4-479. [DOI] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. LYSIS OF BACTERIAL PROTOPLASTS AND SPHEROPLASTS BY STAPHYLOCOCCAL ALPHA-TOXIN AND STREPTOLYSIN S. J Bacteriol. 1965 May;89:1387–1392. doi: 10.1128/jb.89.5.1387-1392.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Grushoff P. Lytic effects of staphylococcal alpha-toxin and delta-hemolysin. J Bacteriol. 1968 Aug;96(2):487–491. doi: 10.1128/jb.96.2.487-491.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W. Disruption of wall-less bacteria by streptococcal and staphylococcal toxins. J Bacteriol. 1966 May;91(5):1677–1680. doi: 10.1128/jb.91.5.1677-1680.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Schwartz L. L. Lysosomal disruption by bacterial toxins. J Bacteriol. 1964 May;87(5):1100–1104. doi: 10.1128/jb.87.5.1100-1104.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHESBRO W. R., HEYDRICK F. P., MARTINEAU R., PERKINS G. N. PURIFICATION OF STAPHYLOCOCCAL BETA-HEMOLYSIN AND ITS ACTION ON STAPHYLOCOCCAL AND STREPTOCOCCAL CELL WALLS. J Bacteriol. 1965 Feb;89:378–389. doi: 10.1128/jb.89.2.378-389.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caird J. D., Wiseman G. M. Purification of the delta toxin os Staphylococcus aureus. Can J Microbiol. 1970 Aug;16(8):703–708. doi: 10.1139/m70-120. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue J. A. Antistaphylococcal hemolysins and delta hemolysin inhibitor in adult human serum. Can J Microbiol. 1969 Aug;15(8):957–959. doi: 10.1139/m69-167. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Evans L., Lack C. H. The action of staphylococcal delta-lysin on lysosomes and lymphocytes. Life Sci. 1969 Jul 15;8(14):677–681. doi: 10.1016/0024-3205(69)90002-2. [DOI] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Yoshida A. The cytopathic action of purified staphylococcal delta-hemolysin. Br J Exp Pathol. 1967 Feb;48(1):11–19. [PMC free article] [PubMed] [Google Scholar]
- Gow J. A., Robinson J. Properties of purified staphylococcal beta-hemolysin. J Bacteriol. 1969 Mar;97(3):1026–1032. doi: 10.1128/jb.97.3.1026-1032.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet F., Plommet M. Hémolysine gamma de staphylococcus aureus: purification et propriétés. Ann Inst Pasteur (Paris) 1970 Jan;118(1):19–33. [PubMed] [Google Scholar]
- HALLANDER H. O. FRACTIONATION OF STAPHYLOCOCCAL TOXINS BY GEL-FILTRATION. Acta Pathol Microbiol Scand. 1963;59:543–552. doi: 10.1111/j.1699-0463.1963.tb01258.x. [DOI] [PubMed] [Google Scholar]
- HOFFMANN E. M., STREITFELD M. M. THE ANTIBIOTIC ACTIVITY ASSOCIATED WITH PREPARATIONS OF DELTA HEMOLYSIN OF STAPHYLOCOCCUS AUREUS. Can J Microbiol. 1965 Apr;11:203–211. doi: 10.1139/m65-026. [DOI] [PubMed] [Google Scholar]
- Hallander H. O. Characterization and partial purification of staphylococcal delta-lysin. Acta Pathol Microbiol Scand. 1968;72(4):586–600. doi: 10.1111/j.1699-0463.1968.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Hallanger H. O., Bengtsson S. Studies on the cell toxicity and species specificity of purified staphylococcal toxins. Acta Pathol Microbiol Scand. 1967;70(1):107–119. doi: 10.1111/j.1699-0463.1967.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Haque R. U., Baldwin J. N. Purification and properties of staphylococcal beta hemolysin. II. Purification of beta hemolysin. J Bacteriol. 1969 Nov;100(2):751–759. doi: 10.1128/jb.100.2.751-759.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Purification and properties of lysozyme produced by Staphylococcus aureus. J Bacteriol. 1968 Feb;95(2):376–384. doi: 10.1128/jb.95.2.376-384.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON A. W., LITTLE R. M. Leucocidal effect of staphylococcal delta-lysin. Can J Microbiol. 1957 Feb;3(1):101–102. doi: 10.1139/m57-012. [DOI] [PubMed] [Google Scholar]
- JACKSON A. W., LITTLE R. M. Staphylococcal toxins. II. Factors affecting hemolysis by delta-lysin. Can J Microbiol. 1958 Oct;4(5):435–444. doi: 10.1139/m58-046. [DOI] [PubMed] [Google Scholar]
- JACKSON A. W., LITTLE R. M. Staphylococcal toxins. III. Partial purification and some properties of delta-lysin. Can J Microbiol. 1958 Oct;4(5):453–461. doi: 10.1139/m58-048. [DOI] [PubMed] [Google Scholar]
- KAYSER A., RAYNAUD M. ETUDE D'UNE DEUXI'EME H'EMOLYSINE (DISTINCTE DE L'H'EMOLYSINE-ALPHA) PR'ESENTE DANS LES FILTRATS DE CULTURE DE LA SOUCHE WOOD 46 DE STAPHYLOCOCCUS AUREUS (H'EMOLYSINE G OU DELTA) Ann Inst Pasteur (Paris) 1965 Feb;108:215–233. [PubMed] [Google Scholar]
- KUSHNER D. J. An evaluation of the egg-yolk reaction as a test for lecithinase activity. J Bacteriol. 1957 Mar;73(3):297–302. doi: 10.1128/jb.73.3.297-302.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser A. Chromatographie d'exclusion des complexes cuivriques des protéines. Application à la toxine staphylococcique. Bull Soc Chim Biol (Paris) 1968 Mar 2;50(1):85–91. [PubMed] [Google Scholar]
- Kayser A. Etude d'une deuxième hémolysine (distincte de la toxine alpha) présente dans les filtrats de culture de la souche Wood 46 de Staphylococcus aureus. C R Acad Sci Hebd Seances Acad Sci D. 1966 Aug 22;263(8):693–695. [PubMed] [Google Scholar]
- Kayser A. Fractionnement d'un complexe hémolytique libéré par Staphylococcus aureus Wood 46, et obtention d'une protéine G, vraisemblablement identifiable a l'hémolysine delta. Ann Inst Pasteur (Paris) 1967 Sep;113(3):351–356. [PubMed] [Google Scholar]
- Kayser A. L'hémolysine staphylococcique delta-G. Action sur les érythrocytes et les stromas, sur les sphéroplastes bactériens et sur le jaune d'oeuf. Bull Soc Chim Biol (Paris) 1969 Jan 30;50(10):1797–1808. [PubMed] [Google Scholar]
- Kleck J. L., Donahue J. A. Production of thermostable hemolysin by cultures of Staphylococcus epidermidis. J Infect Dis. 1968 Jun;118(3):317–323. doi: 10.1093/infdis/118.3.317. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKS J. Recognition of pathogenic staphylococci: with notes on non-specific staphylococcal haemolysin. J Pathol Bacteriol. 1952 Jan;64(1):175–186. doi: 10.1002/path.1700640118. [DOI] [PubMed] [Google Scholar]
- MARKS J., VAUGHAN A. C. T. Staphylococcal delta-haemolysin. J Pathol Bacteriol. 1950 Oct;62(4):597–615. doi: 10.1002/path.1700620411. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MCLEOD J. W. Thermostable staphylococcal toxin. J Pathol Bacteriol. 1963 Jul;86:35–53. doi: 10.1002/path.1700860106. [DOI] [PubMed] [Google Scholar]
- Maheswaran S. K., Smith K. L., Lindorfer R. K. Saphylococcal beta-hemolysin. I. Purification of beta-hemolysin. J Bacteriol. 1967 Aug;94(2):300–305. doi: 10.1128/jb.94.2.300-305.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malveaux F. J., Clemente C. L. Staphylococcal acid phosphatase: extensive purification and characterization of the loosely bound enzyme. J Bacteriol. 1969 Mar;97(3):1209–1214. doi: 10.1128/jb.97.3.1209-1214.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., SELIGMAN A. M. Evidence for the specificity of esterase and lipase by the use of three chromogenic substrates. J Biol Chem. 1949 Nov;181(1):343–355. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., SLADE H. D. THE CELLULAR LOCATION OF THE STREPTOCOCCAL GROUP D ANTIGEN. J Gen Microbiol. 1964 Dec;37:297–305. doi: 10.1099/00221287-37-3-297. [DOI] [PubMed] [Google Scholar]
- STOLLERMAN G. H., BERNHEIMER A. W., MacLEOD C. M. The association of lipoproteins with the inhibition of streptolysin S by serum. J Clin Invest. 1950 Dec;29(12):1636–1645. doi: 10.1172/JCI102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T. Studies on extracellular proteins from Staphylococcus aureus. IV. Separation of alpha-toxin by isoelectric focusing. Biochim Biophys Acta. 1968 Oct 21;168(2):228–242. doi: 10.1016/0005-2795(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I., Krakauer K. Arthritis induced in rabbits by lysates of granulocyte lysosomes. Arthritis Rheum. 1969 Apr;12(2):103–116. doi: 10.1002/art.1780120207. [DOI] [PubMed] [Google Scholar]
- Wiseman G. M., Caird J. D. Phospholipase activity of the delta hemolysin of Staphylococcus aureus. Proc Soc Exp Biol Med. 1968 Jun;128(2):428–430. doi: 10.3181/00379727-128-33029. [DOI] [PubMed] [Google Scholar]
- YOSHIDA A. Staphylococcal delta-hemolysin. I. Purification and chemical properties. Biochim Biophys Acta. 1963 Jun 4;71:544–553. doi: 10.1016/0006-3002(63)91126-0. [DOI] [PubMed] [Google Scholar]