Comorbidity was associated with shorter overall survival but not toxicity or relapse among older women with breast cancer with good functional status.
Abstract
Purpose:
We evaluated associations among comorbidity, toxicity, time to relapse (TTR), and overall survival (OS) in older women with early-stage breast cancer receiving adjuvant chemotherapy.
Methods:
Cancer and Leukemia Group B 49907 (Alliance) randomly assigned women ≥ 65 years old with stages I-III breast cancer to standard adjuvant chemotherapy or capecitabine. We reviewed data from 329 women who participated in the quality of life companion study CALGB 70103 and completed the Physical Health Subscale of the Older American Resources and Services Questionnaire. This questionnaire captures data on 14 comorbid conditions and the degree to which each interferes with daily activities. A comorbidity burden score was computed by multiplying the total number of conditions by each condition's level of interference with function. Outcomes were grade 3 to 5 toxicity, TTR, and OS. Logistic regression was used to evaluate associations between comorbidity and toxicity, and Cox proportional hazards models for TTR and survival.
Results:
Number of comorbidities ranged from 0 to 10 (median 2); the comorbidity burden score ranged from 0 to 25 (median 3). The most common conditions were arthritis (58%) and hypertension (55%). Comorbidity was associated with shorter OS, but not with toxicity or TTR. The hazard of death increased by 18% for each comorbidity (hazard ratio [HR] = 1.18, 95% CI = 1.06 to 1.33) after adjusting for age, tumor size, treatment, node and receptor status. Comorbidity burden score was similarly associated with OS (HR = 1.08; 95% CI, 1.03 to 1.14).
Conclusions:
Among older women enrolled onto a clinical trial, comorbidity was associated with shorter OS, but not toxicity or relapse.
Introduction
Breast cancer is commonly diagnosed among women over 65 years old, yet few older women enroll onto clinical trials, leaving oncologists with limited information about the relationships between comorbidity, treatment toxicity, or treatment outcomes for older women receiving adjuvant therapy. Treatment decision making is often complicated by the presence of comorbidities that are common among older adults.1,2 Comorbidity data, however, are not routinely captured in clinical trials. In practice, clinicians regularly extrapolate data from younger, healthier populations when making adjuvant treatment recommendations to older women. Yet even fit older women are at increased risk for early treatment discontinuation, hematologic toxicity, and treatment-related death compared with younger women in the adjuvant setting.3 The presence of comorbidities has been postulated as one of the explanations of differing toxicity risk by chronologic age. A better understanding of the relationship between comorbid conditions and treatment-associated toxicity would improve informed decision making about the risks and benefits of adjuvant chemotherapy for older women with breast cancer.
Cancer and Leukemia Group B (CALGB) 49907 (Alliance) designed a randomized trial that focused specifically on women age 65 years and older. The parent trial randomly assigned women with early-stage breast cancer to receive standard adjuvant chemotherapy (either doxorubicin-cyclophosphamide [AC] or cyclophosphamide-methotrexate-fluorouracil [CMF]) or capecitabine. Women treated with standard chemotherapy had a lower risk of breast cancer recurrence and death than those treated with capecitabine. A companion study (CALGB-70103) examined quality of life outcomes and captured self-reported comorbidity data before therapy.4,5 We hypothesized that women with a greater number of comorbid conditions would experience more treatment toxicity during adjuvant chemotherapy, a shorter time to relapse (TTR), and reduced overall survival (OS). We also postulated that the impact of comorbid conditions on daily function, rather than the actual number of conditions, would better predict treatment toxicity, and that specific comorbid conditions would be differentially associated with clinical outcomes. The aim of the present study was to investigate the associations among pretreatment comorbidity, treatment toxicity, and the outcomes of disease recurrence and death.
Methods
Setting
Between 2001 and 2006, CALGB 49907 (Alliance) enrolled 633 patients age 65 and older at multiple CALGB-affiliated institutions, with the objective of establishing noninferiority of an oral adjuvant chemotherapeutic agent (capecitabine) to standard chemotherapy (AC or CMF). Patients were eligible if they had an operable breast cancer with negative surgical margins; Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; tumor size > 1 cm; adequate hematological, renal (creatinine clearance ≥ 30 mL/min), and hepatic function; and no medical condition that would make the protocol unreasonably hazardous. Eligible patients had to have an expected survival of more than 5 years from enrollment. Status with respect to estrogen receptor, progesterone receptor, and human epidermal growth factor receptor type 2 (HER2) was not specified as an eligibility criterion. Women were excluded if they had a concurrent malignancy or a previous cancer with a risk of relapse > 30%. Participants were assigned randomly in a 1:1 fashion to standard chemotherapy (CMF or AC, by provider choice) or capecitabine.5 Each participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines that included CALGB-49907 and CALGB-70103.
The quality of life companion study enrolled 367 patients, of whom 350 were eligible. Survey data regarding comorbidity, social support, stressful life events, toxicity, physical function, adherence, and neurobehavioral symptoms were collected before, during, and after completion of adjuvant therapy. The current analysis includes participants in the quality of life companion study who completed the self-reported comorbidity assessment during the baseline visit (N = 329). Of these patients, 171 received standard chemotherapy (AC, n = 99; CMF, n = 72), and 158 received capecitabine.
Measures
Comorbidity was assessed by self-report using a modified version of the Older Americans Resources and Services Questionnaire (OARS) Physical Health subscale (form C-720).6 The Physical Health section obtains information on 14 specific physical comorbid conditions and the degree to which each interferes with the participant's activities, rated from 1 to 3 on a Likert scale. Individual self-reported comorbid conditions assessed by this validated questionnaire include the following categories: other cancers, arthritis or rheumatism or other connective tissue disorder, glaucoma, emphysema or chronic bronchitis, high blood pressure, heart disease, circulation problems, diabetes, stomach or intestinal disorders, osteoporosis, chronic liver or kidney disease, stroke, visual impairment, and hearing impairment.
Comorbidity burden was defined in two ways: (1) total number of comorbid conditions (range, 0 to 14), and (2) a comorbidity burden score to assess the impact of comorbidity on daily function. The comorbidity burden score was calculated by multiplying each individual's positive condition (defined by a “yes” answer on the survey) by the degree of self-reported interference with daily activities.7 Degree of interference was categorized as: 1, “not at all”; 2, “somewhat”; and 3, “a great deal.” The total range of possible scores was 0 to 42.
Variables considered as potential confounders were age, race, performance status, stage, hormone receptor status, HER2 status, type of surgery, and treatment allocation (AC/CMF or capecitabine).
The primary outcomes of interest were incident grade 3 to 5 adverse events (AEs) regardless of attribution (AE, graded according to Common Terminology Criteria for Adverse Events version 3.0), OS and TTR. OS was defined as the time from study entry to death, with data censored at last follow-up. TTR was defined as the time from study entry to the first local, regional, or distant breast cancer recurrence, with data censored at death or last follow-up. Secondary outcomes included dose reduction and treatment discontinuation.
Statistical Analysis
Descriptive statistics were used to evaluate patient characteristics, number of self-reported comorbidities, and adverse effects. Logistic regression models were used to evaluate the associations between self-reported comorbidities on the OARS subscale and the incidence of grade 3 to 5 AEs during the study. Total number of comorbidities and the comorbidity burden score were initially evaluated as continuous measures. When the descriptive analysis demonstrated that the scores were not normally distributed, an exploratory analysis was performed using Box-Cox transformations and categorical assignments for the degree of comorbidity. Number of comorbidities was classified into one of the following groups for categorical analyses: none, 1, 2 to 3, and ≥ 4.
Separate multivariable logistic models were used to evaluate the associations between comorbidity burden (total number and burden score) after adjusting for the effects of age, race, performance status, stage, hormone receptor status, HER2 status, type of surgery (lumpectomy with breast irradiation or mastectomy), and chemotherapy treatment (AC/CMF or capecitabine). Odds ratios (ORs) with 95% CIs were calculated for the incidence of AEs, and a Wald test was used to determine statistically significant effects at a two-sided alpha level of .05. For individual comorbidities, the associations with AEs were evaluated in univariable logistic regression models.
In addition, TTR and OS for the number of comorbidities were estimated using Kaplan-Meier methods. Cox proportional hazard models were used to evaluate the impact of self-reported comorbidities (total number and burden score) on TTR and OS. Multivariable models with the aforementioned covariables were used to evaluate the independent prognostic value, and corresponding hazard ratios (HRs) with 95% CIs were determined. Exploratory univariable modeling was used to evaluate the relationship between specific comorbid conditions and OS; multivariable analyses were limited by small sample size in each disease category. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center using SAS v. 9.2 (Cary, NC). Analyses are based on data collected through April 2012.
Results
Patient Characteristics
Characteristics of the 329 participants are listed in Table 1. The median age was 71 years (range 65 to 89 years). Most subjects (87%) were white and had good performance status (98%), as indicated by an ECOG score of 0 or 1. Almost half (47%) were treated with capecitabine, whereas 30% and 22% were treated with AC and CMF, respectively. Stage was I in 7%, II in 68%, and III in 9%.
Table 1.
Baseline Patient Characteristics (N = 329)
| Characteristic | % | |
|---|---|---|
| Age, years | ||
| Median | 71 | |
| Range | 65-89 | |
| Race | ||
| White | 87 | |
| African American | 11 | |
| Other | 1 | |
| Unknown | 1 | |
| ECOG score | ||
| 0 | 72 | |
| 1 | 26 | |
| Stage grouping | ||
| I | 7 | |
| II | 68 | |
| III | 9 | |
| Hormone receptor positive | 67 | |
| HER2 positive | 15 | |
| Treatment regimen | ||
| CMF | 22 | |
| AC | 30 | |
| Capecitabine | 47 | |
| Total No. of comorbid conditions | ||
| Median | 2 | |
| Range | 0-10 | |
| Comorbidity burden score | ||
| Median | 3 | |
| Range | 0-25 | |
| Individual comorbid conditions | ||
| Arthritis | 59 | |
| Hypertension | 55 | |
| Osteoporosis | 22 | |
| Diabetes | 18 | |
| Circulatory problems | 18 | |
| Heart disease | 16 | |
| Poor eyesight | 16 | |
| Poor hearing | 16 | |
| Gastrointestinal problems | 14 | |
| Chronic pulmonary disease | 9 | |
| Glaucoma | 9 | |
| Cerebrovascular accident | 6 | |
| Other cancers | 3 | |
| Liver or kidney disease | 1 |
Abbreviations: AC, doxorubicin-cyclophosphamide; CMF, cyclophosphamide-methotrexate-fluorouracil; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2.
The median total number of comorbid conditions was 2 (range 0 to 10), and the median comorbidity burden score was 3 (range 0 to 25). The most common individual comorbid conditions were arthritis (59%) and hypertension (55%).
Outcomes
Fifty-two percent of patients experienced at least one grade 3 to 5 AE, with the total number of AEs ranging from 0 to 28. Of these, 28% were hematologic, 12% gastrointestinal, and 3% neurologic. Only one person died of treatment-related toxicity. Treatment doses were modified in 51% of patients; in 44%, these changes were made per protocol. Fifteen percent of patients discontinued therapy early, half as a result of AEs. After a median follow-up of 5.2 years, the overall mortality rate was 25%, with a breast cancer–specific mortality rate of 11%. During follow-up, relapse occurred in 16% of participants.
Associations Between Baseline Comorbidities and Outcomes
There was no association between the number of comorbid conditions reported at study enrollment and incidence of grade 3 or higher AEs (P = .48). Similarly, there was no association between comorbidity burden score and grade 3 or higher AE (P = .42, Table 2). Multivariable analyses were not done because the univariable analyses did not detect significant associations. Results did not differ when cumulative AEs were considered as the outcome (data not shown). No relationships between individual comorbid conditions and toxicity were seen. However, patients with two or more comorbid conditions were more likely to experience a treatment modification (59%) than those with fewer than 2 conditions (46%; P = .03). The majority of dose modifications (80%) were per protocol. Dose modification was not associated with incident toxicity (P = .21) in exploratory analyses. Subjects with two or more comorbid conditions were equally likely to discontinue treatment early compared with those with fewer than two comorbid conditions (15% in each group). There was no association seen between comorbidity and TTR (Table 2).
Table 2.
Relationship Between Comorbidity and Clinical Outcomes
| Comorbidity Variable | Incident AE* |
Time to Relapse† |
Overall Survival† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Total No. comorbid conditions | 0.97 | 0.86 to 1.09 | .60 | 1.09 | 0.95 to 1.26 | 0.23 | 1.18 | 1.06 to 1.33 | < .01 |
| Comorbidity burden score | 0.97 | 0.92 to 1.04 | .42 | 1.03 | 0.97 to 1.10 | 0.37 | 1.08 | 1.03 to 1.14 | < .01 |
Abbreviations: AE, adverse event; HR, hazard ratio; OR, odds ratio; AC, doxorubicin and cyclophosphamide; CMF, cyclophosphamide, methotrexate, fluorouracil.
Any grade 3, 4, or 5 AE.
Adjusted for age, tumor size, chemotherapy treatment arm (AC/CMF v capecitabine), nodal status, and hormone receptor status.
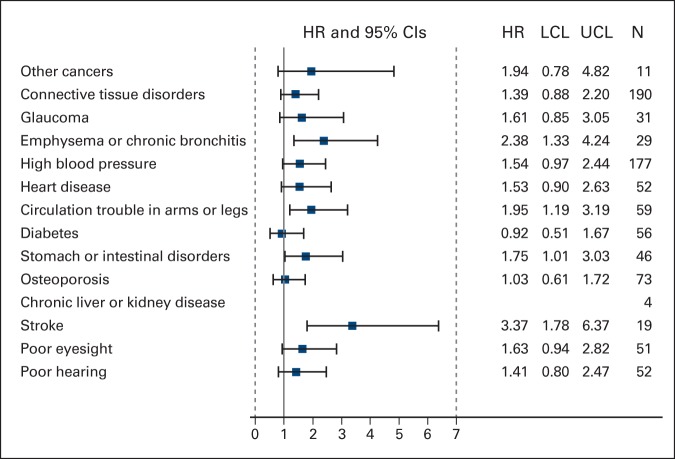
The hazard of death increased by 18% for each additional comorbid condition after adjusting for age, tumor size, treatment, node status, and receptor status (HR = 1.18; 95% CI, 1.06 to 1.33; Table 2). Comorbidity burden score was similarly associated with OS in adjusted analyses (HR = 1.08; 95% CI, 1.03 to 1.14). Presence of four or more comorbid conditions at baseline appeared to be a threshold for shorter OS in this cohort (Figure 1A). Five-year survival rates were similar for patients who reported 0 (90.3%), 1 (85.3%), and 2 to 3 conditions (83.8%) in contrast with patients reporting four or more conditions at baseline (73.3%; P = .002). Among individual comorbid conditions, self-reported emphysema or chronic bronchitis, circulation trouble, and stroke were associated with higher mortality in unadjusted exploratory analyses (Appendix Figure A1, online only).
Figure 1.
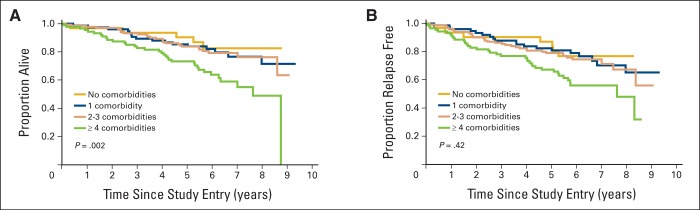
(A) Overall survival and association with numbers of comorbid conditions at baseline. (B) Relapse-free probability and association with number of comorbid conditions at baseline.
Discussion
Because many older cancer patients have comorbidities, investigating the associations between comorbidities and treatment outcomes is important. Data on this topic are scarce. In this study, self-reported comorbidity was not associated with AEs or breast cancer relapse among women with good functional status enrolled on an adjuvant chemotherapy trial. However, comorbidity burden was associated with shorter OS. The presence of four or more conditions appeared to be a threshold for shorter survival in this cohort.
Comorbidity can complicate treatment decision making in the adjuvant setting in several ways. Comorbidity might increase the adverse effects of chemotherapy, altering the risk-benefit balance to the patient. Treatment complications might result in secondary modifications, including dose reductions and early discontinuation, potentially increasing the risk of relapse. Finally, comorbidity alters life expectancy and thereby attenuates estimates of potential benefits from adjuvant therapy. The literature shows that concerns regarding comorbidity influence chemotherapy use for older adults. A survey of breast cancer–specific oncologists found that adjuvant chemotherapy decisions vary on the basis of a patient's health status, functional status, and age.1 A systematic review reported similar associations between increasing comorbidity and declining chemotherapy use among multiple cancer populations.8 Hawfield et al9 reviewed records of 273 women with early-stage breast cancer (mean age 65 years) and found that a Charlson Comorbidity Index (CCI) score of ≥ 2 was associated with 80% lower odds of chemotherapy use.
Few studies, however, have directly investigated the impact of comorbidity on treatment tolerability among older adults in the adjuvant setting. Among 34 articles analyzed in the systematic review by Lee et al, less than one third included a tolerability outcome, and only three evaluated AEs during receipt of adjuvant treatment (two were specific to breast cancer).8,10–12 Retrospective analyses in both older adults who received adjuvant cytotoxic chemotherapy with standard regimens and in those with early-stage breast cancer have shown an association between higher CCI and decreased rate of completion of chemotherapy, breaks in chemotherapy, reduction in dose; development of a grade 3 to 4 neutropenia, grade 3 to 4 constitutional symptoms, and incident grade 3 to 4 toxicity.10,12
In contrast to these findings, our analysis did not reveal an association between incident or cumulative toxicity and comorbidity. Several differences in study design may account for this discrepancy. First, our population was highly selected, and most participants were rated as having excellent performance status (ECOG score 0). Preserved functional status in the setting of comorbidity as seen in our cohort could reflect better compensated or earlier stage comorbid conditions. Alternatively, the types of comorbid conditions may differ between our cohort and those reported above. Although the self-report comorbidity tool used in our study includes most conditions assessed by the commonly studied CCI (exceptions include dementia, hemiplegia, and HIV infection), it also captures conditions that are not considered major comorbidities by the CCI. For example, common conditions in our cohort included arthritis, hypertension, osteoporosis, and sensory impairments, which are not considered comorbidities by the CCI. In both studies mentioned above, 20% to 30% of the study population had at least one major comorbid condition (eg, diabetes, history of myocardial infarction, chronic lung disease, renal dysfunction). Prevalence of these conditions was lower in our clinical trial cohort in part due to specific exclusion criteria (ie, patients with creatinine clearance < 30, elevated bilirubin, or uncompensated heart failure were excluded). In addition, specific comorbid conditions shown to increase toxicity risk among older patients (eg, anemia) were not captured by our self-reported comorbidity tool.13,14 Finally, protocol-driven treatment modifications may have altered the types and severity of toxicity we observed. Our data suggest that among older women with good functional status receiving protocol-directed adjuvant chemotherapy, the presence of self-reported comorbidity does not increase treatment-related toxicity.
Comorbidities have been associated with an increased risk of dying as a result of breast cancer in the adjuvant setting. Data from the Swedish Cancer Registry suggested that women with early-stage breast cancer and a high CCI score at the time of diagnosis had a 47% greater hazard of dying as a result of breast cancer compared with women without significant comorbidity.15 Treatment-related explanations for this association include withheld adjuvant therapy, protocol modifications, and decreased dose intensity.10 Few studies have investigated the association between comorbidity and cancer-specific outcomes in a setting that controls for treatment. Land et al used registry data from Denmark to evaluate the impact of mild to moderate comorbidity (CCI score 1 to 2) compared with no comorbidity among women treated with adjuvant chemotherapy.16 Consistent with our results, they found no association between mild to moderate comorbidity and breast cancer–specific mortality.
Although there is no clear association between baseline comorbidity and relapse, our study is consistent with many prior publications that have demonstrated an association between comorbidity and all-cause mortality among breast cancer survivors.17–21 Controlling for age and treatment type, patients with three or more selected comorbid conditions in a large observational cohort had a 4-fold higher rate of all-cause mortality compared with patients with no comorbid conditions.19 Another study showed that a one-unit increase in the CCI raises the hazard rate of all-cause mortality by approximately 1.4-fold for women treated for breast cancer.17 Our study adds to the literature by controlling for functional status and treatment type in a randomized clinical trial. Our cohort had a lower prevalence of serious comorbid conditions. Nonetheless, we found a clear association between self-reported comorbidity and OS. Our data suggest that simply accounting for the number of selected comorbid conditions reported in patients with good functional status is as predictive as evaluating how much these conditions interfere with daily activity. This provides support for a practical and efficient approach of comorbidity screening in clinical practice and provides evidence that a threshold of four or more comorbid conditions may be considered clinically relevant from the standpoint of its negative impact on life expectancy. This has relevance for adjuvant treatment decision making.
Certain comorbid conditions may be more likely to influence all-cause mortality. For example, patients with breast cancer with pre-existing diabetes may be at increased risk for all-cause mortality compared with those without diabetes,22–24 perhaps because patients with diabetes are less likely to receive standard therapies, which may influence mortality outcomes.25 In addition, the degree to which a comorbid condition is well controlled may also influence mortality risk and should be considered during treatment decision making.26–28 Our analysis did not show an association between diabetes and any outcomes including mortality. However, diabetic patients enrolled onto a clinical trial may represent those with better disease control. Our exploratory analyses did suggest that chronic pulmonary disease and vascular disease (peripheral and cerebral) warrant further study as conditions that increase mortality risk in the adjuvant setting.29
This study has several limitations. Our analyses were restricted to a selected population of women healthy enough to receive chemotherapy on a clinical trial, perhaps resulting in lower comorbidity burden at baseline and minimizing our ability to detect influences of specific comorbidities. However, the results are relevant to women whose performance status remains good despite comorbidities, and sheds light on issues related to multimorbidity that require additional study in less fit populations. Another limitation is the lack of objective assessment of comorbid conditions. However, the literature suggests that self-report is quite reliable compared with medical record review.30,31 Furthermore, this approach remains relevant to clinical practice, as past medical history is often obtained in part via a patient interview. The use of a specific self-report comorbidity scale may be considered a limitation by only accounting for 14 specific conditions. This does, however, provide a framework for standardization of data collection in future trials and in the clinic. Our data set does not include information on mean cumulative dose received or reasons for protocol-specified dose modifications that could further inform the lack of association between comorbidity and toxicity. Finally, our study does not account for AEs that occurred after completion of treatment, or global quality of life.
There are also several strengths of this analysis. Our analysis focuses specifically on older patients with cancer, who have been consistently underrepresented in clinical trials. Using data from a clinical trial increases homogeneity of treatment, to minimize the confounding effect of comorbidities' influence on treatment choice. Similarly, using clinical trial data allows us to take advantage of excellent adjudication of the toxicity and clinical outcomes.
In summary, this study demonstrates that comorbidity is not associated with increased treatment toxicity or relapse for older women with good performance status receiving adjuvant breast cancer chemotherapy. On the other hand, comorbidity burden adversely affects life expectancy and should be considered in treatment decision making regarding expected benefit of adjuvant therapy. Studies in patients with cancer who are less fit would increase the generalizability of this line of research to better reflect the many older adults seen in clinical oncology practice.
Acknowledgment
Supported by National Cancer Institute Grants No. CA31946 to the Alliance for Clinical Trials in Oncology and CA33601 to the Alliance Statistics and Data Center, American College of Surgeons Oncology Group Grant No. CA76001, and North Central Cancer Treatment Group Grant No. CA025224. H.D.K. is funded by a Paul Beeson Career Development Award in Aging Research (K23AG038361; supported by National Institute on Aging, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies), and The Gabrielle's Angel Foundation for Cancer Research. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Appendix
Figure A1.
Forest plot illustrating unadjusted proportional hazard models of overall survival for individual comorbid conditions. Abbreviations: HR, hazard ratio; LCL, lower confidence limit; UCL, upper confidence limit.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Arti Hurria, GTx (C), Seattle Genetics (C) Stock Ownership: None Honoraria: None Research Funding: Arti Hurria, Celgene, GlaxoSmithKline; Eric P. Winer, Genentech Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
Author Contributions
Conception and design: Heidi D. Klepin, Karla V. Ballman, Alice B. Kornblith, Arti Hurria, Eric P. Winer, Clifford A. Hudis, Harvey J. Cohen, Hyman B. Muss, Gretchen Genevieve Kimmick
Collection and assembly of data: Brandelyn N. Pitcher, Hyman B. Muss
Data analysis and interpretation: Heidi D. Klepin, Brandelyn N. Pitcher, Karla V. Ballman, Alice B. Kornblith, Arti Hurria, Harvey J. Cohen, Hyman B. Muss, Gretchen G. Kimmick
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Hurria A, Naeim A, Elkin E, et al. Adjuvant treatment recommendations in older women with breast cancer: A survey of oncologists. Crit Rev Oncol Hematol. 2007;61:255–260. doi: 10.1016/j.critrevonc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 3.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 4.Kornblith AB, Lan L, Archer L, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: A companion study to Cancer and Leukemia Group B 49907. J Clin Oncol. 2011;29:1022–1028. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 7.Ingram SS, Seo PH, Martell RE, et al. Comprehensive assessment of the elderly cancer patient: The feasibility of self-report methodology. J Clin Oncol. 2002;20:770–775. doi: 10.1200/JCO.2002.20.3.770. [DOI] [PubMed] [Google Scholar]
- 8.Lee L, Cheung WY, Atkinson E, et al. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J Clin Oncol. 2011;29:106–117. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 9.Hawfield A, Lovato J, Covington D, et al. Retrospective study of the effect of comorbidity on use of adjuvant chemotherapy in older women with breast cancer in a tertiary care setting. Crit Rev Oncol Hematol. 2006;59:250–255. doi: 10.1016/j.critrevonc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Garg P, Rana F, Gupta R, et al. Predictors of toxicity and toxicity profile of adjuvant chemotherapy in elderly breast cancer patients. Breast J. 2009;15:404–408. doi: 10.1111/j.1524-4741.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–440. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 12.Zauderer M, Patil S, Hurria A. Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat. 2009;117:205–210. doi: 10.1007/s10549-008-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2011;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 14.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berglund A, Wigertz A, Adolfsson J, et al. Impact of comorbidity on management and mortality in women diagnosed with breast cancer. Breast Cancer Res Treat. 2012;135:281–289. doi: 10.1007/s10549-012-2176-4. [DOI] [PubMed] [Google Scholar]
- 16.Land LH, Dalton SO, Jensen MB, et al. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer. 2012;107:1901–1907. doi: 10.1038/bjc.2012.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahern TP, Lash TL, Thwin SS, et al. Impact of acquired comorbidities on all-cause mortality rates among older breast cancer survivors. Med Care. 2009;47:73–79. doi: 10.1097/MLR.0b013e318180913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braithwaite D, Moore DH, Satariano WA, et al. Prognostic impact of comorbidity among long-term breast cancer survivors: Results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012;21:1115–1125. doi: 10.1158/1055-9965.EPI-11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 20.Siegelmann-Danieli N, Khandelwal V, Wood GC, et al. Breast cancer in elderly women: Outcome as affected by age, tumor features, comorbidities, and treatment approach. Clin Breast Cancer. 2006;7:59–66. doi: 10.3816/CBC.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 21.Ording AG, Garne JP, Nyström PMW, et al. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis – a Danish nationwide matched cohort study. PLoS One. 2013;8:e76013. doi: 10.1371/journal.pone.0076013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipscombe LL, Goodwin PJ, Zinman B, et al. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat. 2008;109:389–395. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- 24.Nechuta S, Lu W, Zheng Y, et al. Comorbidities and breast cancer survival: A report from the Shanghai Breast Cancer Survival Study. Breast Cancer Res Treat: 2013;139:227–235. doi: 10.1007/s10549-013-2521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: A systematic review and meta-analysis. J Clin Oncol. 2011;29:40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braithwaite D, Tammemagi CM, Moore DH, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer. 2009;124:1213–1219. doi: 10.1002/ijc.24054. [DOI] [PubMed] [Google Scholar]
- 27.Erickson K, Patterson RE, Flatt SW, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29:54–60. doi: 10.1200/JCO.2010.29.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiderlen M, de Glas NA, Bastiaannet E, et al. Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: A FOCUS study analysis. Ann Oncol. 2013;24:3011–3016. doi: 10.1093/annonc/mdt367. [DOI] [PubMed] [Google Scholar]
- 29.Louwman WJ, Janssen-Heijnen ML, Houterman S, et al. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: A population-based study. Eur J Cancer. 2005;41:779–785. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Bush TL, Miller SR, Golden AL, et al. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriegsman DM, Penninx BW, van Eijk JT, et al. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients' self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49:1407–1417. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]