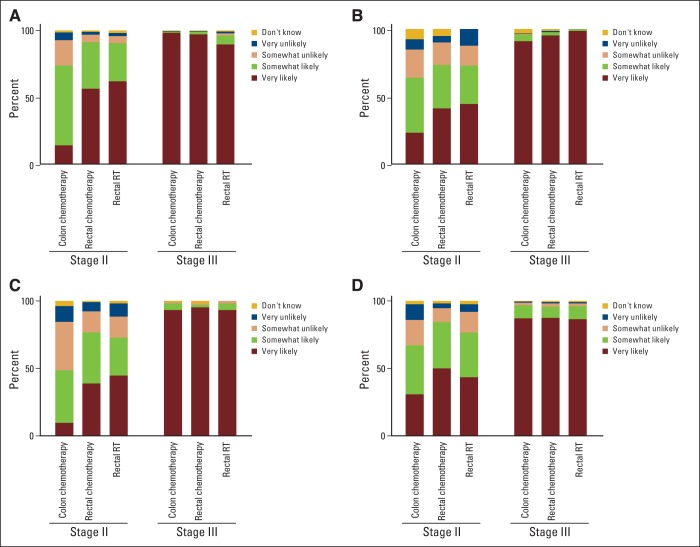
Physicians agreed that the benefits of adjuvant chemotherapy for stage III colon cancer and chemotherapy, and radiation for stage III rectal cancer, outweigh the risks, but were divided over the net benefit of adjuvant therapies for stage II colorectal cancer.
Abstract
Background:
Adjuvant therapy plays a major role in treating colorectal cancer, and physicians' views of its effectiveness influence treatment decisions. We assessed physicians' views of the relative benefits and risks of adjuvant chemotherapy and radiotherapy for stages II and III colon and rectal cancers.
Methods:
The Cancer Care Outcomes Research and Surveillance Consortium surveyed a geographically dispersed population of medical oncologists, radiation oncologists, and surgeons in the United States about the benefits and risks of adjuvant therapies for colorectal cancer. We used logistic regression to assess the association of physician and practice characteristics with beliefs about adjuvant therapies.
Results:
Among 1,296 respondents, > 90% believed the benefits of adjuvant therapies for stage III colorectal cancer outweigh the risks. Only 21.9%, 50%, and 50.4% believed in the net benefit of chemotherapy for stage II colon cancer, chemotherapy for stage II rectal cancer, and radiation for stage II rectal cancer, respectively. Younger physicians were less likely than others to perceive adjuvant therapy for stage II colorectal cancer as beneficial. Medical oncologists were more likely than surgeons and radiation oncologists to endorse the benefits of adjuvant chemotherapy and radiation for stage II rectal cancer, but less likely for stage II colon cancer.
Conclusions:
Physicians largely agreed that the benefits of adjuvant chemotherapy for stage III colon cancer, as well as chemotherapy, and radiation for stage III rectal cancer, outweigh the risks, consistent with strong evidence, but were divided over the net benefit of adjuvant therapies for stage II colorectal cancer, where evidence is inconsistent.
Introduction
Patterns-of-care studies have demonstrated significant variability in colorectal cancer care.1–6 Patients with colorectal cancer who receive guideline-concordant therapy survive longer than those who do not,3,7,8 yet many patients do not receive recommended therapies. Physicians are less likely to offer adjuvant therapy to older patients with colorectal cancer with medical comorbidities than to younger and healthier patients.3,4,9,10 However, few data are available regarding physicians' beliefs about the risks and benefits of adjuvant therapy, even though physicians' recommendations are important determinants of patients' treatment decisions.11
Current national guidelines recommend adjuvant chemotherapy for stage III and high-risk stage II colon cancer, and neoadjuvant chemoradiotherapy and adjuvant chemotherapy for stages II-III rectal cancer.12,13 Features that confer high risk of recurrence in stage II colon cancer include large tumors (T4), bowel perforation or obstruction, lymphovascular invasion, poorly differentiated histology, positive margins, or inadequate lymph node sampling.14–16 Guideline recommendations for patients with stage III colorectal cancer are based on high-quality randomized controlled trials that have demonstrated that adjuvant chemotherapy for stage III colon cancer17–21 and neoadjuvant radiation with or without chemotherapy for stage III rectal cancer22–26 improve outcomes. Treatment of stage II colon and rectal cancers is more controversial.17–20,25,27,28 For stage II colon cancer, studies that assessed survival after adjuvant fluorouracil (FU) chemotherapy after curative resection have been mixed,17,18,20 and no overall survival benefit for adjuvant folinic acid (leucovorin)-FU-oxaliplatin (FOLFOX) chemotherapy has been found, even in high-risk stage II patients in whom recurrence rates approach those of stage III patients.21,29 In stage II rectal cancer, for which radiotherapy primarily impacts local recurrence, pooled data from adjuvant therapy trials demonstrate that pathologically staged T3N0 rectal cancers may be intermediate risk and therefore may not require adjuvant radiotherapy.30 However, it may be premature to omit radiotherapy for cT3N0 tumors given the uncertainties of clinical staging. Chemotherapy for rectal cancer is also controversial; a randomized study showed no survival benefit with adjuvant FU.31 The adjuvant chemotherapy guidelines for rectal cancer are based on extrapolations from colon cancer trials.
We surveyed a geographically dispersed, representative population of US medical oncologists, radiation oncologists, colorectal surgeons, and general surgeons to understand how physicians who treat colorectal cancer perceive the balance of benefits and harms of adjuvant therapy. We also examined how physician and practice characteristics correlated with the more varied beliefs about the benefits of adjuvant therapy for stage II colon and rectal cancer.
Methods
Design
The Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) collected information from patient surveys, medical records, and physician surveys for approximately 5,000 patients with colorectal cancer diagnosed during 2003 to 2005 in Northern California, Los Angeles County, North Carolina, Iowa, or Alabama or who received care in one of five large health maintenance organizations or 15 Veterans Affairs Medical Centers.32,33 This analysis used only physician survey data. The study was approved by human subjects committees at all participating institutions.
Population
As described previously,9 physicians named by patients as providing important roles in their care were surveyed from 2004 to 2007 (97% of surveys were mailed between January 2005 and May 2006). Contact information for 6,871 physicians was verified, and 4,188 (61.0%) responded. Respondents did not differ by sex (P = .97). Radiation oncologists and those who graduated from medical school before 1976 or after 1989 versus 1976 to 1989 responded more frequently (both P ≤ .005).
We restricted the sample to the 1,382 physicians who self-identified as surgeons, radiation oncologists, or medical oncologists; cared for more than one patient with colorectal cancer in the past year; and were not still in training. We focused on the 1,296 physicians with complete data on the six questions of primary interest (described below).
Survey
To assess physicians' beliefs about the benefits versus risks of adjuvant colorectal cancer therapies, each physician was asked, “For an otherwise healthy 55-year-old man with colorectal cancer, how likely is it that the benefits outweigh the risks for each of the following treatments? (1) adjuvant chemotherapy for stage II colon cancer, (2) adjuvant chemotherapy for stage III colon cancer, (3) adjuvant chemotherapy for stage II rectal cancer, (4) adjuvant chemotherapy for stage III rectal cancer, (5) adjuvant radiotherapy for stage II rectal cancer, (6) adjuvant radiotherapy for stage III rectal cancer.” Physicians responded “very unlikely,” “somewhat unlikely,” “somewhat likely,” “very likely,” or “don't know.” Physicians also reported specialty, age, United States/Canadian medical graduate status, practice site, whether they practice at a National Cancer Institute (NCI)–designated cancer center, number of colorectal cancer patients cared for in the last month, whether they enroll patients onto clinical trials, teaching involvement, attendance at tumor board meetings, percentage of patients in managed care, and base clinical income. Variables were categorized as shown in Table 1.
Table 1.
Percentage of Physicians Who Reported That the Benefits of Adjuvant Therapy Are Very Likely to Outweigh the Risks for Stage II Colon and Rectal Cancers
| Physician Characteristic | No. | % | Chemotherapy for Stage II Colon Cancer | χ2 P | Chemotherapy for Stage II Rectal Cancer | χ2 P | Radiotherapy for Stage II Rectal Cancer | χ2 P |
|---|---|---|---|---|---|---|---|---|
| All physicians | 21.9 | 50 | 50.4 | |||||
| Specialty | ||||||||
| Medical oncologist | 466 | 36 | 14.2 | < .001 | 56.4 | < .001 | 62 | < .001 |
| Radiation oncologist | 211 | 16 | 23.2 | 41.2 | 44.6 | |||
| Colorectal surgeon/surgical oncologist | 103 | 8 | 9.7 | 38.8 | 44.7 | |||
| General surgeon | 516 | 40 | 30.8 | 50 | 43.4 | |||
| Age, years | ||||||||
| < 40 | 228 | 18 | 16.9 | < .001 | 48.5 | .1 | 45.9 | .35 |
| 40-49 | 400 | 31 | 17.7 | 48.5 | 50.7 | |||
| 50-54 | 217 | 17 | 19.3 | 49.2 | 51.5 | |||
| 55-59 | 217 | 17 | 25.8 | 46.6 | 48.3 | |||
| ≥ 60 | 228 | 18 | 33.2 | 58.3 | 55.2 | |||
| Missing | 6 | 0 | ||||||
| US medical graduate | ||||||||
| Yes | 1,088 | 84 | 21.5 | .38 | 48.2 | .002 | 48.6 | .002 |
| No | 201 | 16 | 24.3 | 59.9 | 60.3 | |||
| Missing | 7 | 1 | ||||||
| Practice site | ||||||||
| Office, solo | 150 | 12 | 35 | < .001 | 53.4 | .41 | 56.1 | .27 |
| Office, single-specialty group | 346 | 27 | 21.1 | 53.3 | 48.8 | |||
| Office, multi-specialty group | 103 | 8 | 21.9 | 53.1 | 55.1 | |||
| HMO | 243 | 19 | 19.8 | 48.6 | 52.3 | |||
| VA/government | 102 | 8 | 11.8 | 47.1 | 52.9 | |||
| Hospital | 352 | 27 | 21.6 | 46.3 | 46.1 | |||
| NCI cancer center | ||||||||
| Yes | 300 | 23 | 22.2 | .87 | 49.8 | .96 | 46.5 | .12 |
| No/don't know | 974 | 75 | 21.8 | 50.1 | 51.6 | |||
| Missing | 22 | 2 | ||||||
| No. of patients per month | ||||||||
| ≤ 2 | 325 | 25 | 29.9 | < .001 | 47 | .38 | 46.2 | < .001 |
| 2 to 5 | 395 | 30 | 21.2 | 51.6 | 44.1 | |||
| 5 to 10 | 269 | 21 | 17.4 | 48 | 52.7 | |||
| >10 | 276 | 21 | 18.2 | 53.1 | 61.8 | |||
| Missing | 31 | 2 | ||||||
| Enroll patients onto clinical trials | ||||||||
| Yes | 719 | 55 | 18.2 | < .001 | 50.8 | .52 | 53.9 | .002 |
| No | 523 | 40 | 27.3 | 48.9 | 45.2 | |||
| Missing | 54 | 4 | ||||||
| Teaching | ||||||||
| None | 654 | 50 | 21.8 | .18 | 50.7 | .56 | 49.7 | .61 |
| 1-5 d/mo | 289 | 22 | 25.5 | 51.3 | 53 | |||
| ≥ 6 d/mo | 332 | 26 | 19.1 | 47.4 | 49.5 | |||
| Missing | 21 | 2 | ||||||
| Attend tumor board | ||||||||
| Weekly | 705 | 54 | 18.3 | < .001 | 48.8 | .64 | 51.2 | .78 |
| Monthly | 326 | 25 | 22.4 | 50.7 | 49.9 | |||
| Quarterly or less often | 250 | 19 | 31.3 | 52.3 | 48.7 | |||
| Missing | 15 | 1 | ||||||
| % patients in managed care | ||||||||
| 0-20 | 319 | 25 | 20.2 | .15 | 51.5 | .8 | 51.6 | .68 |
| 21-49 | 267 | 21 | 21.9 | 50.8 | 47 | |||
| 50-78 | 316 | 24 | 26.1 | 50.5 | 51 | |||
| 79-100 | 286 | 22 | 19.4 | 47.5 | 51.3 | |||
| Missing | 108 | 8 | ||||||
| Base clinical income | ||||||||
| Mostly fee-for-service | 485 | 37 | 28.5 | .002 | 51.8 | .79 | 50.4 | .71 |
| Mixture fee-for-service and capitation | 332 | 26 | 17.2 | 47.6 | 42.5 | |||
| Salary, productivity based | 391 | 30 | 18.9 | 48.5 | 51.6 | |||
| Salary, not productivity based | 42 | 3 | 19.4 | 50.5 | 49.7 | |||
| Missing | 46 | 4 |
Abbreviations: HMO, health maintenance organization; VA, Veterans Affairs; NCI, National Cancer Institute.
Statistical Analysis
For most variables, item nonresponse ranged from less than 2% to 3%. No subjects were missing the dependent variables of interest on the basis of the inclusion criteria described above. We used multiple imputation to impute missing data.34,35 We categorized physicians' reports about the relative benefits outweighing risks of each treatment as “very likely” versus “somewhat likely/somewhat unlikely/very unlikely/don't know.” “Don't know” responses were ≤ 3% for all scenarios.
We examined physician and practice characteristics associated with believing that the benefits very likely outweigh the risks of adjuvant therapy for stage II colorectal cancer (nearly all physicians endorsed the net benefits of adjuvant therapies for stage III disease). We used logistic regression to evaluate the association of independent variables with P < .20 in bivariable analyses with any of the therapies with the likelihood of responding that benefits very likely outweigh the risks of adjuvant therapy. All tests of statistical significance were two-sided. Statistical analyses were performed using the SAS version 9.2 statistical package (SAS Institute, Cary, NC).
Results
The 1,296 physicians were medical oncologists (36%), general surgeons (40%), radiation oncologists (16%), and surgeons or surgical oncologists (8%); had a median age of 50 years; and saw a median of five patients with colorectal cancer per month (Table 1). On average, the survey respondents were 23 years post–medical school graduation, and 94% were board certified.
There was consensus among physicians that the benefits of adjuvant therapy outweigh the risks for an otherwise healthy middle-aged patient with stage III colorectal cancer (Figure 1), with nearly all indicating that the benefits of adjuvant chemotherapy in stage III colon cancer (92%), and adjuvant chemotherapy (93%) and radiation (90%) for stage III rectal cancer were very likely to outweigh the risks.
Figure 1.
Unadjusted frequencies of physician responses regarding the likelihood of benefits outweighing the risks of adjuvant therapy in an asymptomatic, otherwise healthy 55-year-old male patient with colon or rectal cancer. (A) medical oncologists, (B) radiation oncologists, (C) surgical oncologists or colorectal surgeons, (D) general surgeons. RT, radiotherapy.
There was weaker consensus regarding stage II colon and rectal cancers (Figure 1). Twenty-two percent and 46% of physicians reported that chemotherapy in stage II colon cancer was very likely and somewhat likely to have a net benefit, respectively. Physicians were more enthusiastic, but still divided, over adjuvant therapy in stage II rectal cancer, with about half indicating that the benefits of both chemotherapy and radiotherapy were very likely to outweigh the risks.
Because nearly all physicians agreed on adjuvant therapies for stage III disease, we focused additional analyses on stage II colorectal cancer. In unadjusted and adjusted analyses, physician specialty was strongly associated with beliefs regarding the net benefit of treatment for stage II colorectal cancer (Tables 1 and 2). Compared with medical oncologists, radiation oncologists (odds ratio [OR] = 2.29; 95% CI, 1.39 to 3.79) and general surgeons (OR = 2.79; 95% CI, 1.77 to 4.40) were more likely to report that the benefits of chemotherapy for stage II colon cancer were very likely to outweigh the risks. For stage II rectal cancers, radiation oncologists (OR = 0.54; 95% CI, 0.36 to 0.80) and surgical oncologists (OR = 0.50; 95% CI, 0.31 to 0.79) were less likely than medical oncologists to report that the benefits of chemotherapy were very likely to outweigh risks, and radiation oncologists (OR = 0.59; 95% CI, 0.40 to 0.88), surgeons (OR = 0.54; 95% CI, 0.38 to 0.77), and surgical oncologists (OR = 0.50; 95% CI, 0.32 to 0.81) were less likely than medical oncologists to report that the benefits of radiation were very likely to outweigh risks (Table 2).
Table 2.
Adjusted Odds Ratios and 95% CIs for Physicians Who Reported Benefit of Adjuvant Therapy Very Likely to Outweigh Risk for Stage II Colon and Rectal Cancers
| Characteristic | Chemotherapy for Stage II Colon Cancer |
Chemotherapy for Stage II Rectal Cancer |
Radiotherapy for Stage II Rectal Cancer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | OR | CI | P | |
| Specialty | |||||||||
| Radiation oncologist | 2.29 | 1.39 to 3.79 | .001 | 0.54 | 0.36 to 0.80 | .002 | 0.59 | 0.40 to 0.88 | .009 |
| General surgery | 2.79 | 1.77 to 4.40 | < .001 | 0.76 | 0.54 to 1.09 | .13 | 0.54 | 0.38 to 0.77 | < .001 |
| Colorectal surgeon/surgical oncologist | 0.68 | 0.32 to 1.44 | .31 | 0.50 | 0.31 to 0.79 | .004 | 0.50 | 0.32 to 0.81 | .004 |
| Medical oncologist | 1.0 | 1.0 | 1.0 | ||||||
| Age, years | |||||||||
| < 40 | 0.48 | 0.30 to 0.78 | .003 | 0.65 | 0.44 to 0.96 | .03 | 0.66 | 0.45 to 0.98 | .04 |
| 40-49 | 0.49 | 0.33 to 0.74 | .001 | 0.66 | 0.47 to 0.93 | .02 | 0.85 | 0.60 to 1.20 | .36 |
| 50-54 | 0.50 | 0.32 to 0.80 | .003 | 0.64 | 0.43 to 0.93 | .02 | 0.79 | 0.54 to 1.17 | .24 |
| 55-59 | 0.69 | 0.45 to 1.07 | .10 | 0.56 | 0.38 to 0.83 | .004 | 0.68 | 0.46 to 1.00 | .048 |
| ≥ 60 | 1.0 | 1.0 | 1.0 | ||||||
| US medical graduate | |||||||||
| Yes | 1.0 | 1.0 | 1.0 | ||||||
| No | 1.27 | 0.86 to 1.89 | .23 | 1.60 | 1.16 to 2.21 | .004 | 1.38 | 1.00 to 1.92 | .053 |
| Practice type | |||||||||
| VA/government | 1.0 | 1.0 | 1.0 | ||||||
| HMO | 1.85 | 0.85 to 4.00 | .12 | 1.26 | 0.73 to 2.16 | .40 | 1.06 | 0.62 to 1.82 | .82 |
| Office, solo | 2.66 | 1.16 to 6.09 | .02 | 1.06 | 0.57 to 1.98 | .85 | 1.21 | 0.65 to 2.26 | .55 |
| Office, single-specialty group | 2.14 | 1.00 to 4.59 | .051 | 1.28 | 0.75 to 2.19 | .36 | 0.86 | 0.50 to 1.47 | .58 |
| Office, multispecialty group | 2.37 | 1.00 to 5.65 | .051 | 1.31 | 0.70 to 2.46 | .40 | 1.18 | 0.63 to 2.21 | .61 |
| Hospital | 1.89 | 0.92 to 3.88 | .08 | 1.17 | 0.71 to 1.91 | .54 | 0.99 | 0.60 to 1.62 | .97 |
| NCI cancer center | |||||||||
| Yes | 1.0 | 1.0 | 1.0 | ||||||
| No or don't know | 0.89 | 0.63 to 1.27 | .52 | 0.92 | 0.70 to 1.22 | .58 | 1.17 | 0.88 to 1.56 | .27 |
| No. of colorectal patients per month | |||||||||
| ≤ 12 | 0.81 | 0.49 to 1.33 | .41 | 0.92 | 0.62 to 1.34 | .65 | 0.72 | 0.48 to 1.08 | .12 |
| > 2 and ≤ 5 | 0.65 | 0.41 to 1.03 | .07 | 1.10 | 0.78 to 1.55 | .59 | 0.60 | 0.42 to 0.85 | .005 |
| > 5 and ≤ 10 | 0.65 | 0.39 to 1.07 | .09 | 0.89 | 0.63 to 1.26 | .50 | 0.79 | 0.55 to 1.14 | .20 |
| > 10 | 1.0 | 1.0 | 1.0 | ||||||
| Enroll patients onto clinical trials | |||||||||
| Yes | 1.0 | 1.0 | 1.0 | ||||||
| No | 1.00 | 0.71 to 1.43 | .98 | 1.02 | 0.76 to 1.36 | .92 | 0.89 | 0.67 to 1.20 | .44 |
| Teaching | |||||||||
| No teaching | 0.80 | 0.53 to 1.21 | .28 | 1.00 | 0.72 to 1.37 | .98 | 0.92 | 0.67 to 1.26 | .60 |
| Teach 1-5 d/mo | 1.25 | 0.80 to 1.95 | .33 | 1.00 | 0.71 to 1.42 | .99 | 0.94 | 0.66 to 1.33 | .72 |
| ≥ 6 d/mo | 1.0 | 1.0 | 1.0 | ||||||
| Attend tumor board | |||||||||
| Weekly | 1.0 | 1.0 | 1.0 | ||||||
| Monthly | 0.96 | 0.67 to 1.39 | .85 | 1.06 | 0.79 to 1.42 | .69 | 1.09 | 0.81 to 1.46 | .58 |
| Quarterly or less often | 1.40 | 0.94 to 2.09 | .10 | 1.15 | 0.81 to 1.64 | .42 | 1.20 | 0.85 to 1.70 | .30 |
| % patients in managed care | |||||||||
| 0-20 | 1.00 | 0.60 to 1.66 | .99 | 1.19 | 0.81 to 1.75 | .37 | 0.99 | 0.66 to 1.47 | .95 |
| 21-49 | 1.14 | 0.69 to 1.90 | .60 | 1.17 | 0.79 to 1.73 | .43 | 0.83 | 0.55 to 1.24 | .35 |
| 50-78 | 1.22 | 0.76 to 1.95 | .41 | 1.18 | 0.80 to 1.74 | .39 | 1.08 | 0.75 to 1.57 | .67 |
| 79-100 | 1.0 | 1.0 | 1.0 | ||||||
| Base clinical income | |||||||||
| Mixture fee-for-service and capitation | 1.0 | 1.0 | 1.0 | ||||||
| Salary, productivity based | 1.48 | 0.61 to 3.57 | .38 | 1.08 | 0.56 to 2.07 | .82 | 1.52 | 0.79 to 2.92 | .21 |
| Salary, not productivity based | 1.19 | 0.49 to 2.85 | .70 | 1.05 | 0.55 to 2.01 | .88 | 1.46 | 0.75 to 2.81 | .26 |
| Mostly fee-for-service | 1.70 | 0.71 to 4.05 | .23 | 1.09 | 0.57 to 2.09 | .79 | 1.47 | 0.76 to 2.86 | .25 |
Abbreviations: HMO, health maintenance organization; NCI, National Cancer Institute; OR, odds ratio; VA, Veterans Affairs.
Physician age was also significantly associated with beliefs about the net benefit of adjuvant therapy. Younger physicians were less likely than older physicians to believe that the benefits of adjuvant therapies for stage II colorectal cancers were very likely to outweigh the risks (Table 2).
Physicians who were not US medical graduates were more likely to endorse the net benefit of adjuvant chemotherapy for stage II rectal cancer, but graduation from a non-US medical institution was not associated with beliefs regarding chemotherapy for stage II colon cancer or radiotherapy for stage II rectal cancer. Regarding practice setting, compared with physicians in Veterans Affairs Medical Centers, those in office-based solo practices (OR = 2.66; 95% CI, 1.16 to 6.09) were more likely to believe that the benefits very likely outweigh the risks of adjuvant chemotherapy for stage II colon cancer. Physicians in single-specialty (OR = 2.14; 95% CI, 1.00 to 4.59) and multispecialty office-based practices (OR = 2.37; 95% CI, 1.00 to 5.65) were also more likely than physicians in Veterans Affairs settings to believe that the benefits of chemotherapy for stage II colon cancer outweighed risks, but these differences were not statistically significant.
NCI cancer center status, clinical trials participation, tumor board participation, proportion of patients in managed care, and base clinical income were not associated with beliefs about the relative risk and benefits of adjuvant therapy for stage II colon or rectal cancers (Table 2).
Discussion
This large, multiregional study of US oncologists and surgeons demonstrated widespread consensus among physicians that the benefits of adjuvant chemotherapy and chemoradiotherapy outweigh the risks for healthy, middle-aged patients with stage III colon and rectal cancer, consistent with the strong evidence from randomized trials and guideline recommendations. In contrast, physicians had divergent opinions about the net benefit of adjuvant therapies for stage II colorectal cancers. The limited endorsement of adjuvant chemotherapy for patients with stage II colon cancer likely reflects the unclear evidence of benefit in this setting17,18,20 and varied interpretations of existing data by individual physicians. Some physicians may believe that the relative benefit of adjuvant therapy in stage III patients may extend to stage II patients, yet perhaps as a result of small samples in randomized trials, the absolute benefit cannot be detected. Alternatively, trials have not clearly demonstrated a survival advantage, so physicians may be reluctant to recommend therapy without well-established and/or sizeable benefits.
Guidelines also recommend chemotherapy and radiation for all patients with stage II rectal cancer, even those without high-risk features; however, only half of physicians in our study believed that chemotherapy and radiation were very likely to have net benefit in this setting. Some experts have suggested that low-risk stage II, as well as stage II or III disease located high in the rectum, might be adequately treated by surgery and chemotherapy alone.36,37 Moreover, the benefit of radiotherapy has primarily been in local control rather than in overall survival. These perspectives may explain the incomplete adoption of neoadjuvant chemoradiotherapy for locally advanced rectal cancers; in 2006, only 60% of patients with stage II or III rectal cancer received radiotherapy.38,39 The benefit of chemoradiotherapy over chemotherapy alone in stage II or III rectal cancer is currently being studied in the Alliance N1048 phase II/III randomized trial (clinicaltrials.gov NCT01515787).
The additional benefit of adjuvant chemotherapy in stage II rectal cancer, particularly in patients with a good response to chemoradiotherapy, is controversial. When chemoradiotherapy is delivered preoperatively, postoperative adjuvant chemotherapy is not always administered.2,6 We found, somewhat paradoxically, that medical oncologists were more likely than radiation oncologists and surgeons to endorse the net benefits of adjuvant chemotherapy and radiotherapy for stage II rectal cancers, but less likely to endorse adjuvant chemotherapy for stage II colon cancer. Because radiation oncologists and general surgeons are less often involved in treating colon cancer patients, they may be less familiar with the limited indications for chemotherapy in stage II disease.
We also observed that older physicians were more likely than their younger colleagues to endorse the net benefit of adjuvant therapies for stage II colorectal cancer. Physicians in office-based solo practices were more likely than Veterans Affairs physicians to believe that chemotherapy has net benefit for stage II colon cancer, potentially resulting from differences in the patients they see or financial incentives for providing chemotherapy, although we found no association with the structure of physicians' base clinical income. Also, foreign medical graduates were more likely than US graduates to endorse the net benefit of chemotherapy for patients with stage II rectal cancer, although they did not differ from US graduates in other scenarios.
This study has several limitations. First, physicians reported their beliefs about adjuvant therapies for hypothetical patients. Clinical vignettes, however, have been previously validated as a method for studying clinical practice.40 The study is also subject to nonresponse bias, although response rates were relatively high. Furthermore, the survey questions themselves did not include details about certain clinical considerations such as preoperative versus postoperative timing of adjuvant therapy, and high- versus low-risk features in stage II disease or details of the adjuvant regimens (eg, whether they contained oxaliplatin, an agent that considerably augments toxicity).19 In addition, the lack of consensus about the benefits of chemotherapy and radiation for stage II rectal cancer may reflect the preference for administration of chemoradiotherapy in the neoadjuvant setting after publication of a randomized trial that compared pre- and postoperative treatment in 2004.24 The survey questions did not permit distinction between physicians whose beliefs were influenced by such nuances in the literature and those who lacked knowledge.
Our findings are based on data mostly collected between January 2005 and May 2006, which may limit their applicability to current clinical practice. For example, clinicians today occasionally use molecular testing and genetic profiling to stratify risk of recurrence, and these assays were not routinely performed when our survey was administered.41 Nevertheless, the results of several landmark trials of adjuvant therapies in locally advanced colorectal cancer19,22,24,26 were available when our survey was conducted, and practice guidelines indicating which patients should receive chemotherapy and radiation were already established and have not changed substantially since then. Future work will determine the extent to which genetic and molecular information influences physician recommendations for adjuvant therapy.
In conclusion, we found nearly all physicians agreed with evidence-based guidelines regarding the net benefit of adjuvant therapy for stage III colorectal cancers, but there was no consensus about the treatment of stage II tumors. Our results suggest that, in the absence of strong evidence from clinical trials, individual physician characteristics, such as age, specialty, and practice type, may inappropriately play a role in determining adjuvant treatment. Although clinical trials large enough to establish a survival advantage for stage II colorectal cancer are unlikely, studies using molecular profiling to identify the highest risk patients may help to refine treatment recommendations. The disagreement over management of stage II disease also suggests a role for a formal consensus-making process, such as the RAND/UCLA Appropriateness Methodology, to develop quality indicators in the delivery of colorectal cancer adjuvant therapy. In the absence of such consensus guidelines, given the variability in the beliefs of physicians with different specialty training and experience, multidisciplinary input may help to optimize decisions about adjuvant treatment for patients with stage II colorectal cancer.
Acknowledgment
Supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network [U01CA093332], Harvard Medical School/Northern California Cancer Center [U01CA093324], RAND/UCLA [U01CA093348], University of Alabama at Birmingham [U01CA093329], University of Iowa [U01CA093339], University of North Carolina [U01CA093326]) and a Department of Veterans Affairs grant to the Durham VA Medical Center (CRS 02-164).
K.A.G. and N.L.K. contributed equally.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
Author Contributions
Conception and design: Anthony C. Wong, Karyn A. Goodman, Nancy L. Keating
Collection and assembly of data: Katherine L. Kahn, Talya Salz, Selwyn O. Rogers Jr, Nancy L. Keating
Data analysis and interpretation: Anthony C. Wong, Shannon Stock, Deborah Schrag, Katherine L. Kahn, Talya Salz, Mary E. Charlton, Nancy L. Keating, Karyn A. Goodman
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Sinclair AH, Schymura MJ, Boscoe FP, et al. Measuring colorectal cancer care quality for the publicly insured in New York State. Cancer Med. 2012;1:363–71. doi: 10.1002/cam4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham NS, Gossey JT, Davila JA, et al. Receipt of recommended therapy by patients with advanced colorectal cancer. Am J Gastroenterol. 2006;101:1320–1328. doi: 10.1111/j.1572-0241.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 3.Cronin DP, Harlan LC, Potosky AL, et al. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101:2308–2318. doi: 10.1111/j.1572-0241.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 4.Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972–979. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romanus D, Weiser MR, Skibber JM, et al. Concordance with NCCN colorectal cancer guidelines and ASCO/NCCN quality measures: An NCCN institutional analysis. J Natl Compr Canc Netw. 2009;7:895–904. doi: 10.6004/jnccn.2009.0059. [DOI] [PubMed] [Google Scholar]
- 6.Khrizman P, Niland JC, ter Veer A, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: A National Comprehensive Cancer Network analysis. J Clin Oncol. 2013;31:30–38. doi: 10.1200/JCO.2011.40.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119:1593–1601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanoff HK, Carpenter WR, Stürmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30:2624–2634. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26:2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 10.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders T, Skevington S. Do bowel cancer patients participate in treatment decision-making? Findings from a qualitative study. Eur J Cancer Care (Engl) 2003;12:166–175. doi: 10.1046/j.1365-2354.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [DOI] [PubMed]
- 13.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [DOI] [PubMed]
- 14.Petersen VC, Baxter KJ, Love SB, et al. Identification of objective pathological prognostic determinants and models of prognosis in Dukes' B colon cancer. Gut. 2002;51:65–69. doi: 10.1136/gut.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51:503–507. doi: 10.1007/s10350-008-9246-z. [DOI] [PubMed] [Google Scholar]
- 16.Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008:CD005390. doi: 10.1002/14651858.CD005390.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 18.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–1363. [PubMed] [Google Scholar]
- 19.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 20.Quasar Collaborative Group. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 21.Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 23.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 24.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 25.Ceelen W, Fierens K, Van Nieuwenhove Y, et al. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: A systematic review and meta-analysis. Int J Cancer. 2009;124:2966–2972. doi: 10.1002/ijc.24247. [DOI] [PubMed] [Google Scholar]
- 26.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 27.Cercek A, Weiser MR, Goodman KA, et al. Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J Clin Oncol. 2010;28(suppl) abstr 3649. [Google Scholar]
- 28.Wu X, Zhang J, He X, et al. Postoperative adjuvant chemotherapy for stage II colorectal cancer: A systematic review of 12 randomized controlled trials. J Gastrointest Surg. 2012;16:646–55. doi: 10.1007/s11605-011-1682-8. [DOI] [PubMed] [Google Scholar]
- 29.Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: Subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30:3353–3360. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 30.Wo JY, Mamon HJ, Ryan DP, et al. T3N0 rectal cancer: Radiation for all? Semin Radiat Oncol. 2011;21:212–219. doi: 10.1016/j.semradonc.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Tepper JE, O'Connell M, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: Analysis of stage, sex, and local control–Final report of intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 32.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute. Cancer Care Outcomes Research and Surveillance Consortium. http://appliedresearch.cancer.gov/cancors.
- 34.He Y, Zaslavsky AM, Harrington DP, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little RJ. Statistical Analysis with Missing Data. New York, NY: Wiley; 1986. [Google Scholar]
- 36.Lai LL, Fuller CD, Kachnic LA, et al. Can pelvic radiotherapy be omitted in select patients with rectal cancer? Semin Oncol. 2006;33:S70–S74. doi: 10.1053/j.seminoncol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: A pooled analysis. J Clin Oncol. 2004;22:1785–96. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 38.Baxter NN, Rothenberger DA, Morris AM, et al. Adjuvant radiation for rectal cancer: Do we measure up to the standard of care? An epidemiologic analysis of trends over 25 years in the United States. Dis Colon Rectum. 2005;48:9–15. doi: 10.1007/s10350-004-0792-8. [DOI] [PubMed] [Google Scholar]
- 39.Mak RH, McCarthy EP, Das P, et al. Adoption of preoperative radiation therapy for rectal cancer from 2000 to 2006: A Surveillance, Epidemiology, and End Results patterns-of-care study. Int J Radiat Oncol Biol Phys. 2011;80:978–984. doi: 10.1016/j.ijrobp.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 40.Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: A prospective validation study. Ann Intern Med. 2004;141:771–780. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]
- 41.Benson AB, 3rd, Hamilton SR. Path toward prognostication and prediction: An evolving matrix. J Clin Oncol. 2011;29:4599–4601. doi: 10.1200/JCO.2011.37.8646. [DOI] [PubMed] [Google Scholar]