Abstract
Regulatory T cell (Treg) therapy has the potential to induce transplantation tolerance so that immunosuppression and associated morbidity can be minimized. Alloantigen-reactive Tregs (arTregs) are more effective at preventing graft rejection than polyclonally expanded Tregs (PolyTregs) in murine models. We have developed a manufacturing process to expand human arTregs in short-term cultures using good manufacturing practice-compliant reagents. This process uses CD40L-activated allogeneic B cells to selectively expand arTregs followed by polyclonal restimulation to increase yield. Tregs expanded 100- to 1600-fold were highly alloantigen reactive and expressed the phenotype of stable Tregs. The alloantigen-expanded Tregs had a diverse TCR repertoire. They were more potent than PolyTregs in vitro and more effective at controlling allograft injuries in vivo in a humanized mouse model.
Keywords: Cellular therapy, clinical application, regulatory T cells, tolerance induction
Introduction
Regulatory T cells (Tregs) are essential for self-tolerance (1). In experimental models of transplantation, Tregs are necessary and, under certain experimental conditions, sufficient in establishing transplantation tolerance (2–5). Three Phase I trials evaluating the safety of Treg cell therapy in graft-versus-host disease (GvHD) have been reported and all showed minimal toxicity and suggested possible efficacy (6–8). A Phase I trial of Treg therapy in children with new-onset type 1 diabetes also showed slower disease progression without serious adverse events (9). These findings inspired many to consider applying Treg therapy to solid organ transplantations so that immunosuppression can be minimized or withdrawn.
Alloantigen-reactive Tregs (arTregs) are more effective than polyclonally expanded Tregs (PolyTreg) in inducing tolerance in experimental models of transplantation (10–12). We have estimated that the numbers of Tregs needed for efficacy for humans are in the range of several billion for PolyTregs and 10 times less for arTregs (13). Several approaches have been reported for selective expansion of human arTregs (12,14–16), and none has demonstrated expansion under good manufacturing practice (GMP)-compliant conditions. In this study, we report a robust process for manufacturing clinical-grade human arTregs.
Methods
Cells
Normal donors were consented for whole blood donation. Alternatively, deidentified apheresis products from normal donors were obtained from the UCSF Blood Center. Peripheral blood mononuclear cells (PBMCs) were isolated as described previously (17) and used fresh or after cryopreservation in CryoStor CS10 freezing medium (BioLife Solutions, Bothell, WA). Spleens were from cadaveric organ donors with research consent. All procedures were approved by the authorities at UCSF and King’s College London.
Generation of CD40L-expressing K562 cells
Lentiviral vectors encoding human CD40L (NM_000074), CD64 (BC032634), DRA (BC071659) and DRB0401 (18) were produced, and transduction and cloning were performed as previously described (19,20). Stable expression of transduced genes was verified by flow cytometry using antibodies to CD40L (TRAP1), HLA-DR (G46-6) and CD64 (10.1).
Generation of CD40L-stimulated B cells (CD40L-sBc)
B cells were enriched from PBMCs or spleens using the untouched B cell enrichment kit (Invitrogen, Carlsbad, CA), and cultured with irradiated 3T3-CD40L cells (40 Gy) as described (21). The CD40L-sBc were irradiated (30 Gy) and used to stimulate Tregs or cryopreserved in CryoStor CS10 until use. For GMP-compliant expansions, B cells were purified using CD19 positive selection on a CliniMACS (Miltenyi Biotech, Auburn, CA), stimulated with irradiated K562-CD40L cells (100 Gy) in transferrin-containing X-VIVO15 medium (Lonza, Walkersville, MD) supplemented with 10% human AB serum (Valley Biomedical, Winchester, PA), GMP grade IL-4 (Miltenyi) and Cyclosporine A (Teva Pharmaceuticals, North Wales, PA).
Mixed lymphocyte reaction (MLR)
Responder PBMCs labeled with 1.25 µM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) were stimulated with irradiated allogeneic CD40L-sBc (2 sBc per PBMC) or with irradiated allogeneic PBMCs (5 stimulators per responder). The cultures were harvested after 84–96 h and stained with antibodies to CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), a fixable viability dye, FOXP3 (clone 206D), and HELIOS (clone 22F6). Flow cytometry was performed on Fortessa (BD Biosciences) and analyzed using FACSdiva (BD Biosciences) or FlowJo software (Treestar, Ashland, OR).
Treg expansion
Tregs were isolated using a BD FACSAria II (BD Biosciences) based on the phenotype of CD4+CD127lo/−CD25+ and PolyTreg expansions were performed as previously described (17). The clinically compliant sorting utilized GMP mAbs generated and provided by Noel Warner (BD Biosciences). For arTreg expansions, the cultures were maintained in OpTmizer Medium (Invitrogen) supplemented with GlutaMAX (Invitrogen), Penicillin/Streptomycin and 2% human AB serum or in X-VIVO15 medium with 10% human AB serum. Fluorescence-activated cell sorting (FACS) purified Tregs were mixed with CD40L-sBc at a 4:1 sBc to Treg ratio. The cultures were maintained with 300 IU/ml human IL-2 until day 9 or 11, when the cells were restimulated with new irradiated sBc or with GMP-grade anti-CD3 and anti-CD28-coated (anti-CD3/CD28) beads at a 4:1 sBc to T cell or 1:1 bead to T cell ratio. Cultures were fed 3 days later and harvested on day 5 after restimulation. Viability of the cells was assessed using trypan blue exclusion.
Flow cytometry
Phenotype of expanded Tregs was assessed using the following three flow cytometric panels: (1) CD8 (clone SK1), CD4 (clone SK3), CD3 (clone SK7) and CD19 (clone SJ25C1); (2) CD4, CD62L (clone SK11), CD27 (clone L128) and FOXP3 (clone 206D; BioLegend, San Diego, CA); and (3) CD4, CD25 (clone 2A3), HELIOS (clone 22F6; BioLegend) and FOXP3. For some experiments, interferon gamma (IFNγ) production by expanded arTregs were assessed as previously described (22). The CD40L-sBc were stained with antibodies to HLA-DR (clone G46-6), CD80 (clone L307.4), CD86 (clone 2331) and CD19 (clone HIB19). The stained cells were analyzed on a FACSCalibur or AccuriC6 (BD Biosciences, San Diego, CA). All antibodies were from BD Biosciences unless otherwise noted.
Treg specificity assay
Expanded Tregs were labeled with 1.25µM CFSE and stimulated with allogeneic or autologous CD40L-sBc, anti-CD3/CD28 beads, or left unstimulated in media containing 30 IU/mL IL-2. After 72 h, the cells were collected and stained with anti-CD4 and propidium iodide and analyzed on an AccuriC6.
TCRβ repertoire analysis
Genomic DNA was extracted from 0.25 × 106 to 1 × 106 freshly isolated Tregs and ex vivo expanded PolyTregs and arTregs. The DNA was submitted to Adaptive Biotechnologies (Seattle, WA) for survey level TCRβ sequencing. Analyses of the sequencing data including determining the clonality index and repertoire similarities were done using algorithms developed by Adaptive Biotechnologies.
In vitro suppression assays
Titrated numbers of expanded Tregs were mixed with 3 × 104 PBMCs from the Treg donor in V-bottom 96-well plates in triplicates. The cells were stimulated with irradiated PBMCs from the sBc or third-party donors for 7 days, and incorporation of 3[H] thymidine during the final 16–20 h of culture was used to measure proliferation. Cultures containing no Tregs were used as controls.
Treg-specific demethylated region (TSDR) methylation assay
Genomic DNA from 0.5 × 106 expanded Tregs was analyzed using licensed reagents from Epiontis GmbH (Berlin, Germany) according to established protocol (23). Percentages of demethylated TSDR were calculated as: [mean copy numbers of unmethylated DNA/(mean copy numbers of unmethylated + mean copy numbers of methylated DNA)] × 100. For female Tregs, the percentages calculated above were multiplied by 2 to correct for X-chromosome inactivation.
Humanized mouse model of skin transplantation
De-identified human skin was obtained from surgery patients with informed consent. The skin was transplanted onto 8- to 12-week-old BALB/c.Rag2−/− γc−/− mice and allowed to engraft for 6 weeks before the recipient mice were injected with 10 × 106 HLA-mismatched CD25-depleted PBMCs. Some mice were co-injected with 2 × 106 PolyTregs or arTregs. Histological analysis of the grafts was performed 6 weeks after PBMC injections. For the total duration of these experiments, 100 µg anti-mouse Gr1 (Bio X Cell, West Lebanon, NH) was injected intraperitoneally every 4–5 days to deplete mouse granulocytes. All procedures were conducted in accordance with institutional guidelines. Frozen sections of human skin grafts were fixed with 5% paraformaldehyde and stained with antibodies against human antigens ki67 (cat. # ab15580; Abcam, Cambridge, MA), CD45 (clone HI30; eBioscience), CD3 (cat. # A0452; Dako, Carpenteria, CA), FOXP3 (clone 259D/C7; eBioscience), involucrin (clone SY5) and CD31 (cat. # ab28364; Abcam), followed by incubation with appropriate fluorochrome-conjugated secondary antibodies and mounted with Prolong Gold Anti-fade Reagent with 4-6-diamidino-2-phenylindole (DAPI; Invitrogen). Quantitative assessment of immunofluorescence results was done by counting four to six nonoverlapping fields preformed by an individual blinded to the treatment conditions.
Statistics
Statistical analyses were performed using GraphPad Prism version 5.00 (GraphPad Software, San Diego CA).
Results
CD40L-sBc are potent stimulators of arTregs
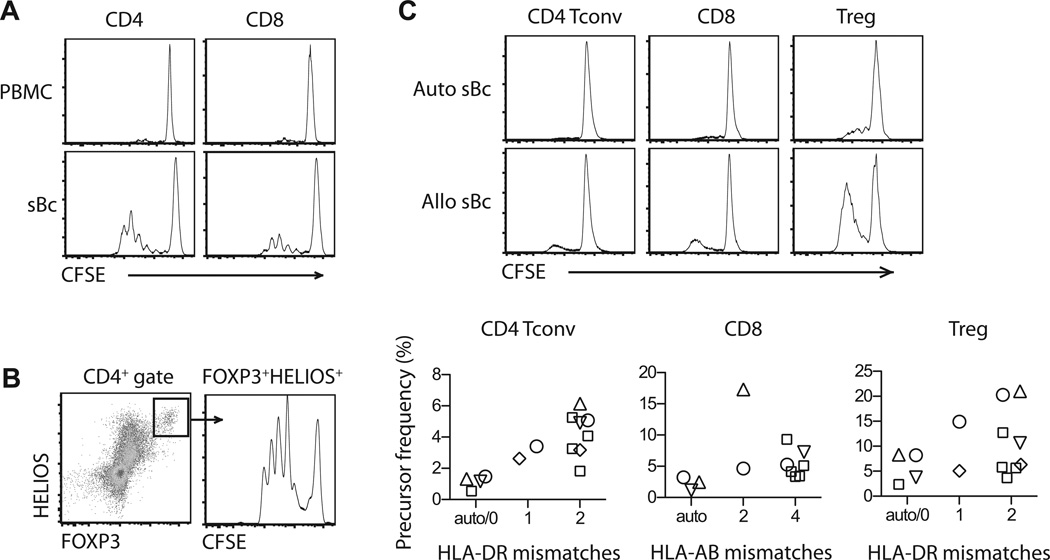
Using a one-way MLR, we found CD40L-sBc were markedly more potent than PBMCs at stimulating proliferation of CD4+ T cells, CD8+ T cells and CD4+FOXP3+HELIOS+ Tregs (Figure 1A and B). To determine if the proliferation was in response to alloantigens expressed on CD40L-sBc, we compared the stimulatory capacity of autologous CD40L-sBc and allogeneic CD40L-sBc with varying degree of HLA mismatches to the responders. We found a trend of higher frequencies of responding CD4+ conventional T cells (Tconv) and Tregs with more HLA-DR mismatches and higher frequencies of responding CD8+ T cells with more HLA-AB mismatches (Figure 1C). These results demonstrated that CD40L-sBc were potent allogeneic stimulators and prompted us to explore the utility of CD40L-sBc in selective expansion of arTregs.
Figure 1. CD40L-sBc potently stimulate T cell proliferation.
(A and B) PBMC and CD40L-sBc from the same donor were compared for their ability to stimulate proliferation of alloreactive T cells in a one-way MLR. The responder PBMCs were labeled with CFSE before MLR and the cultures were harvested on day 4 for flow cytometric analysis. Representative CFSE dilution profiles of CD4+ and CD8+ T cells (A) and CD4+FOXP3+HELIOS+ Tregs (B) are shown. The data are a representative of at least 10 independent experiments. (C) Autologous CD40L-sBc and allogeneic CD40L-sBc with different degree of HLA mismatches with responder cells were compared in their ability to stimulation proliferation of CD4+ Tconv, CD8+ T cells and Treg cells. Each symbol represents the same responder. Results area summary of 15 different stimulator and responder combinations. CD40L-sBc, CD40L-stimulated B cells; CFSE, carboxyfluorescein succinimidyl ester; MLR, mixed lymphocyte reaction; PBMC, peripheral blood mononuclear cell; Tconv, conventional CD4+ T cells; Treg, regulatory T cell.
Generation of GMP-compliant CD40L-expressing cells
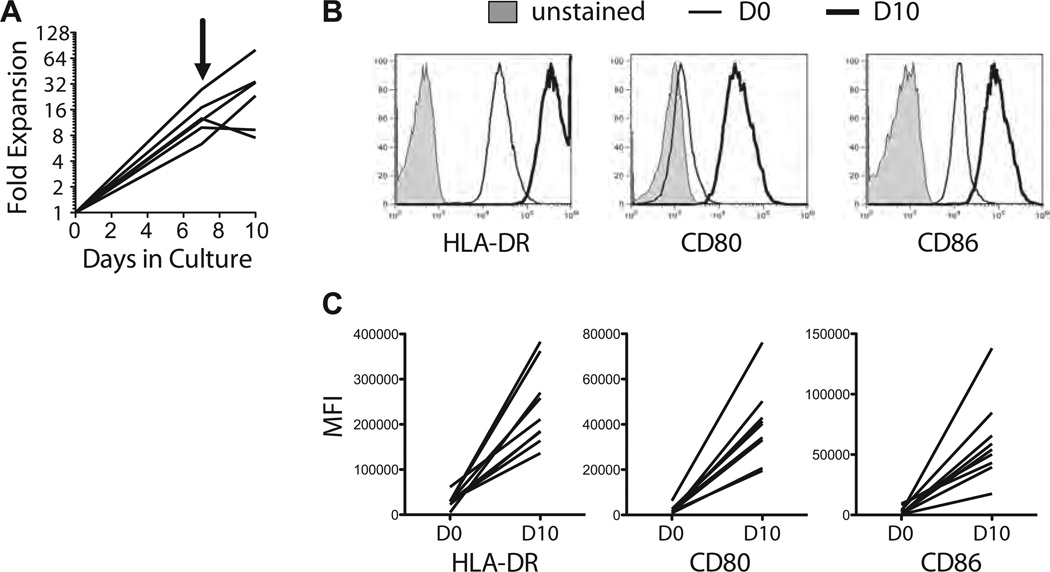
A GMP-compatible human CD40L-expressing cell line, KT64.CD40L.HLADR0401 (abbreviated as K-CD40L), was generated to enable manufacture of clinical-grade arTregs. We used lentiviral transduction to express CD40L in the myeloleukemia cell line K562, which has been used as cancer vaccines and artificial antigen presenting cells for clinical applications (24–27). The additional CD64 and HLADR0401 genes were intended for other applications and do not interfere with CD40L stimulation of sBc. Two rounds of stimulation with the K-CD40L cells on days 0 and 7 and a constant supply of IL-4 led to 10- to 50-fold expansion of purified B cells (Figure 2A). When compared to freshly isolated B cells, the CD40L-sBc expressed significantly higher levels of HLA-DR, CD80 and CD86 (Figure 2B and C), consistent with their enhanced potency in stimulating T cells.
Figure 2. Generation of CD40L-sBc using K-CD40L cells.
(A) The expansion of purified B cells in the 10-day culture is shown. The arrow indicates the time of restimulation. (B and C) Expression of HLA-DR, CD80, and CD86 in freshly isolated B cells and day 10 CD40L-sBc was compared using flow cytometry. Sample overlay histograms are shown in (B), and charts summarizing results from independent experiments are shown in (C). The data are summary of six independent experiments. CD40L-sBc, CD40L-stimulated B cells; K-CD40L, CD40L-expressing cell line; KT64.CD40L.HLADR0401; MFI, mean fluorescence intensity.
CD40L-sBc robustly induce arTreg expansion
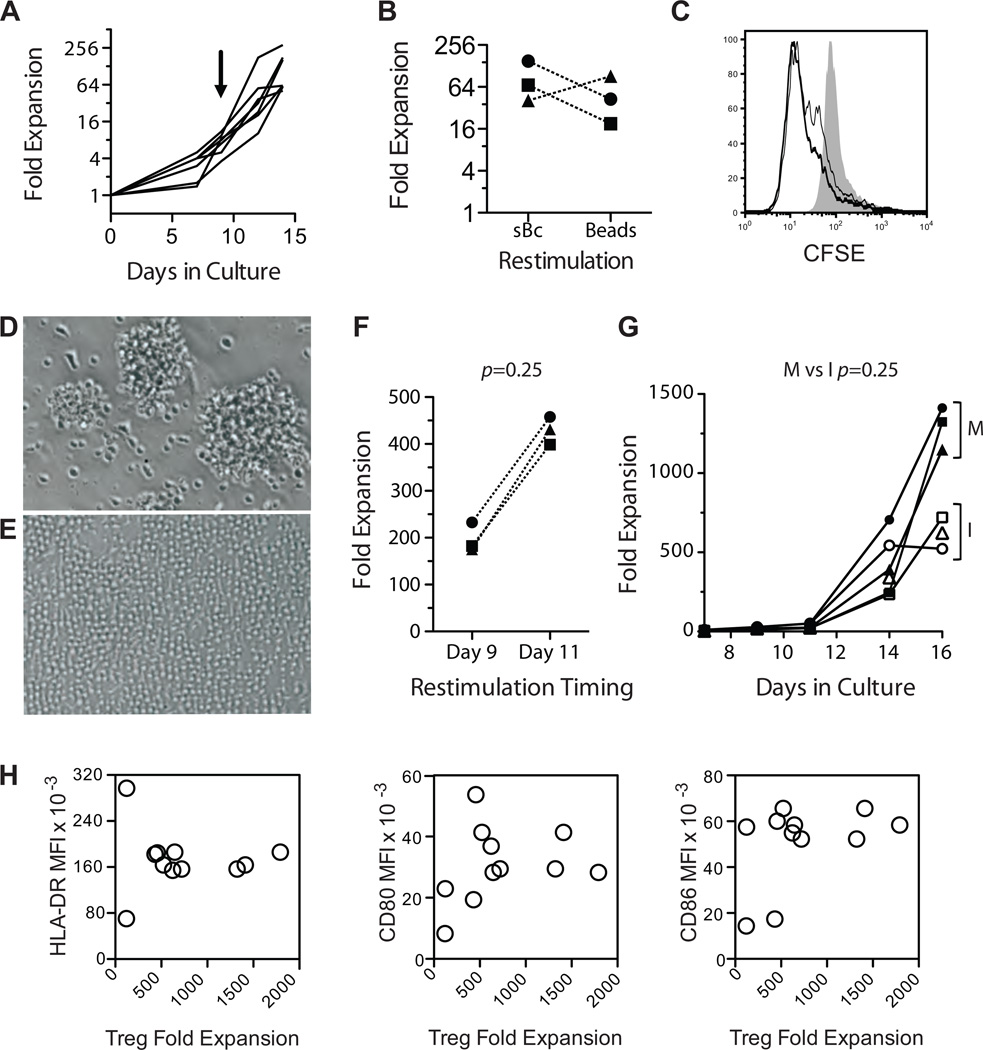
We have previously reported that polyclonal expansion of FACS purified CD4+CD127lo/−CD25+ Tregs using two rounds of stimulations (days 0 and 9) with anti-CD3/CD28 beads (17). For expanding arTregs, we compared two rounds of stimulation with CD40L-sBc versus primary CD40L-sBc stimulations followed by anti-CD3/CD28 restimulation. Two stimulations with CD40L-sBc led to 50- to 300-fold expansion of Tregs (Figure 3A), similar to that achieved when CD40L-sBc were replaced with beads during restimulation (Figure 3B). Tregs expanded either way were highly reactive to the sBc used for their expansion (Figure 3C). We decided to use bead restimulation for arTreg expansion for the ease of standardization and implementation.
Figure 3. Selective expansion of arTregs using CD40L-sBc.
(A)Allogeneic sBc were used to stimulate FACS purified Tregs on days 0 and 9. Fold expansion of Treg in the 14-day culture in six independent experiments is shown. The arrow indicates the time of restimulation. (B) Tregs were stimulated with CD40L-sBc for 9 days and then the cultures were split with half restimulated with CD40L-sBc from the same donor and the other half with anti-CD3 and anti-CD28-coated beads. Fold expansion on day 14 of three independent paired cultures is shown (p=0.75, Wilcoxon matched-pairs signed rank test). (C) Alloreactivity of expanded Tregs was determined by labeling the expanded Tregs with CFSE before restimulation with the same CD40L-sBc used for expansion (thick line), anti-CD3 and anti-CD28-coated beads (thin line) or syngeneic CD40L-sBc (shaded histogram). (D and E) Appearances of Treg cultures on days 9 (D) and 11 (E) after primary stimulation are shown. Data represent results from at least 10 independent cultures. (F) Tregs were stimulated with CD40L-sBc for 9 or 11 days before restimulation with anti-CD3 and anti-CD28-coated beads. The cultures were harvested 5 days after restimulation, and total fold expansions in three paired cultures were compared (p = 0.25, Wilcoxon matched-pairs signed rank test). (G) Tregs were stimulated with CD40L-sBc for 11 days before restimulation with anti-CD3 and anti-CD28-coated beads from Invitrogen (open symbols) or Miltenyi Biotec (closed symbols). Cell expansions over time in three paired cultures are shown. Wilcoxon matched-pairs signed rank test was used to compare the difference in total fold expansion on day 16 (p = 0.25). (H) XY scatterplots showing a correlation of arTreg expansion and mean fluorescence intensity (MFI) of HLA-DR, CD80 and CD86 expressed on different CD40L-sBc preparations. The data are a summary of 11 independent arTreg cultures. arTregs, arTreg, alloantigen-reactive Tregs; CD40L-sBc, CD40L-stimulated B cells; CFSE, carboxyfluorescein succinimidyl ester; FACS, fluorescence activated cell sorting; Tregs, regulatory T cells.
One unit of blood yields an average of 5 million Tregs after FACS purification. With 50-to 300-fold expansions, we would be able to produce between 250 million to 1.5 billion arTregs, which may fall short of our estimated efficacy dose (13). We therefore explored conditions to improve arTreg expansion. The CD40L-sBc-stimulated Tregs continued to cluster and blast on day 9 after stimulation (Figure 3D), suggesting that the Tregs were still activated and might undergo activation-induced cell death if restimulated at this time. Delaying restimulation until day 11 when the cells appeared more rested (Figure 3E) consistently improved overall expansion (Figure 3F). The source of the anti-CD3/CD28 beads also affected the rate of Treg expansion (Figure 3G). In contrast, we found that variation in HLA-DR, CD80 and CD86 expression on CD40L-sBc did not correlate with arTreg expansion (Figure 3H), suggesting that the potency of the CD40L-sBc was not strictly correlated with the absolute amount of these molecules as long as a threshold was met. Overall, by optimizing restimulation timing and restimulation regents, arTregs routinely expanded 100- to 1600-fold.
In vitro characterization of arTregs
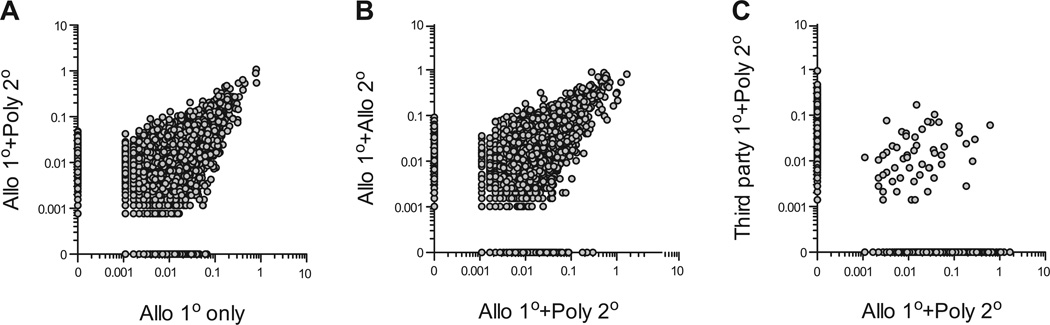
High-throughput TCR sequencing was used to compare the repertoires of freshly isolated Tregs and various expanded Tregs. arTregs were less diverse than freshly isolated Tregs and PolyTregs (Table 1), consistent with selective expansion using the CD40L-sBc stimulation. arTreg repertoire remained diverse with cumulative frequencies of top 10 clones representing less than 7% of the total repertoire and very low clonality indexes. We found 85% TCR repertoire similarity between the Tregs after primary CD40L-sBc stimulation and after additional anti-CD3/CD28 bead restimulation (Figure 4A). Consistently, arTregs expanded with two rounds of CD40L-sBc or primary CD40L-sBc and secondary bead restimulation had 93% similarity in TCRβ usage (Figure 4B). These results suggest that polyclonal restimulation did not appreciably alter the arTreg repertoire. Last, Tregs isolated from the same individual expanded using two distinct allogeneic CD40L-sBc have very little overlap in their TCR repertoires (Figure 4C), demonstrating alloantigen selective Treg expansion and effective depletion of nonreactive cells using this protocol.
Table 1.
TCR repertoire analysis of Tregs using high-throughput TCR β chain sequencing
| 1° stim | 2° stim | n | % Unique reads | Frequency of top 10 clones (%) | Clonality1 |
|---|---|---|---|---|---|
| None | None | 5 | 10.23 ± 5.39 | 1.77 ± 0.96 | 0.037 ± 0.007 |
| Poly | Poly | 3 | 11.36 ± 2.09 | 1.37 ± 0.71 | 0.036 ± 0.018 |
| Allo | None | 2 | 5.87 ± 1.45 | 6.41 ± 2.09 | 0.122 ± 0.016 |
| Allo | Allo | 2 | 2.34 ± 1.31 | 5.76 ± 1.82 | 0.133 ± 0.025 |
| Allo | Poly | 4 | 3.12 ± 1.39 | 6.86 ± 2.27 | 0.127 ± 0.027 |
Clonality is a measurement of repertoire diversity calculated using an ImmunoSeq online analysis tool. The value is between 0 and 1. Clonality of 0 indicates most diverse repertoire, and clonality of 1 indicates monoclonality. Tregs, regulatory T cells.
Figure 4. Treg TCR repertoire analyses using high-throughput TCR β chain sequencing.
(A) An xy scatterplot was used to compare TCR β chain usage by Tregs after primary CD40L-sBc (Allo) stimulation (x-axis) or after CD40L-sBc (Allo) stimulation and anti-CD3/28 bead (Poly) restimulation (y-axis), showing 85% similarity between the two samples. Each circle represent one unique TCR β chain nucleotide sequence, and data points on the x- and y-axis are present in one sample but absent in the other. The data represent results from two independent experiments. (B) An xy scatterplot was used to compare TCR β chain usage by Tregs after primary CD40L-sBc stimulation and anti-CD3/CD28 bead restimulations (x-axis) and after two rounds of alloantigen stimulations (y-axis) showing 93% similarity between the two samples. The data represent results from two independent experiments. (C) Tregs purified from one donor was split into two equal parts and subjected to primary stimulation with CD40L-sBc from two different allogeneic B cell donors (Allo and Third party) followed by polyclonal restimulation. A comparison of TCR β chain usage by the two arTreg preparations showed 2% overlap. similarity CD40L-sBc, CD40L-stimulated B cells; Treg, regulatory T cell.
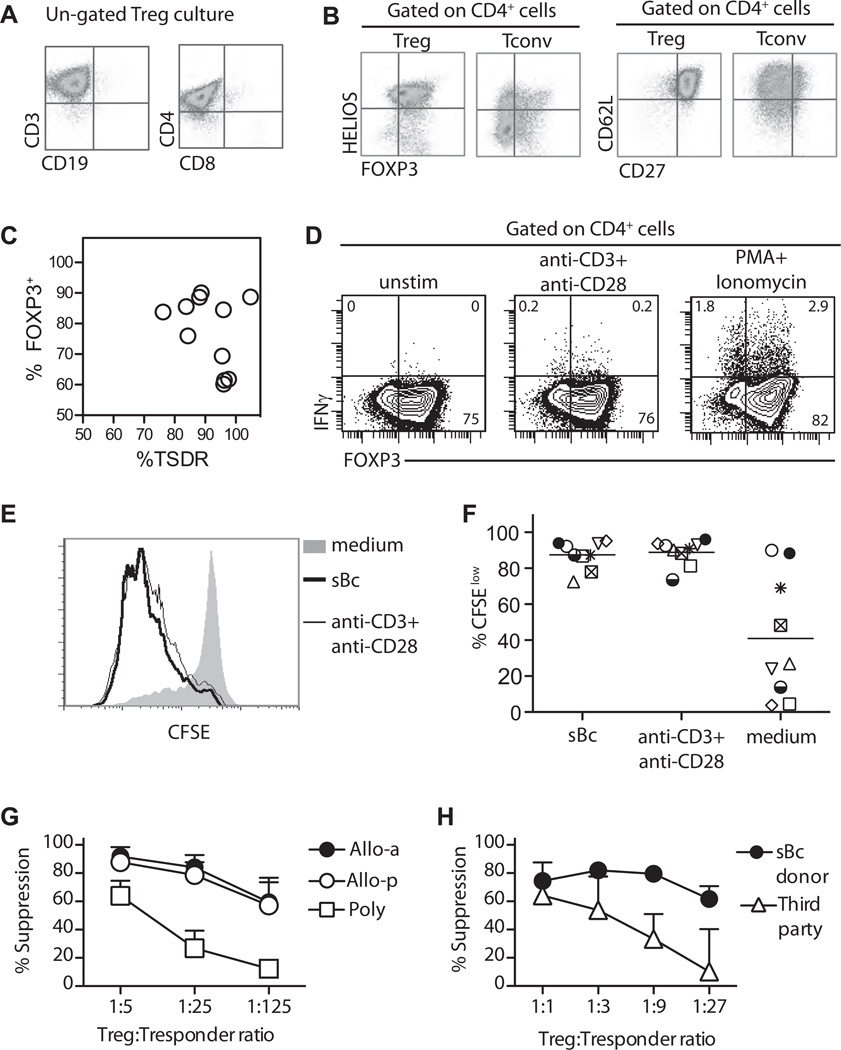
Tregs expanded with this protocol are on average >95% viable and CD3+CD4+ with minimal contamination with CD8+ T cells and CD19+ B cells (Figure 5A and Table 2). The majority of the CD4+ T cells were FOXP3+HELIOS+ and co-expressed CD27 and CD62L (Figure 5B and Table 2), distinct from the pattern expressed by similarly expanded Tconv cells (Figure 5B). The majority of the expanded Tregs had demethylated TSDR (Figure 5C, Table 2) and did not produce IFNγ after TCR or mitogenic stimulations (Figure 5D). These results suggested that arTreg were stable and the ex vivo expansion did not lead to increase IFNγ expression as we previously reported (22). To determine the reactivity of the expanded Tregs, we restimulated Tregs harvested on day 16 with CD40L-sBc from the same donor. On average 87.5% (range 72.5–95.2%) of the alloantigen expanded Tregs proliferated in response to restimulation by the same sBc, similar to the proliferation induced using anti-CD3/CD28 beads (average 88.8%, range 73.6–96%), demonstrating that the vast majority of the Tregs were reactive to the alloantigens expressed by the CD40L-sBc (Figure 5E and F).
Figure 5. Phenotype, alloantigen reactivity, and in vitro function of Tregs expanded with CD40L-sBc.
(A and B) Flow cytometric profiles of ungated (A) and CD4 gated (B) Treg cultures. Data are representative of at least 14 independent experiments. (C) Correlation between percentages of demethylated TSDR and FOXP3 from 11 independent cultures. (D) IFN-γ expression by arTregs after 4 h in vitro stimulation as indicated. (E) Alloreactivity of Tregs expanded with primary allogeneic sBc stimulation and polyclonal restimulation on day 11 was determined as described in Figure 3B. An example of overlay histogram is shown. (F) A summary of seven independent cultures analyzed as described in (C) is shown. Each symbol represents one independent Treg culture. (G) A summary of in vitro suppression by Tregs expanded with two rounds of stimulation with allogeneic CD40L-sBc (closed circles, Allo-a, n = 3), allogeneic sBc primary stimulation followed by polyclonal restimulation (open circles, Allo-p, n = 8), or two rounds of polyclonal stimulations (open squares, Poly, n = 5) is shown. Responders are PBMC from the Treg donor, and stimulators are PBMC from the sBc donor. Data shown are mean ± SEM suppression observed in three to eight independent experiments. Two-way analysis of variance (ANOVA) with Bonferroni multiple comparison test was used to determine the statistical significance of the differences. Suppression at 1:5 ratio by different groups of Tregs is not significantly different. Suppression by PolyTregs is significantly lower when compared to Allo-a Tregs (p < 0.001 at 1:25 ratio and p < 0.01 at 1:125 ratio), or when compared to Allo-p Tregs (p < 0.0001 at 1:25 ratio and p < 0.001 at 1:125 ratio). Allo-a and Allo-p Tregs are not significantly different from each other at all ratios. (H) Suppression by CD40L-sBc expanded Tregs stimulated by PBMC from the sBc donors (closed circles) or third-party donors (open triangles) is shown. Data shown are mean±SEM suppression observed in six independent experiments. Two-way ANOVA with Bonferroni multiple comparison test was used to determine the statistical significance of the differences. Suppression at 1:1 and 1:3 ratios stimulated by sBc and third-party donors is not significantly different. Suppression stimulated by sBc donor at 1:9 and 1:27 ratios is significantly lower when compared to that stimulated by third-party donors (p < 0.001 at 1:9 ratio and p < 0.001 at 1:27 ratio). arTregs, arTreg, alloantigen-reactive Tregs; CD40L-sBc, CD40L-stimulated B cells; CFSE, carboxyfluorescein succinimidyl ester; INFγ, interferon gamma; PBMC, peripheral blood mononuclear cells; PolyTregs, polyclonally expanded Tregs; Tconv, conventional CD4+ T cells; Tregs, regulatory T cells; TSDR, Treg-specific demethylated region.
Table 2.
Phenotype of expanded arTregs
| CD3+ | CD4+ | FOXP3+ | TSDR | HELIOS+ | CD62L+ CD27+ | CD8+ | CD19+ | |
|---|---|---|---|---|---|---|---|---|
| Mean | 97.1 | 97.1 | 83.0 | 94.0 | 88.2 | 85.4 | 0.5 | 0.2 |
| SD | 2.6 | 1.9 | 10.8 | 15.5 | 6.6 | 6.4 | 0.2 | 0.2 |
| N | 14 | 14 | 14 | 10 | 14 | 10 | 14 | 14 |
arTregs, arTreg, alloantigen-reactive Tregs; TSDR, Treg-specific demethylated region.
Consistent with the phenotype and the enhanced alloantigen recognition, the expanded arTregs were highly suppressive when activated in vitro by PBMCs from the same donor as the CD40L-sBc (Figure 5G). arTregs were 5- to 25-fold more potent at suppressing MLR than PolyTregs (Figure 5G), consistent with previous reports of 5- to 32-fold increase in potency by alloreactive Tregs (12,14,28–30). arTreg expanded by restimulation with CD40L-sBc or anti-CD3/CD28 beads had identical suppressive activity (Figure 5G), demonstrating that polyclonal restimulation did not alter their alloreactivity or suppressive activity in vitro. In addition, arTregs were 9–27 times more suppressive when stimulated by the relevant PBMC than when stimulated by third-party cells (Figure 5H). Together, our results show that CD40L-sBc expanded Tregs had enriched reactivity and suppressive activity toward the alloantigens expressed by the B cells used for their expansion.
arTregs are superior at protecting skin allografts in vivo
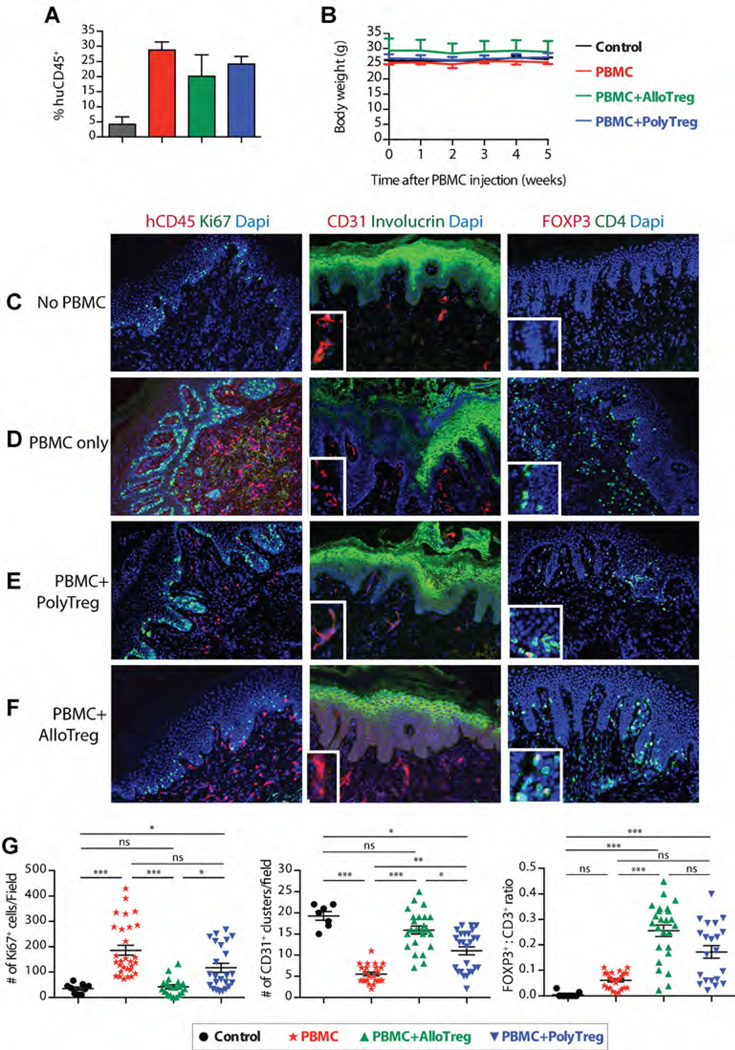
Using our recently described model of alloimmune-mediated injury of human skin allografts (12), we compared the potency of arTregs and PolyTregs. BALB/c.Rag2−/− γc−/− mice were transplanted with human skin from a HLA-DR0401+ donor before adoptive transfer of allogeneic PBMC depleted of CD25+ cells alone or in combination with different preparations of syngeneic Tregs at a ratio 5:1 effector cells/Treg. PBMC donors were HLA-DR0401− and arTregs from these donors were expanded using HLA-DR0401+ CD40L-sBc. Grafts were monitored until rejection or until 6 weeks after PBMC reconstitution when they were collected for histological analysis. Levels of human leukocyte engraftment in spleens were similar in mice that received PBMCs alone or in combination with Tregs (Figure 6A). For the duration of these experiments, all mice maintained stable body weight, suggesting a lack of GvHD (Figure 6B), consistent with our previous report of this model (31).
Figure 6. Suppression of skin allograft injury by PolyTregs and arTregs in vivo in a humanized mouse model.
BALB/c.Rag2−/− γc−/− mice were transplanted with human skin and reconstituted with PBMC allogeneic to the skin donor. (A) PBMC reconstitution was determined at the end of the experiment, demonstrating that co-infusion of Tregs did not significantly alter the extent of PBMC reconstitution. (B) Body weight of the BALB/c.Rag2−/− γc−/− mice in four experimental groups was assessed to determine general health status, demonstrating that PBMC infusion did not induce GvHD. (C–F) Skin graft injury was assessed using three-color immunofluorescence microscopy and representative results are shown. (G) Immunofluorescence micrograph images were analyzed by counting four to six high-powered visual fields per stain for each graft. Quantitative results from four experimental groups were then compared. One-way analysis of variance with Kruskal–Wallis test and Dunn’s multiple comparison posttest was used to determine the statistical significance of the differences (*p<0.05, **p<0.01, ***p<0.001). arTregs, arTreg, alloantigen-reactive Tregs; GvHD, graft-versus-host disease; PBMC, peripheral blood mononuclear cells; PolyTregs, polyclonally expanded Tregs; Tregs, regulatory T cells.
Compared to the skin grafts in no PBMC control animals (Figure 6C), grafts in the PBMC alone group showed intense human CD45+ mononuclear cell infiltrates with concomitant increase in keratinocyte proliferation, loss of involucrin and decreased vascularization as indicated by the reduction in clustered CD31+ cells in the dermis (Figure 6D). These changes revealed active inflammation and loss of dermo-epidermal integrity mediated by the allogeneic human leukocytes. All these inflammatory parameters were reduced by co-injection of PolyTregs, correlating with an increase in FOXP3+ cells (Figure 6E). Moreover, skin grafts in the arTreg group were nearly completely protected from histological features of graft injuries and were indistinguishable from those in control grafts except for the infiltration of FOXP3+ cells (Figure 6F). Quantitative analysis of histological findings demonstrated significant reduction in Ki67+ keratinocytes and an increase in CD31+ cell clusters, correlating with significantly higher FOXP3+ to CD3+ cell ratio in grafts of arTreg-treated mice when compared with the PolyTreg group (Figure 6G). These results suggest improved efficacy of arTregs in protecting allografts in vivo.
Discussion
Producing sufficient Tregs during ex vivo expansion has been a major challenge in applying Treg therapy to humans (13). By stimulating highly purified Tregs with potently antigenic CD40L-sBc, we were able to achieve 100- to 1600-fold expansion in 16 days. The expanded Tregs had diverse TCR repertoire; retained Treg-specific phenotype were enriched for alloantigen reactivity, and were more potent at suppressing alloimmune responses in vitro and in vivo when compared to expanded PolyTregs. Critical parameters that contributed to the success of this protocol were the purity of the Tregs at the beginning of the culture, the potency of the CD40L-sBc and the conditions of restimulation.
Naïve B cells failed to induce expansion of Tregs without the addition of anti-CD28 agonist antibodies (30), consistent with the notion that Treg expansion depends on costimulation through CD28 (32). We found that stimulating B cells with CD40L induced nearly 20-fold increase of CD80 and CD86 expression, which may underlie their potency as Treg stimulators. One advantage of using B cells is their relative abundance and ease of expansion when compared to dendritic cells. In the setting of living donor transplant, we estimate that 100mL of peripheral blood from an organ donor would generate enough CD40L-sBc to expand 5 × 106 Tregs purified from 1 U of blood, which could yield 1 billion arTregs after 200-fold expansion. For deceased donor transplant, donor spleen can be used as a source of CD40L-sBc without prior purification of B cells because their high abundance (data not shown). Our results demonstrate that it is feasible to mass produce highly pure and potent arTregs using GMP-compliant reagents in short-term cultures. Previous reports show Tregs can be expanded from uremic pretransplant patients (33,34). Current efforts are focused on applying this protocol to expand Tregs isolated from pretransplant patient with end-stage organ diseases to enable two planned phase I trials in liver and kidney transplantations. We believe that efficacy of Treg therapy in transplantation depends on the number and quality of Treg products in addition to the timing of Treg infusion and adjunct immunosuppression (5,13).
Acknowledgments
This study was supported by research funds from the Nicholas Family, UCSF Department of Surgery, JDRF, UCSF CTSI, NIH (R34 AI095135, P30DK063720) and British Heart Foundation, UK. MW was a recipient of a Genentech Graduate Student Fellowship, NS is the recipient of a Clinical Research Fellowship from the Medical Research Council (MRC). The research was also funded by the National Institute for Health Research (NIHR), Biomedical Research Centre based at Guy’s, St Thomas’ NHS Foundation Trust, and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors acknowledge the support of the MRC Centre for Transplantation.
Abbreviations
- arTreg
alloantigen-reactive Tregs
- CD40L-sBc
CD40L-stimulated B cells
- CFSE
carboxy-fluorescein succinimidyl ester
- DAPI
4-6-diamidino-2-phenylindole
- DC
dendritic cells
- FACS
fluorescence-activated cell sorting
- GMP
good manufacturing practice
- GvHD
graft-versus-host disease
- INFγ
interferon gamma
- MLR
mixed lymphocyte reaction
- PBMCs
peripheral blood mononuclear cells
- PolyTregs
polyclonally expanded Tregs
- Tconv
conventional CD4+ T cells
- Treg
regulatory T cells
- TSDR
Treg-specific demethylated region
Footnotes
Authorship Contributions
ALP, NS, AM, ML, MW, GLS, ET, MAM, WL, A Lares, KL, and A Laing performed experiments and analyzed data. RIL, JLR and JAB provided critical advice and review of the studies. QT and GL directed the research and wrote the manuscript together with ALP and NS.
Disclosure
The authors of this manuscript have conflicts of interests to disclose as described by the American Journal of Transplantation. JAB and QT are co-inventors on two patents on regulatory T cell therapy. JAB has received reagents and equipment from BD Biosciences in support of developing regulatory T cell therapy.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldmann H, Adams E, Cobbold S. Reprogramming the immune system: Co-receptor blockade as a paradigm for harnessing tolerance mechanisms. Immunol Rev. 2008;223:361–370. doi: 10.1111/j.1600-065X.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cel therapy in transplantation. J Mol Cell Biol. 2011;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2010;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 9.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Fueyo A, Sandner S, Habicht A, et al. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J Immunol. 2006;176:329–334. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang JY, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002076. 83ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: How many cells do we need? Curr Opin Organ Transplant. 2012;17:349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 14.Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4(pos) CD25(high) T cells for immunotherapy. PLoS ONE. 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenen HJ, Fasse E, Joosten I. CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol. 2005;174:7573–7583. doi: 10.4049/jimmunol.174.12.7573. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 20.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of costimulatory molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zand MS, Bose A, Vo T, et al. A renewable source of donor cells for repetitive monitoring of T- and B-cell alloreactivity. Am J Transplant. 2005;5:76–86. doi: 10.1111/j.1600-6143.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- 22.McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2010;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieczorek G, Asemissen A, Model F, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 24.Ye Q, Loisiou M, Levine BL, et al. Engineered artificial antigen presenting cells facilitate direct and efficient expansion of tumor infiltrating lymphocytes. J Transl Med. 2011;9:131. doi: 10.1186/1479-5876-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler MO, Friedlander P, Milstein MI, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002207. 80ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BD, Kasamon YL, Kowalski J, et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin Cancer Res. 2010;16:338–347. doi: 10.1158/1078-0432.CCR-09-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrello IM, Levitsky HI, Stock W, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML) Blood. 2009;114:1736–1745. doi: 10.1182/blood-2009-02-205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–5680. doi: 10.1182/blood-2011-02-337097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran GT, Hodgkinson SJ, Carter NM, et al. Interleukin-5 (IL-5) promotes induction of antigen specific CD4+CD25+ T regulatory cells that suppress autoimmunity. Blood. 2012;119:4441–4450. doi: 10.1182/blood-2011-12-396101. [DOI] [PubMed] [Google Scholar]
- 30.Chen LC, Delgado JC, Jensen PE, Chen X. Direct expansion of human allospecific FoxP3+CD4+ regulatory T cells with allogeneic B cells for therapeutic application. J Immunol. 2009;183:4094–4102. doi: 10.4049/jimmunol.0901081. [DOI] [PubMed] [Google Scholar]
- 31.Ali N, Flutter B, Sanchez Rodriguez R, et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rgammanull mice display a T-effector memory phenotype. PLoS ONE. 2012;7:e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golovina TN, Mikheeva T, Suhoski MM, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berglund D, Korsgren O, Lorant T, Schneider K, Tufveson G, Carlsson B. Isolation, expansion and functional assessment of CD4+CD25+FoxP3+ regulatory T cells and Tr1 cells from uremic patients awaiting kidney transplantation. Transpl Immunol. 2012;26:27–33. doi: 10.1016/j.trim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Berglund D, Karlsson M, Biglarnia AR, et al. Obtaining regulatory T cells from uraemic patients awaiting kidney transplantation for use in clinical trials. Clin Exp Immunol. 2013;173:310–322. doi: 10.1111/cei.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]