Abstract
Carbapenem-resistant Acinetobacter species are increasingly recognized as major nosocomial pathogens, especially in patients with critical illnesses or in intensive care. The ability of these organisms to accumulate diverse mechanisms of resistance limits the available therapeutic agents, makes the infection difficult to treat, and is associated with a greater risk of death. In this review, we provide an update on the epidemiology, resistance mechanisms, infection control measures, treatment, and outcomes of carbapenem-resistant Acinetobacter infections.
Keywords: Acinetobacter baumannii, Colistin, Drug therapy
INTRODUCTION
Acinetobacter species are aerobic gram-negative bacilli that are ubiquitous in natural environments such as soil and water. These species are occasionally found as commensals on the skin and throat and in secretions of healthy people. It was previously believed that Acinetobacter species were not a common human pathogen and that they were uncommon causes of infection. However, Acinetobacter species are now one of the most common organisms isolated from hospital environments and hospitalized patients.1 Acinetobacter species have excellent biofilm-producing ability and intrinsic and acquired resistance to various antibiotic agents, all of which facilitate their survival in hospital environments. For these reasons, Acinetobacter species are frequently found on the skin and in the respiratory and urinary tracts of hospitalized patients.
MICROBIOLOGY AND TAXONOMY
The genus Acinetobacter belongs to the subclass γ-Proteobacteria, family Moraxellaceae, and comprises a heterogeneous group of aerobic nonhemolytic gram-negative coccobacilli, which are usually found in diploid formation or chains of variable length. Acinetobacter spp. are oxidase-negative, catalase-positive, indole-negative, and nitrate-negative. However, identification of individual species by their phenotypic traits is difficult, and identification of individual species by use of current automated or manual commercial systems will require further confirmation testing. Nowadays, with advancements in molecular bacterial taxonomy, particularly 16S rDNA sequencing and DNA-DNA hybridization, species can be distinguished more accurately. Currently, the genus Acinetobacter contains 34 formally named species (Table 1).2
TABLE 1.
Updated list of validated named species of Acinetobacter
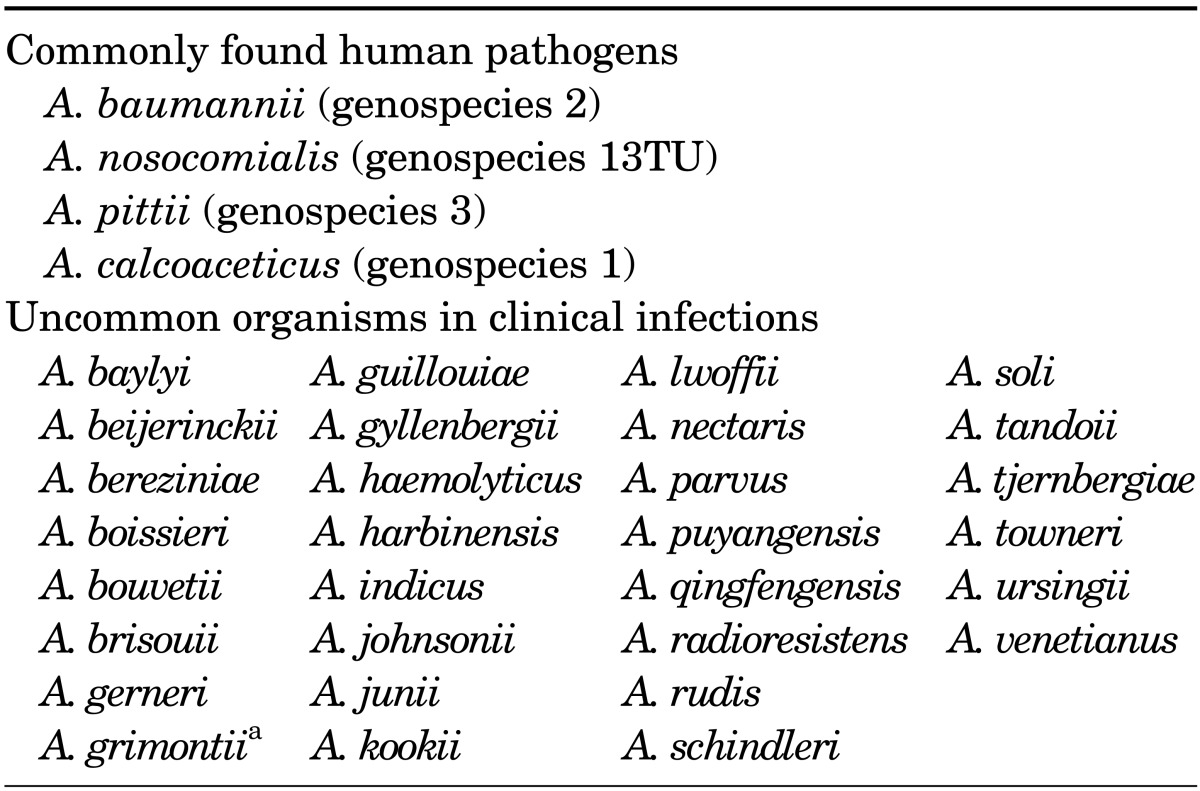
aSynonym of A. junii.
Among the Acinetobacter species, Acinetobacter baumannii, Acinetobacter pittii (genospecies 3), and Acinetobacter nosocomialis (genospecies 13TU) (together forming the "A. baumannii complex") are closely related; they are considered important nosocomial pathogens and account for most clinically significant infections. These three species together with another closely related Acinetobacter species commonly found in the natural environment, Acinetobacter calcoaceticus, are grouped under the term "A. calcoaceticus-A. baumannii complex" (ACB complex).
PATHOGENESIS
Acinetobacter was initially considered to be an organism of low virulence, and little is known about its virulence mechanisms and host responses. Several specific potential virulence mechanisms related to the ability of Acinetobacter species to adhere to, colonize, and invade human epithelial cells have been identified. However, despite recent advances, many questions regarding the virulence and pathogenesis of Acinetobacter species remain unanswered.
The pathogenic determinants include pilus-mediated biofilm formation,3 an outer membrane protein A associated with apoptosis in human cells,4,5 an iron-acquisition system,5,6 lipopolysaccharides,7 and a quorum-sensing system.8 Biofilm formation can be considered a key virulence factor of many A. baumannii isolates, including carbapenem-resistant strains. A biofilm constitutes living bacteria attached to a surface as sessile communities,9 which enables bacteria to withstand host immune defense mechanisms, antibiotics, and hydrodynamic shear forces. This allows A. baumannii to colonize and persist on biotic and abiotic surfaces, causing infections associated with indwelling medical devices. Recent studies have shown that ACB complex species are threefold more likely to form a biofilm at a liquid-solid interface than are non-ACB complexes at 25℃ (80-91% versus 5-24%).10
RESISTANCE MECHANISMS
Acinetobacter species are a common cause of nosocomial infections, and some Acinetobacter isolates are resistant to all or almost all β-lactam antibiotics, aminoglycosides, and quinolones. However, controversy exists over the terms "multidrug resistance" (MDR), "extreme drug resistance" (XDR), and "pandrug resistance" (PDR) in gram-negative pathogens, and a consensus on their definitions is needed. The most recent recommended updated definitions are as follows:11 MDR refers to a pathogen being nonsusceptible to at least one agent in more than three antimicrobial categories. XDR refers to a pathogen being nonsusceptible to at least one agent in all but less than two categories. PDR refers to a pathogen being nonsusceptible to all antimicrobial agents (Table 2).
TABLE 2.
Antimicrobial categories and agents used to define multidrug resistance (MDR), extreme drug resistance (XDR), and pandrug resistance (PDR) in Acinetobacter species

MDR: nonsusceptible to ≥1 agent in ≥3 antimicrobial categories, XDR: nonsusceptible to ≥1 agent in all but ≤2 categories, PDR: nonsusceptible to all antimicrobial agents listed.
Adapted from reference 11.
Resistance mechanisms include inherent antibiotic resistance, antimicrobial-degrading enzymes, efflux pumps, target modification, and porin deficiency. The most important mechanism, however, is the endless capacity of Acinetobacter species to acquire antibiotic resistance, leading to MDR and even PDR.12 There are many mechanisms of antibiotic resistance; here we discuss only resistance to carbapenem and colistin.
Resistance to carbapenems is often mediated by serine oxacillinases (OXAs; Amber class D), which are encoded by the blaOXA-23, blaOXA-40, blaOXA-58, and metallo-β-lactamases (MBLs; Amber class B) genes of the VIM, IMP, and SIM families.13,14 OXAs are not inhibited by clavulanic acid and have been found in most regions of the world, whereas MBLs mediate resistance to carbapenems and all other β-lactamases, with the exception of aztreonam.14,15 Carbapenem resistance in A. baumannii isolates is most frequently due to OXA production, whereas MBL production is more prevalent in non-baumannii Acinetobacter isolates.13,16 In A. baumannii, the level of carbapenem resistance provided by OXAs is considerably lower than that mediated by MBLs. In particular, some of these enzymes do not hydrolyze meropenem.
The Acinetobacter mechanism of resistance to colistin differs from the usual mechanism in gram-negative pathogens,17 and investigations of these additional regulatory factors are ongoing. Currently, two main hypotheses regarding the resistance mechanisms exist. The first is the loss of lipopolysaccharide,18 and the second is a mutation in the genes encoding the PmrA and B proteins, which are related to increased expression of the PmrAB system and amino acid sequence alterations.19 Although acquired colistin resistance remains rare among clinical Acinetobacter isolates,20 some Acinetobacter species seem to possess intrinsic resistance to colistin without multidrug resistance.21 The emergence of colistin resistance has provided a safe and accurate method of determining the susceptibility of Acinetobacter species in a clinical setting. A comparison of the Vitek 2, MicroScan, and Etest methods with the agar dilution method for assessment of colistin susceptibility22 revealed that MicroScan was unreliable, whereas Etest and Vitek 2 were suitable for identification of colistin-resistant and colistin-susceptible Acinetobacter isolates.
EPIDEMIOLOGY
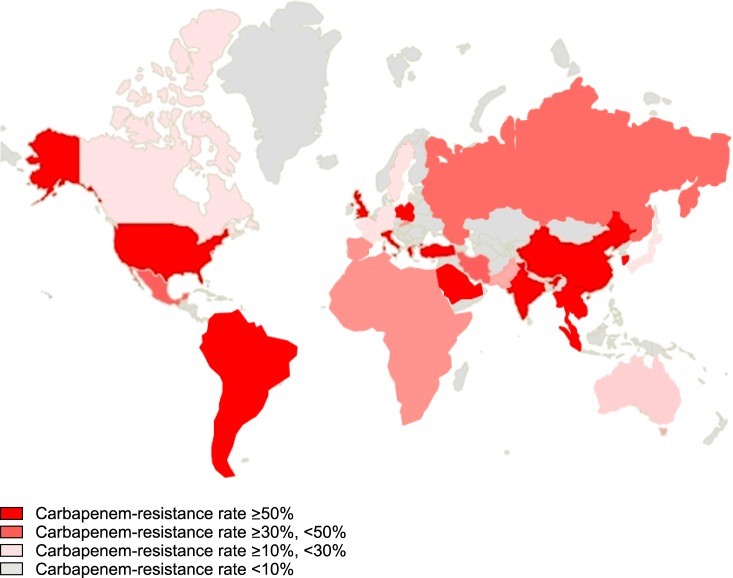
Carbapenemases have been reported increasingly frequently in Enterobacteriaceae and Acinetobacter species over the past 10 years worldwide (Fig. 1). The incidence of infections caused by carbapenem-resistant gram-negative bacilli in intensive care units (ICUs) has also increased; this now represents a global problem. Nosocomial outbreaks and endemic infections in ICUs are now commonplace because of the widespread use of broad-spectrum antibiotics and medical devices as well as an increase in the number of immunocompromised hosts.
FIG. 1.
Global epidemiology of carbapenem-resistant Acinetobacter strains.
Acinetobacter species comprise 8.4% of ventilator-associated pneumonia and 2.2% of central-line-associated bloodstream infections in the USA. Carbapenem resistance accounts for 65% of A. baumannii pneumonia in the USA and Europe,23 and clonal complex 92 was the most frequently identified worldwide.24 A recent study showed that >60% of A. baumannii isolates causing hospital-acquired pneumonia in Asian countries were PDR and carbapenem-resistant. Clonal complex 92, corresponding to global clone 2, was most prevalent, and OXA-23 oxacillinase was responsible for the majority of carbapenem resistance in the USA and Europe.25
CLINICAL FEATURES
Acinetobacter species have become a key concern owing to their ability to cause epidemics and nosocomial infections. Because of mechanisms that facilitate colonization of patients or devices used in hospital settings, Acinetobacter catheter or device-related infections are clinically important. A. baumannii is increasingly frequently responsible for nosocomial pneumonia in ICUs, predominantly ventilator-associated pneumonia, the incidence of which has increased from 4% (1986) to 7% (2003).26 Bloodstream infections due to A. baumannii account for 1% to 2% of all nosocomial bloodstream infections.27,28 A. baumannii is also a cause of urinary tract infections, commonly in patients with urinary tract catheters; surgical-site infections; and nosocomial meningitis.26
TREATMENT OPTIONS
Most A. baumannii strains are resistant to antibiotics such as penicillin, ampicillin, macrolides and second- and third-generation cephalosporins, ciprofloxacin, and chloramphenicol. Infections caused by antibiotic-susceptible Acinetobacter strains can be treated with ceftazidime, carbapenems, sulbactam, piperacillin/tazobactam, aminoglycosides, and quinolones (e.g., levofloxacin/ciprofloxacin) or cefepime, alone or in combination. Single-drug therapies with aminoglycosides are generally not recommended because of the high failure rates in Pseudomonas aeruginosa infections.29 However, the proportion of Acinetobacter infections caused by resistant strains is increasing and outbreaks of strains with PDR have been reported.30 The rate of Acinetobacter resistance to aminoglycosides and piperacillin/tazobactam is higher in Asian and European countries than in the USA.31
A number of studies have reported promising results regarding the efficacy of sulbactam against A. baumannii infections; indeed, in cases of sulbactam-susceptible A. baumannii, sulbactam was the preferred treatment option.32,33 For carbapenem-resistant Acinetobacter baumannii (CRAB) infections, sulbactam is more effective than polymyxins.34 Most studies prescribed 8 to 9 g of sulbactam per day in several doses, assuming normal renal function.34,35 Sulbactam is usually manufactured as a compound with ampicillin at a fixed ratio of 2:1. Although sulbactam appears to be effective against CRAB infections, an increasing number of sulbactam-resistant A. baumannii strains have been isolated; a recent study from Taiwan reported that 70% of clinical isolates were resistant to sulbactam.36
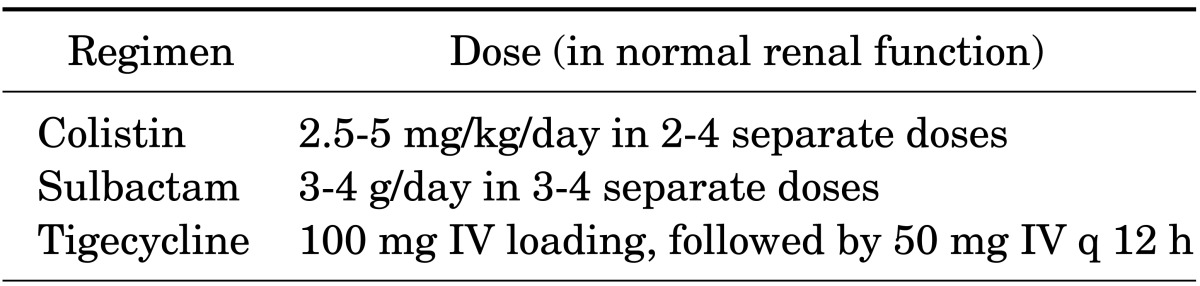
Another treatment option for CRAB infections is the polymyxins. Among them, polymyxins B and E (colistin) are suitable for clinical use and additional studies have been conducted on colistin and polymyxin B. Colistin can be injected into the bloodstream, ventricles, and spinal cord or can be administered as an inhalant. Owing to its renal toxicity, polymyxin had been one of the least-used antibiotics in practice. However, recently, the emergence of MDR in gram-negative bacteria has resulted in an increase in their use. Use of polymyxins for A. baumannii infections is clinically useful; however, a total cumulative dose of colistin appears to be associated with nephrotoxicity.37 Therefore, in patients requiring prolonged treatment, possible renal damage should be considered. Pharmacokinetic data on colistin are scarce and no consensus on the optimum method of its administration has been reached. The usual recommended dose for adults with normal renal function is 2.5 to 5 mg/kg/day as an intravenous colistin base in two to four doses (Table 3). However, a recent study suggested that a high dose with an extended-interval regimen can achieve better results.38 Because colistin has difficulty penetrating the blood-brain barrier, intra-ventricular administration of colistin is recommended for CRAB infections and Acinetobacter meningitis, as well during the removal of infected hardware.39,40
TABLE 3.
Antibiotics used in infections caused by carbapenem-resistant Acinetobacter species
Colistin-based combination therapies in patients with CRAB infections remain controversial. In a randomized controlled clinical trial, a significant increase in the microbiological eradication rate was seen for a colistin and rifampicin combination, compared with colistin monotherapy, in serious XDR A. baumannii infections. However, 30-day mortality was not reduced by the addition of rifampicin to colistin.41 In another randomized trial, a colistin/fosfomycin combination exhibited a significantly enhanced microbiological response compared to colistin alone. However, although the clinical outcomes and mortality rates were lower in the combination therapy group, the difference was not statistically significant.42 In a prospective observational study, combination therapy was not associated with a reduced mortality rate in MDR A. baumannii infections.43 A recent meta-analysis indicated that various colistin-based combination therapy regimens showed no evidence of a benefit compared with monotherapies in the treatment of infections with carbapenem-resistant gram-negative bacteria.44
Tigecycline, which has shown in vitro activity against Acinetobacter species in skin and soft tissue infections45 as well as in complicated intra-abdominal infections,46 could be used as alternative therapy for CRAB infections. However, the use of tigecycline in patients with CRAB infections is limited, because the two major clinical CRAB infections are hospital-acquired pneumonia and central- catheter-related bloodstream infections. Tigecycline has been associated with an increased risk of mortality compared with other agents, most markedly among patients with hospital-acquired pneumonia.47 Moreover, tigecycline rapidly enters the tissues following administration, which results in low serum levels, making it inappropriate for the treatment of Acinetobacter bacteremia.48
A recent study found that aspergillomarasmine A, a natural fungal product purified from Aspergillus versicolor, was a rapid and potent inhibitor of carapenemase, including NDM and VIM, and fully restored the activity of meropenem against Enterobacteriaceae, Acinetobacter spp., and Pseudomonas spp. possessing either VIM or NDM-type alleles both in vitro and in mice.49 Further research is essential to evaluate the clinical efficacy, safety, and usefulness of this product and its derivatives.
OUTCOMES
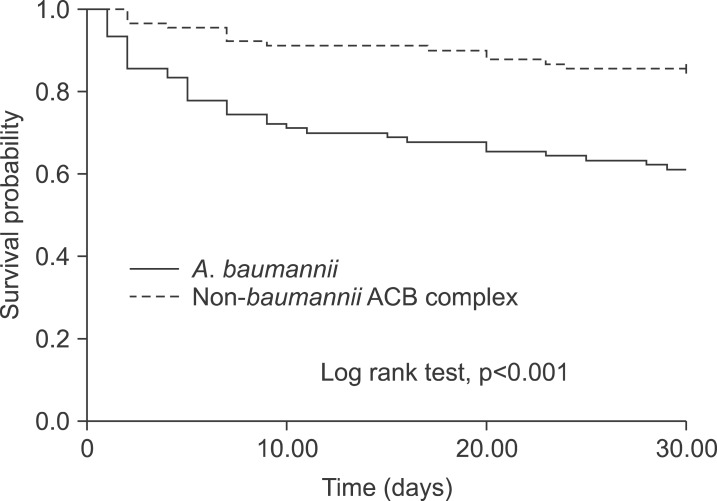
As mentioned previously, the ACB complex accounts for most nosocomial infections. Owing to its critical role in nosocomial infections and high mortality rates, much effort has focused on identifying the factors associated with the outcomes of Acinetobacter species infections. Several factors have been reported to influence the outcomes of Acinetobacter infections, including species differences, antibiotic resistance, and use of inappropriate empirical therapies. Although carbapenem resistance of other non-A. baumannii species has been documented, carbapenem resistance occurs most frequently in A. baumannii.50 This makes determination of the factors directly related to outcomes problematic. In an American study of 295 bloodstream infections caused by Acinetobacter species, the crude mortality rate of A. baumannii was higher than those of A. pittii and A. nosocomialis (36.9%, 13.0%, and 16.4%, respectively).51 Another study in Taiwan reported that bacteremia due to MDR stains appeared to be associated with a poor outcome, rather than A. baumannii itself.52 In our previous study, A. baumannii species, rather than antibiotic resistance, was found to be associated with mortality (Fig. 2).53 A study using a Galleria mellonella animal model reported that larvae survival was higher during infection with a non-baumannii ACB complex strain than with carbapenem-susceptible and carbapenem-resistant A. baumannii strains without appropriate treatment. No significant difference in survival was observed between larvae infected with carbapenem-susceptible and carbapenem-resistant A. baumannii.54 These data suggest that the factors contributing to the outcomes of Acinetobacter infections are more likely associated with differences in the virulence of individual species rather than carbapenem resistance. However, further investigation of the innate virulence of the various genospecies, and how these findings could be implemented in daily practice, should be carried out. Patients infected with A. baumannii had a mortality rate >30%. The poor outcome emphasizes the importance of aggressive infection control for prevention of mortality and the urgent need for new therapeutic agents and vaccines.
FIG. 2.
Kaplan-Meier curves of survival in 90 patients with A. baumannii bacteremia and 90 patients with non-baumannii ACB complex bacteria. Adapted from reference 53.
INFECTION CONTROL AND PREVENTION
Acinetobacter species are increasingly present in healthcare settings, either as occasional outbreaks or as endemic pathogens. Successful control has been reported in many healthcare facilities.55,56,57,58,59,60,61 However, once Acinetobacter has become endemic, it becomes difficult to eradicate from a healthcare facility. For this reason, early recognition, aggressive control of spread, and prevention of the establishment of endemic strains are crucial. Strategies for control of Acinetobacter outbreaks include active surveillance cultures, environmental surveillance cultures, improved hand hygiene, cleaning and disinfection of the environment, cohort nursing, isolation of patients in single rooms, restriction of access to the ICU, appropriate antibiotic use, and closing wards for cleaning and disinfection.62
MDR A. baumannii contamination of gloves, gowns, and hands of healthcare workers occurs after contact with a source patient.63 Acinetobacter is easily transmitted from healthcare workers and the general environment, including medical equipment, to patients. The ability to form a biofilm and the presence of dormant cells enables Acinetobacter to survive for several weeks on abiotic surfaces under dry conditions.64 Therefore, thorough disinfection of a potentially contaminated environment is also important, as is the use of a closed tracheal suction system and vascular devices to prevent Acinetobacter contamination.65 Although some studies have demonstrated successful control of infection without isolation of infected or colonized patients,66 implementation of precautions during contact and isolation of patients is generally encouraged.67,68 The use of patients' own equipment might facilitate the control of outbreaks, and the maintenance of a good hand hygiene regimen for healthcare workers is also important.69 Because previous exposure to antibiotics is considered a risk factor for outbreaks and antibiotic resistance, restriction of the use of broad-spectrum antibiotics may also contribute to a reduction in infections.70
Once endemic in a healthcare unit, it is extremely difficult to eradicate A. baumannii. Whether this organism can be reduced by strict infection control measures after becoming endemic is still under debate. A study of ICU patients showed that a multifaceted intervention featuring active surveillance and environmental cleaning resulted in a sustained reduction in the rate of XDR A. baumannii colonization and infection and reduced the cost of antibiotics and hospitalization.71 Further well-designed randomized controlled trials are needed to validate the effects of these strategies in endemic settings.
Footnotes
None declared.
References
- 1.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 2.Euzéby JP. List of bacterial names with standing in nomenclature: a folder available on the internet. Int J Syst Bacteriol. 1997;47:590–592. doi: 10.1099/00207713-47-2-590. [DOI] [PubMed] [Google Scholar]
- 3.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 4.Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 5.Dorsey CW, Beglin MS, Actis LA. Detection and analysis of iron uptake components expressed by Acinetobacter baumannii clinical isolates. J Clin Microbiol. 2003;41:4188–4193. doi: 10.1128/JCM.41.9.4188-4193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jankowski S, Grzybek-Hryncewicz K, Fleischer M, Walczuk M. Susceptibility of isolates of Acinetobacter anitratus and Acinetobacter lwoffii to the bactericidal activity of normal human serum. FEMS Microbiol Immunol. 1992;4:255–260. doi: 10.1111/j.1574-6968.1992.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 7.Haseley SR, Pantophlet R, Brade L, Holst O, Brade H. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter junii strain 65. Eur J Biochem. 1997;245:477–481. doi: 10.1111/j.1432-1033.1997.t01-1-00477.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, et al. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007;21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 10.Martí S, Rodríguez-Baño J, Catel-Ferreira M, Jouenne T, Vila J, Seifert H, et al. Biofilm formation at the solid-liquid and air-liquid interfaces by Acinetobacter species. BMC Research Notes. 2011;4:5. doi: 10.1186/1756-0500-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Chuang YY, Huang YC, Lin CH, Su LH, Wu CT. Epidemiological investigation after hospitalising a case with pandrug-resistant Acinetobacter baumannii infection. J Hosp Infect. 2009;72:30–35. doi: 10.1016/j.jhin.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 15.Gordon NC, Wareham DW. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents. 2010;35:219–226. doi: 10.1016/j.ijantimicag.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009;33:520–524. doi: 10.1016/j.ijantimicag.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park YK, Choi JY, Shin D, Ko KS. Correlation between overexpression and amino acid substitution of the PmrAB locus and colistin resistance in Acinetobacter baumannii. Int J Antimicrob Agents. 2011;37:525–530. doi: 10.1016/j.ijantimicag.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012;67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Shin JH, Park KH, Kim JH, Shin MG, Suh SP, et al. Identification, genotypic relation, and clinical features of colistin-resistant isolates of Acinetobacter genomic species 13BJ/14TU from bloodstreams of patients in a university hospital. J Clin Microbiol. 2014;52:931–939. doi: 10.1128/JCM.02868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SY, Shin JH, Lee K, Joo MY, Park KH, Shin MG, et al. Comparison of the Vitek 2, MicroScan, and Etest methods with the agar dilution method in assessing colistin susceptibility of bloodstream isolates of Acinetobacter species from a Korean university hospital. J Clin Microbiol. 2013;51:1924–1926. doi: 10.1128/JCM.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell DJ, Sader HS, Flamm RK, Jones RN. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012) Int J Antimicrob Agents. 2014;43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents. 2013;41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Choi JY, Kim HW, Kim SH, Chung DR, Peck KR, et al. Spread of carbapenem-resistant Acinetobacter baumannii global clone 2 in Asia and AbaR-type resistance islands. Antimicrob Agents Chemother. 2013;57:5239–5246. doi: 10.1128/AAC.00633-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaynes R, Edwards JR National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 27.Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000;31:690–697. doi: 10.1086/314040. [DOI] [PubMed] [Google Scholar]
- 28.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 29.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87:540–546. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 30.Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, Fernández-Cuenca F, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009;30:257–263. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 31.Falagas ME, Karveli EA, Siempos II, Vardakas KZ. Acinetobacter infections: a growing threat for critically ill patients. Epidemiol Infect. 2008;136:1009–1019. doi: 10.1017/S0950268807009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JY, Kim CO, Park YS, Yoon HJ, Shin SY, Kim YK, et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med J. 2006;47:63–69. doi: 10.3349/ymj.2006.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smolyakov R, Borer A, Riesenberg K, Schlaeffer F, Alkan M, Porath A, et al. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hosp Infect. 2003;54:32–38. doi: 10.1016/s0195-6701(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61:1369–1375. doi: 10.1093/jac/dkn128. [DOI] [PubMed] [Google Scholar]
- 35.Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 2008;56:432–436. doi: 10.1016/j.jinf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Yang SC, Chang WJ, Chang YH, Tsai YS, Yang TP, Juan CW, et al. Prevalence of antibiotics resistance and OXA carbapenemases genes in multidrug-resistant Acinetobacter baumannii isolates in central Taiwan. Eur J Clin Microbiol Infect Dis. 2010;29:601–604. doi: 10.1007/s10096-010-0878-2. [DOI] [PubMed] [Google Scholar]
- 37.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–543. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis. 2012;54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–255. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 41.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 42.Sirijatuphat R, Thamlikitkul V. Colistin versus Colistin plus Fosfomycin for Treatment of Carbapenem - Resistant Acinetobacter baumannii Infections: A Preliminary Study. Antimicrob Agents Chemother. 2014 Jun 30; doi: 10.1128/AAC.02435-13. pii: AAC.02435-13. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Cortes LE, Cisneros JM, Fernandez-Cuenca F, Bou G, Tomas M, Garnacho-Montero J, et al. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii : analysis of a multicentre prospective cohort. J Antimicrob Chemother. 2014 Jun 25; doi: 10.1093/jac/dku233. pii: dku233. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother. 2014 May 28; doi: 10.1093/jac/dku168. pii: dku168. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Montravers P, Bassetti M, Dupont H, Eckmann C, Heizmann WR, Guirao X, et al. Efficacy of tigecycline for the treatment of complicated skin and soft-tissue infections in real-life clinical practice from five European observational studies. J Antimicrob Chemother. 2013;68(Suppl 2):ii15–ii24. doi: 10.1093/jac/dkt141. [DOI] [PubMed] [Google Scholar]
- 46.Eckmann C, Montravers P, Bassetti M, Bodmann KF, Heizmann WR, Sánchez García M, et al. Efficacy of tigecycline for the treatment of complicated intra-abdominal infections in real-life clinical practice from five European observational studies. J Antimicrob Chemother. 2013;68(Suppl 2):ii25–ii35. doi: 10.1093/jac/dkt142. [DOI] [PubMed] [Google Scholar]
- 47.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54:1699–1709. doi: 10.1093/cid/cis270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 49.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, et al. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schleicher X, Higgins PG, Wisplinghoff H, Körber-Irrgang B, Kresken M, Seifert H. Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosocomialis in Germany over a 5-year period (2005-2009) Clin Microbiol Infect. 2013;19:737–742. doi: 10.1111/1469-0691.12026. [DOI] [PubMed] [Google Scholar]
- 51.Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, Edmond MB, et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect. 2012;64:282–290. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, Lee PI, et al. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J Antimicrob Chemother. 2011;66:1839–1846. doi: 10.1093/jac/dkr200. [DOI] [PubMed] [Google Scholar]
- 53.Park KH, Shin JH, Lee SY, Kim SH, Jang MO, Kang SJ, et al. The clinical characteristics, carbapenem resistance, and outcome of Acinetobacter bacteremia according to genospecies. PLoS One. 2013;8:e65026. doi: 10.1371/journal.pone.0065026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chusri S, Chongsuvivatwong V, Rivera JI, Silpapojakul K, Singkhamanan K, McNeil E, et al. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob Agents Chemother. 2014;58:4172–4179. doi: 10.1128/AAC.02992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carbonne A, Naas T, Blanckaert K, Couzigou C, Cattoen C, Chagnon JL, et al. Investigation of a nosocomial outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a hospital setting. J Hosp Infect. 2005;60:14–18. doi: 10.1016/j.jhin.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 56.De Jong G, Duse A, Richards G, Marais E. Back to basics--optimizing the use of available resources during an outbreak of multi-drug resistant Acinetobacter spp. J Hosp Infect. 2004;57:186–187. doi: 10.1016/j.jhin.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Denton M, Wilcox MH, Parnell P, Green D, Keer V, Hawkey PM, et al. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. J Hosp Infect. 2004;56:106–110. doi: 10.1016/j.jhin.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Idzenga D, Schouten MA, van Zanten AR. Outbreak of Acinetobacter genomic species 3 in a Dutch intensive care unit. J Hosp Infect. 2006;63:485–487. doi: 10.1016/j.jhin.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Kraniotaki E, Manganelli R, Platsouka E, Grossato A, Paniara O, Palù G. Molecular investigation of an outbreak of multidrug-resistant Acinetobacter baumannii, with characterisation of class 1 integrons. Int J Antimicrob Agents. 2006;28:193–199. doi: 10.1016/j.ijantimicag.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Longo B, Pantosti A, Luzzi I, Placanica P, Gallo S, Tarasi A, et al. An outbreak of Acinetobacter baumannii in an intensive care unit: epidemiological and molecular findings. J Hosp Infect. 2006;64:303–305. doi: 10.1016/j.jhin.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Pimentel JD, Low J, Styles K, Harris OC, Hughes A, Athan E. Control of an outbreak of multi-drug-resistant Acinetobacter baumannii in an intensive care unit and a surgical ward. J Hosp Infect. 2005;59:249–253. doi: 10.1016/j.jhin.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Cookson B. The working party guidance on the control of multi-resistant AcinetobacterOutbreaks. [Internet] [place unknown]: 2006. Aug 29, [Cited 2009 April 1]. Available from: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Acinetobacter/Guidelines/ [Google Scholar]
- 63.Morgan DJ, Liang SY, Smith CL, Johnson JK, Harris AD, Furuno JP, et al. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol. 2010;31:716–721. doi: 10.1086/653201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gayoso CM, Mateos J, Méndez JA, Fernández-Puente P, Rumbo C, Tomás M, et al. Molecular mechanisms involved in the response to desiccation stress and persistence in Acinetobacter baumannii. J Proteome Res. 2014;13:460–476. doi: 10.1021/pr400603f. [DOI] [PubMed] [Google Scholar]
- 65.Podnos YD, Cinat ME, Wilson SE, Cooke J, Gornick W, Thrupp LD. Eradication of multi-drug resistant Acinetobacter from an intensive care unit. Surg Infect (Larchmt) 2001;2:297–301. doi: 10.1089/10962960152813331. [DOI] [PubMed] [Google Scholar]
- 66.Wilks M, Wilson A, Warwick S, Price E, Kennedy D, Ely A, et al. Control of an outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus colonization and infection in an intensive care unit (ICU) without closing the ICU or placing patients in isolation. Infect Control Hosp Epidemiol. 2006;27:654–658. doi: 10.1086/507011. [DOI] [PubMed] [Google Scholar]
- 67.Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268–1274. doi: 10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 68.Wybo I, Blommaert L, De Beer T, Soetens O, De Regt J, Lacor P, et al. Outbreak of multidrug-resistant Acinetobacter baumannii in a Belgian university hospital after transfer of patients from Greece. J Hosp Infect. 2007;67:374–380. doi: 10.1016/j.jhin.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Husni RN, Goldstein LS, Arroliga AC, Hall GS, Fatica C, Stoller JK, et al. Risk factors for an outbreak of multi-drug-resistant Acinetobacter nosocomial pneumonia among intubated patients. Chest. 1999;115:1378–1382. doi: 10.1378/chest.115.5.1378. [DOI] [PubMed] [Google Scholar]
- 70.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977-2000. Infect Control Hosp Epidemiol. 2003;24:284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 71.Apisarnthanarak A, Pinitchai U, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ Thammasat University Pandrug-Resistant Acinetobacter baumannii Control Group. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis. 2008;47:760–767. doi: 10.1086/591134. [DOI] [PubMed] [Google Scholar]