Abstract
Nonalcoholic fatty liver disease (NAFLD) is quickly becoming one of the most prominent causes of liver disease worldwide. The increasing incidence of NAFLD is tied to the obesity epidemic and the subsequent metabolic derangements brought along with it. Current efforts to elucidate the mechanism and causes of the disease have answered some questions, but much remains unknown about NAFLD. The aim of this article is to discuss the current knowledge regarding the pathogenesis of the disease, as well as the current and future diagnostic, preventative, and therapeutic options available to clinicians for the management of NAFLD.
Keywords: Nonalcoholic fatty liver disease, Steatohepatitis, Nonalcoholic steatohepatitis, Pathogenesis, Treatments, Diagnosis, Management, Metformin, Vitamin E, Thiazolidinedione, Imaging, Prevalence
Core tip: Given the increasing prevalence of the disease, Nonalcoholic fatty liver disease (NAFLD), adequate knowledge regarding the disease is becoming especially important for physicians and patients. Despite this, since its description in 1981 in pregnant women, NAFLD has been a difficult to understand and treat for both scientists and clinicians. Current efforts to elucidate the mechanism and causes of the disease have answered some questions, but much remains unknown about NAFLD. The aim of this article is to discuss the current knowledge regarding the pathogenesis of the disease, as well as the current and future diagnostic, preventative, and therapeutic options available to clinicians for the management of NAFLD.
INTRODUCTION
The problem of obesity has grown tremendously through the 20th century and into the 21st century, slowly transforming into an epidemic. Along with it, nonalcoholic fatty liver disease (NAFLD) has become one of the major diseases plaguing the nation and world. In the United States, NAFLD is the most common cause of liver disease, representing over 75% of the chronic liver disease[1]. It also is one of the most common indications for liver transplantation, contributing a major burden to both the morbidity and mortality of the nation. NAFLD is a disease of all ages, and the disease has been reported in children as young as 2 years of age. The prevalence of fatty liver increases with age in adults, and while the exact incidence of the disease is unknown, In the United States National Health and Nutrition Examination Survey, 6% of overweight, and 10% of obese adolescents had an elevated alanine aminotransferase (ALT), although alcohol use was not excluded[2]. Results from prevalence studies done internationally have varied widely, with recent studies done in Japan[3,4] and England[5] indicating the prevalence of the disease has nearly doubled over the last twenty years[1]. This increase was even more dramatic in adolescent populations, where the incidence increased 174%[6].
Given the increasing prevalence of the disease, adequate knowledge regarding the disease is becoming especially important for physicians and patients. Despite this, since its description in 1980, nonalcoholic steatohepatitis (NASH) has been a difficult to understand and treat for both scientists and clinicians[7]. Current efforts to elucidate the mechanism and causes of the disease have answered some questions, but much remains unknown about NAFLD. The aim of this article is to discuss the current knowledge regarding the pathogenesis of the disease, as well as the current and future diagnostic, preventative, and therapeutic options available to clinicians for the management of NAFLD.
The spectrum of NAFLD is a continuum ranging from simple steatosis to NASH and finally cirrhosis. The defining characteristic of the disease is the presence of greater than normal lipid deposition within the liver with the absence of excessive alcohol consumption defined as > 20 g/d for men and 10 g/d for women. Steatosis is the presence of lipid within the cytoplasm of hepatocytes, the criteria for which is defined in the literature as being either hepatic lipid levels above the 95th percentile for healthy individuals (about >55 mg/g liver)[8], greater than 5% of the liver’s weight[9], or found in greater than 5% of hepatocytes histologically[10]. NASH is defined as steatosis in the presence of hepatocyte damage, inflammation and/or subsequent scarring and replacement of the tissue with type I collagen. Approximately 10%-29% of patients with NASH will develop cirrhosis within a 10 year period[11].
The implications for an individual who develops cirrhosis are grave no matter the cause. The liver is perhaps the most multifunctional solid organ in the body, and is involved in detoxification, nutrient storage, and glucose homeostasis. Insult to any of these processes can lead to liver disease. In the case of NAFLD numerous derangements can be found in the liver’s capacity to process lipid, and the cause of which has been linked to multifactorial alterations in genetics, intestinal flora, diet, adipose tissue, hormone regulation and the immune system.
PATHOGENESIS
Normal liver processing of lipid
The liver is one of the principle regulators of lipid in the body (Figure 1). Fatty acids in the liver used for triglyceride (TG) synthesis are provided by diet, adipose tissue or de novo synthesis from excess glucose. In the postprandial state, chylomicrons transport dietary fats into the lymphatics and eventually the systemic circulation, where they will be hydrolyzed by lipoprotein lipase (LPL) or delivered to the liver. Excess carbohydrates from the diet also promote the de novo synthesis of free fatty acids; acetyl-coenzyme A excess derived from glucose is shuttled into the lipogenesis pathways in the cytoplasm and mitochondria.
Figure 1.
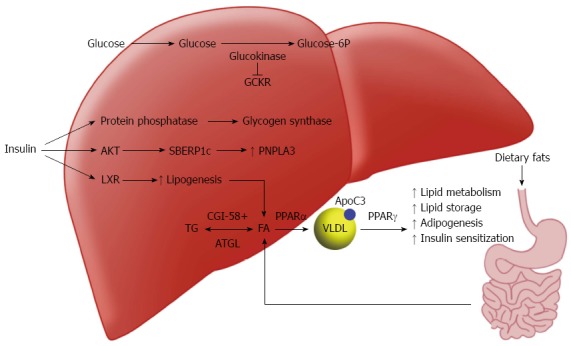
Schematic of protein and gene pathways involved in lipid processing and implicated in non-alcoholic fatty liver disease. LXR: Liver X receptor; PNPLA3: Patatinlike phospholipase domain-containing 3 gene; PPAR: Peroxisome proliferators activated receptors; SREBP-1c: Sterol regulatory element-binding protein.
Insulin serves a regulator for this pathway by activating acetyl-CoA carboxylase (ACC) via sterol regulatory element-binding protein 1c (SREBP-1c)[12] and allowing the conversion of acetyl CoA into malonyl-CoA. Glucose itself at high levels can activate lipogenesis by binding the carbohydrate response element binding protein (ChREBP)[13]. ChREBP increases de novo synthesis of FFA through the regulation of numerous enzymes including pyruvate kinase, fatty acid synthase and ACC[14]. Another important regulator of lipid in the liver, liver x receptor (LXR), was uncovered in the last twenty years[15]. While the mechanisms of LXR are not fully understood, it is clear that it is involved in a positive feedback loop with SREBP-1c[16]. Mice with knockouts of LXR have substantial decreases in lipogenesis, and LXR has been shown to down regulate many enzymes involved in de novo lipogenesis[17].
In the fasting state, insulin and glucose levels drop off and lipogenesis slows. Glucagon and epinephrine levels increase subsequently increasing the activity of several lipases including hormone sensitive lipase (HSL) and adipocyte TG lipase (ATGL)[18]. The hydrolysis of TG by these lipases creates FFA for the liver to process having three different destinies: beta oxidation for energy creation, reesterification and storage within hepatocytes, or processed with lipoproteins or phospholipids and exported as cholesterol[8].
Genetics
Multiple rare genetic conditions, which cause dysfunction of the normal liver processing of nutrients and lipids, can result in steatosis (Table 1). In a 2011 review by Hooper et al[19] mutations which contributed to either an increase in lipid synthesis/uptake or a decrease in hydrolysis/export were associated with NAFLD. Examples of such diseases include: glycogen storage diseases[20], ATGL defects, or very-low-density lipoproteins mutations (such as those involving the surface protein apoB or its transfer protein MTTP) have been shown to cause steatosis[19]. However, many of the genetic mutations described in the study are rare single protein or enzyme mutations that do not explain the vast majority of the cases of NAFLD.
Table 1.
Genes and proteins Implicated in non-alcoholic fatty liver disease
| Gene | Protein | Function |
| APOc3 | Apolipoprotein C3 | Surface component of VLDL and inhibits LPL[227] |
| ATGL | Adipose triglyceride lipase | Catalyzes the initial step in triglyceride hydrolysis[228] |
| CGI-58 | Comparative Gene Identification-58 | Activator of triglyceride hydroxylases (including ATGL)[228] |
| GCKR | Glucokinase regulatory Protein | Downregulation of glucokinase[229] |
| LXR | Liver X Receptor | Transcription factor for numerous target genes involved in glucose and lipid metabolism[230] |
| PNPLA3 | Palatin-like phospholipase-containing protein 3 | Unknown, possible triglyceride hydroxylase[16] |
| PPARα | Peroxisome proliferator-activated receptor α | Nuclear receptor that promotes the catabolism of fatty acids[231] |
| PPARγ | Peroxisome Proliferator-Activated Receptor γ | Nuclear receptor that promotes storage and processing of fatty acids[232] |
| PPP1R3B | Glycogen Binding Subunit of Protein Phosphatase 1 | Subunit in Protein Phosphatase which activates glycogen synthase[233] |
| SBERP1c | Sterol regulatory element-binding protein | Transcription factor for genes involved in de novo lipogenesis[234] |
ATGL: Adipocyte TG lipase; LPL: Lipoprotein lipase; VLDL: Very-low-density lipoproteins.
Despite this finding, the heritability of NAFLD has been demonstrated to be approximately 39% in a recent study comparing the presence of fatty liver in siblings and parents of patients[21]. Furthermore it has been demonstrated that men have an increased prevalence of NASH, and women typically develop the disease later than men (in the sixth decade of life vs the fourth)[22]. Hispanics have the highest prevalence of the disease, 45%, vs 33% for non-Hispanic whites and 24% for African Americans[23]. Further comparison between the groups showed different odds ratio for certain disease modifying risks in Latinos when compared to whites[24].
Efforts to elucidate the genetic cause for this difference has pointed towards a missense mutation, I148M, in the patatin-like phospholipase domain-containing 3 gene (PNPLA3)[25], which has been linked to susceptibility and progression of the disease. Studies have shown differences in the prevalence of the mutation among races, which may help explain the differences in risk between races[26,27]. PNPLA3 is a protein involved in the SREBP1c pathway[28], however the exact mechanism of its involvement in steatosis has not yet been elucidated. Recent studies have demonstrated the interaction of PNPLA3 as a regulator of lipid homeostasis[29]. Other genetic variants, with prevalence differences between ethnicities, such as NCAN, PPP1R3B, and GCKR have been linked to increases in hepatic steatosis[30]. The mechanisms of these differences has not been well elucidated to date, further research should be done to perhaps reveal the mechanisms leading to steatosis and NASH.
Metabolic changes
The peroxisome proliferators activated receptors (PPAR) are a group of nuclear receptors involved in regulation of fatty acid metabolism and storage. PPARα is a regulator of β-oxidation and PPARγ is involved in insulin sensitivity and triglyceride storage. PPARα increases the β-oxidation, uptake and clearance of fatty acids[31]. Knockout models of PPARα have shown a marked development of steatosis, implicating a possible role in the disease[32]. Studies have also documented the presence of certain SNPs (Leu162Va) and their correlation with progression of NAFLD[33], however other studies have contested this[34]. Further studies are still required to establish the importance of the genetic polymorphism. Fibrates act as agonists at PPARα and have shown some promise as a treatment option.
PPARγ has also shown connection to NAFLD. In murine models of the disease, PPARγ has been found at increased levels in the livers of mice[35]. Additionally a Pro12Ala SNP in the gene for PPARγ has been shown to be protective against liver injury[33]. However, as was the case for the Leu162Va SNP in PPARα, other studies have contested this correlation including a recent meta-analysis by Wang et al[36] The effect of SNPs in the PPAR’s requires further investigation in order to conclusively determine their importance in NAFLD. The thiazolidinediones (TZDs) are the pharmacologic activators of PPARγ.
Hormone changes
Insulin resistance, frequently found in those with metabolic syndrome, obesity and/or diabetes, is frequently considered the key factor in developing hepatic steatosis. The number of diabetic patients from 1988 to 2008 has nearly tripled[37] and the obesity rate has increased by about 56%[38]; it is therefore not surprising that over the same period the prevalence of NAFLD has nearly doubled. While the presence of peripheral, and specifically hepatic, insulin resistance in fatty liver is commonly accepted, there remains controversy whether the insulin resistance is caused by NAFLD or vice versa[39].
Studies of animal models for fatty liver have been observed to concurrently develop hepatic steatosis and insulin resistance, suggesting that steatosis is a factor in the development of insulin resistance[8,40]. However, mice with more specific mutations for hepatic steatosis do not develop insulin resistance[41]. Furthermore, patients with AKT2 mutations, a secondary messenger for insulin in glucoregulation, develop hepatic steatosis due to insulin resistance[42]. Other patients with mutations in apoB, ATGL, or CGI58 (a protein involved in the regulation of lipolysis and implicated in Chanarin-Dorfman syndrome) develop hepatic steatosis without insulin resistance[19,43-46]. The inconsistent associations between hepatic steatosis and insulin resistance in different mutations suggests a complex interaction between the two phenomena; these observations have led to the hypothesis that the presence of insulin resistance is dependent on the location of TG accumulation.
While further research is needed to determine the cause and effect relationship between insulin resistance and hepatic steatosis, a strong consensus remains that insulin resistance is frequently concurrently present with NAFLD. The pathogenesis of fatty liver in insulin resistance is strongly linked to the two major biochemical pathways insulin affects in the liver. The key factor in the progression of fatty accumulation is that the lipogenetic pathway remains sensitive to the effects of insulin, while the gluconeogenesis pathways, which are normally inhibited by insulin, become resistant. The result of this process is hyperglycemia, which stimulates the body to produce more insulin. This insulin hypersecretion stimulates lipogenesis, as the SREBP-1c mediated pathway maintains its response[47].
Inflammation
The progression of steatosis to NASH is a frequently encountered clinical scenario, associated with worse outcomes for patients. A key-defining feature of the NASH is the presence of inflammation and subsequent fibrosis. Change of concentration in several inflammatory cytokines has been implicated in this progression. A frequently implicated, and targeted, cytokine in this process is tumor necrosis factor alpha (TNFα) which has been linked to both insulin resistance and progression to NASH[48,49]. TNFα mediated insulin resistance has been correlated with increased levels of SREBP-1c and hepatic steatosis[50]. Serum and liver levels of TNFα have been correlated to increased levels of inflammation, steatosis and histologic damage in patients with NASH[51-54]. Furthermore, anti-TNFα therapy has shown promise in the management of NAFLD, this is discussed further in the treatment section.
Interleukin-6 (IL-6) has also been shown to be increased in fatty liver disease. These increased levels have been correlated to the development of insulin resistance[55], diabetes[56] and fatty liver in both animals and humans[57,58]. Additionally, like TNFα, expression of IL-6 has been correlated with severity of disease in patients with NASH[59]. While the mechanism of these correlations and whether chronic exposure is a primary or secondary effect of NAFLD is not clear, IL-6 has been identified early in liver disease as a regenerative factor and chronically as a mediator of inflammation, apoptosis and liver damage/scarring[60].
Diet and physical activity
The role of nutritional status and physical activity in the general health of the population is of growing interest in nearly all fields of medicine. While diet and physical activity are difficult measures to control precisely and study both prospectively and retrospectively, there appears to be a link between diet and physical activity and the pathogenesis of fatty liver disease. The link between increased calorie intake and sedentary lifestyle with metabolic syndrome and insulin resistance has been demonstrated in numerous studies[61], and the connection of metabolic syndrome and insulin resistance with NAFLD has also been established[4,10,62].
Furthermore, retrospective and observational data has suggested that individuals with NAFLD have lower activity levels[63]. Limited studies have shown a correlation between both dietary fat[64] and carbohydrate intake[65,66] with the level of fat deposition in patients. Similarly, the combination of both increased carbohydrate and fat intake was observed in patients with NASH[63]. Strong evidence exists for a significant link between soda and fructose consumption with NAFLD and NASH[67-69]. Low intake of antioxidants such as vitamin E, zinc and polyunsaturated fatty acids have also been shown to have a connection with NAFLD[65,70,71]. Recent studies have shown an inverse correlation of vitamin D levels and levels of fatty liver and NASH in children[72]. However despite these links, much of the evidence remains weak and it remains difficult to monitor healthy individuals and their diets long term and monitor the rates of progression to diseased states. Further controlled studies must be done to truly establish the risk of NAFLD with certain diets and activity levels.
Gut microbiota
Intestinal flora has been implicated in numerous diseases of the GI tract. Recent work has also suggested that the microbiota in the gut has a role in obesity. In a study by Bäckhed et al[73] mice were raised in a sterile environment and were observed to have 40% less body fat, the mice were subsequently transferred with the microbiota from the control mice and had a 60% increase in body fat. These mice also saw an increase in hepatic TG content. It has been postulated that this results via numerous mechanisms including: increased energy yield from food, increasing gut permeability and low grade inflammation, modulation of choline metabolism, regulation of bile acid metabolism, and/or increasing ethanol production from bacterial sources[74]. Several studies have been done reviewing the effects of modifying the intestinal flora on NAFLD; they are discussed further in the treatment section.
Other factors
Obstructive sleep apnea (OSA) has also been connected to NAFLD and NASH. A 2012 meta-analysis demonstrated that patients with OSA had a pooled odds ratio of 2.01 for the presence of NAFLD by histological, radiological or biochemical criteria[75]. While the exact cause of this correlation is not clear, increased inflammation as well increased levels of hypoxia inducible factors have been proposed as explanations for the connection[76]. It has also been suggested that due to both OSA’s and NAFLD’s association with obesity that the connection between OSA and NAFLD are merely coincidental, however recent evidence in the pediatric population has demonstrated a link regardless of obesity[77]. Despite this link, the use of continuous positive airway pressure to treat OSA has not been shown to improve AST and ALT levels in OSA patients[78].
Choline, a micronutrient found in legumes and egg yolks, deficiency has also been shown to have an association with NAFLD. Mice genetically or dietarily deprived of choline develop fatty liver[79,80]. This connection has also been demonstrated in humans, however at this time it is unclear if choline supplementation can reverse the associated fatty liver[81,82].
DIAGNOSIS
Initial evaluation
The diagnosis of NAFLD requires (1) there is hepatic steatosis by imaging or histology; (2) there is no significant alcohol consumption; (3) no competing etiologies are present for hepatic steatosis; and (4) there are no co-existing causes for chronic liver disease[83]. Patients with NAFLD are typically asymptomatic, and when present, manifest with vague symptoms such as fatigue and abdominal discomfort[84]. On physical exam, it is useful to examine risk factors for NAFLD such as an increased body mass index (BMI), weight, and elevated blood pressure[85]. Furthermore, as there exists an association between metabolic syndrome and NAFLD[86], clinical indications of insulin resistance should raise suspicion for NAFLD.
It is important to rule out common causes of liver injury, such as alcohol, drug use, and viral hepatitis as well as other co-existing etiologies for chronic liver disease including alpha-1 antitrypsin deficiency, hemochromatosis, autoimmune liver disease (types 1 and 2), chronic viral hepatitis, and Wilson’s disease[83,85]. Accordingly, laboratory tests for alpha-1 antitrypsin deficiency, antinuclear antibody, smooth muscle antibody, anti-liver/kidney microsomes antibody, anti-liver cytosol antigen, serum ferritin and transferrin levels, HFE genetic testing, as well as ceruloplasmin levels should be obtained[87].
Elevated alanine aminotransferase and aspartate aminotransferase levels may indicate the presence of hepatic steatosis, inflammation, or fibrosis, however their utility in the diagnosis of NASH is limited because of their low specificity, sensitivity, and prognostic value[88,89]. Even more, cohort studies have shown that ALT levels are within normal limits in nearly 80% of patients with fatty liver, and aminotransferase levels tend to fall over time, even with progressing fibrosis[23,90]. For these reasons, other diagnostic methods are needed for confirming the suspected diagnosis of NAFLD.
Screening
NAFLD is often diagnosed incidentally following a routine lab panel or an imaging study done for other reasons. Despite this, screening for NAFLD is not recommended in the general population[91]. Even screening of higher-risk individuals such as those with diabetes[92] have not been recommended due to uncertainties surrounding diagnostic tests, treatment options, long-term benefits, as well as cost-effectiveness of screening[83]. Although recent findings suggest that family members of children with NAFLD demonstrate a higher risk for NAFLD[21,93], there have been no conclusive studies that prove the utility of screening of family members at the time being. Several attempts have been made at creating risk assessment scores, to stratify patients by their risk factors in order to screen only those with the highest likelihood of disease. However, to date none of these scores have gained widespread acceptance.
Prediction models
Numerous attempts have been made at creating a noninvasive scoring model to assess fibrosis in NAFLD. The most extensively researched model is the NAFLD Fibrosis Score, which incorporates age, BMI, AST/ALT ratio, platelet count, and albumin[94]. In a large cohort study of patients who were diagnosed with liver biopsy-proven NAFLD, the test was shown to have a AUROC of 0.84, sensitivity of 82%, specificity of 98%, PPV of 90% and NPV of 93%[94]. Furthermore, the NAFLD fibrosis scoring used two cutoff scores, thus dividing patients into low-probability fibrosis, high-probability fibrosis, and indeterminate. Of the determinable patients, 90% of patients were accurately diagnosed. However, one major limitation of the scoring system is that about 25% of patient were indeterminate[94]. Nevertheless, the NAFLD Fibrosis Score has proven clinical utility, which has been further validated by a metanalysis[89].
Another model that has also demonstrated promise in the assessment of fibrosis is Fibrotest which uses an undisclosed formula, which incorporates the age of a patient, α2 macroglobulin, total bilirubin, GGT, and apolipoprotein A1[95]. The AUROC score for fibrosis ranged from 0.75 to 0.86 with a sensitivity of 77%, specificity of 98%, PPV 90%, and NPV 73%. While validated by other studies[96], the model had some limitations, including an inability to classify one third of the patients, as well an inability to distinguish between mild to moderate fibrosis[95].
Other scoring models for fibrosis include the FIB-4[97], European Liver Fibrosis Test (ELF)[98], BARD[99]. the NICE model[100], and NASH test[101]. Each of these predictive models incorporates various lab values and patient characteristics (Table 2). While each test has relative strengths statistically (Table 3), the most externally validated test remains the NAFLD Fibrosis Score. With a high specificity, the test has a good ability to rule-in NAFLD, making it a useful tool in deciding when to progress to liver biopsy. While many other models have shown some clinical utility, the studies have been limited by small sample sizes and currently lack repeat studies for validation. Therefore, currently the NAFLD Fibrosis Score is the most widely applicable model and has value in limiting the number of liver biopsies necessary to diagnose NAFLD[83].
Table 2.
Summary of included variables in scoring methods for non-alcoholic fatty liver disease
| NAFLD Fibrosis Score | Fibrotest | FIB-4 | ELF | BARD | NICE | NASH test | |
| Age | × | × | × | × | × | ||
| BMI | × | × | |||||
| AST | × | × | × | × | |||
| ALT | × | × | × | x | × | ||
| Platelet Count | × | × | |||||
| Albumin | × | ||||||
| α2-MG | × | × | |||||
| Total bilirubin | × | × | |||||
| Apo A1 | × | × | |||||
| GGT | × | × | |||||
| PIIINP | × | ||||||
| HA | × | ||||||
| TIMP | × | ||||||
| DM | × | ||||||
| MS | × | ||||||
| CK-18 | × | ||||||
| Sex | × | ||||||
| Height | × | ||||||
| Weight | × | ||||||
| HG | × | ||||||
| TGL | × | ||||||
| CL | × |
α2-MG: α2 macroglobulin; ALT: Alanine aminotransferase; ApoA1: Apolipoprotein A1; AST: Aspartate aminotransferase; BMI: Body mass index; CK-18: Cytokeratin-18; CL: Cholesterol levels; DM: Diabetes mellitus; ELF: European Liver Fibrosis Test; GGT: Gamma-glutamyl transpeptidase; HA: Hyaluronic acid; HG: Haptoglobin; MS: Metabolic syndrome; PIIINP: Aminoterminal propeptide of type III collagen; TGL: Triglyceride levels; TIMP: Tissue inhibitor of metalloproteinases; NASH: Nonalcoholic steatohepatitis.
Table 3.
Statistical analysis of various scoring methods for non-alcoholic fatty liver disease
| NAFLD fibrosis score | Fibrotest | FIB-4 | ELF | BARD | NICE | NASH test | |
| AUROC | 0.84 | 0.75-0.86 | 0.86 | 0.87 | 0.81 | 0.88 | 0.79 |
| Sens (%) | 82 | 77 | 85 | 89 | 84 | 33 | |
| Spec (%) | 98 | 98 | 65 | 96 | 86 | 94 | |
| PPV (%) | 90 | 90 | 36 | 80 | 43 | 44 | 66 |
| NPV (%) | 93 | 73 | 95 | 98 | 96 | 98 | 81 |
NAFLD: Nonalcoholic fatty liver disease; AUROC: Area under the receiver operating characteristic curve; ELF: European Liver Fibrosis Test; PPV: Positive predictive value; NPV: Negative predictive value; Sens: Sensitivity; Spec: Specificity; NASH: Nonalcoholic steatohepatitis.
Serum markers
There have been a number of serum markers that have been proposed for the diagnosis of NAFLD, but very few that have been extensively researched. In particular, cytokeratin-18 fragments have shown the most promise in the diagnosis of NASH[84,89]. CK18 is a major intermediate filament protein of the liver, which is cleaved by caspases during apoptosis. One meta-analysis showed that using enzyme assays of cleaved cytokeratin-18 fragments, specifically M30, which is an indicator of hepatocyte apoptosis, may have clinical utility showing a sensitivity of 66% and specificity of 82% in diagnosing NASH[102]. While CK18 has shown promising results as a potential biomarker, currently this assay is not commercially available and there is no established cut-off value for determining the presence of steatohepatitis[83].
Other serum biomarkers that have been evaluated for the diagnosis of NASH include various cytokines, acute phase proteins, and oxidative stress markers. Some of the cytokines that have been evaluated include IL-6, TNFα, and CC-chemokine ligand-2. While some studies showed elevated IL-6 in the serum of NASH patients[59,103], others have showed no difference between NASH and steatosis[104]. Due to the lack of consistent correlation between studies, there is currently little clinical utility in the sole measurement of IL-6 at this time.
Similarly, cytokines such as TNFα and adiponectin have shown equivocal results in terms of their clinical utility. As mentioned, TNFα is a proinflammatory cytokine, which plays a role in the pathogenesis of NAFLD. While several studies showed elevated levels of TNFα in NASH patients and animal models[52,103,105,106], the clinical significance of these results has not yet been found. Adiponectin, which has been shown to have antilipogenic and anti-inflammatory effects and is thought to be reduced in NASH[52], has also shown conflicting results when studied as a marker[52,104,107,108].
One chemokine, CC-chemokine ligand-2 (CCL2), also known as monocyte chemoattractant protein-1 (MCP-1), is responsible for the recruitment of macrophages during liver inflammation, and contributes to the progression of NASH[109]. One study showed increased levels of CCL2 in those diagnosed with NAFLD by ultrasound, but these patients did not receive a liver biopsy, warranting the need for further evaluation of CCL2 as a biomarker[110].
Two acute phase reactants, C-reactive protein (CRP) and pentatrix-3, have been studied in the diagnosis of NASH. While Yoneda et al[111] did show elevation of high sensitivity CRP compared to cases of simple nonprogressive steatosis, other studies failed to show such results[53,112]. One study utilized biopsy-proven NAFLD patients to show increased levels of pentatrix-3 in NASH patients compared to non-NASH patients[113]. Even more, pentatrix-3 was also shown to be able to assess the degree of fibrosis. However, more extensive studies are needed at this time to prove the overall clinical utility of the acute phase reactant.
There are many other less studied serum markers that still hold the potential to have clinical Utility. One such marker is serum prolidase enzyme activity (SPEA), which is involved in the breakdown of the final step of collagen[114]. SPEA was shown to be significantly elevated in patients with NASH compared with steatosis[114]. Another potential biomarker is the soluble receptor for advanced glycation end products (sRAGE), which is involved in a similar pathway as that of metabolic syndrome. One study showed a 0.77 AUROC for predicting NASH[115]. Currently, oxidative stress pathway markers have shown little evidence of utility in the diagnosis of NASH, despite its known involvement in the pathogenesis of NASH[116]. In fact, standard blood oxidative stress markers were shown to not predict the extent hepatic steatosis[117].
Radiological evaluation
Similar to serum markers, the radiologic evaluation of NAFLD is of wide interest as a possible noninvasive method for evaluating and diagnosing steatosis and NASH. Ultrasonography (US) is a cheap, fast, and widely available imaging technique with applications for fatty liver. US has been reported to have a sensitivity ranging from 60% to 94% and a specificity of 66% to 95%[118]; lower sensitivities are frequently observed in patients with mild disease[118]. Limitations of US include it being subject to interobserver variability, difficulty in obese patients, and the high proportion of NAFLD patients with coexisting obesity[118]. Contrast enhanced ultrasonography has been reported to have an increased sensitivity (in one study 65.8%) in finding fatty liver infiltrations[119], which nearly rivals that of a computed tomography scan (CT). Contrast-enhanced ultrasound, however it lacks clinical validation and is not commercially available at this time.
Transient elastography (TE) (Fibroscan®) is a tool developed in the last decade, designed to measure liver stiffness via ultrasound[120]. TE measures stiffness in kilopascals by introducing low frequency vibrations to the tissue and measuring the propagation of the energy. Presently TE has been thoroughly investigated for use in other diseases such as hepatitis C, however it has been less studied in steatosis and NASH[121]. Additionally, the use of TE is largely for staging of levels of fibrosis, but, as mentioned below, the results of TE can be of diagnostic utility in patients with inconclusive results in other tests. Like US, TE is subject to difficulties of use in obese patients, with one study reporting a 75% success rate in determining stiffness in obese patients vs a 97% success rate in patients with a BMI below 30 kg/m2[120]. Overall TE can be a beneficial tool, if available to the clinician, in monitoring disease status in NAFLD and NASH.
Incidental finding of hepatic steatosis is frequently found when a patient receives a CT. CT without contrast is a useful modality for assessing the presence and amount of steatosis, with a sensitivity ranging from 82% to 95% and a specificity approaching 100%[85,122]. One of three findings is diagnostic of steatosis on CT: absolute hepatic attenuation of less than 40 Hounsfield units (HU), a hepatic attenuation value <10 HU compared with the spleen and a spleen to liver attenuation ratio < 0.8[118]. Despite the clinical applicability, and being relatively well validated, CT scans are expensive, expose the patient to ionizing radiation and can provide less accurate results in patients with underlying liver disease.
Magnetic resonance spectroscopy (MRS) represents the most sensitive and specific imaging modality, with both values being greater than 90% in most studies[93] and near 100% accuracy in detecting steatosis[123]. MRS is able to compare adjacent tissues by their differing hydrogen content. Using MRS, clinicians are able to calculate the fraction of the liver composed of fat. This so called fat fraction is said to be abnormal when it is greater than 5.56%[123]. Like CT, MRS has a high cost and also exposes patients to radiation (albeit a lower amount than CT), making its clinical utility relatively low.
Liver biopsy
Currently, liver biopsy remains the gold-standard for the diagnosis of NASH as it serves as the only means of distinguishing hepatic steatosis from steatohepatitis through examination of liver histology[85]. In hepatic steatosis, is characterized by the presence of intracellular fat in more than 5% of hepatocytes. Steatohepatitis, on the other hand, has distinctive morphological features similar to that of alcoholic hepatitis including Mallory hyaline, hepatocyte ballooning, as well as the presence of polymorphonuclear leukocytes and perisinusoidal fibrosis in zone 3 of the acinus[124]. However, there are limitations to the use of a liver biopsy including cost, sampling error, and interobserver/intraobserver variability. One study showed that overall interobserver and intraobserver agreement scores for the diagnosis of NASH ranged from only 0.66 to 0.90 and 0.61 to 0.62, respectively[125]. Furthermore, the rate of sampling error complicates the ability to diagnose the histological changes of NASH as a biopsy only samples 0.00002% of the liver[126]. Some studies have shown that NASH may be incorrectly missed in up to 24% of cases, and the fibrosis stage is discordant in 22%-37% of cases[125,127]. In addition, a liver biopsy also carries some morbidity and very rare mortality risk, with a 1-3 risk for major complications and death in 0.01%[128].
According to current AGA guidelines, a liver biopsy should only be considered in patients with NAFLD who are at increased risk to have steatohepatitis and advanced fibrosis, such as those who have metabolic syndrome[83]. Furthermore, a liver biopsy should also be considered in patients with suspected NAFLD that may also have other explaining etiologies for steatosis and co-existing chronic liver diseases[83]. Currently, the most externally validated non-invasive method for determining when to obtain a liver biopsy is the NAFLD Fibrosis Score[94]. It incorporates factors including age, glycemia, BMI, platelet count, albumin, and AST/ALT to predict the presence for advanced fibrosis[89]. In one proposed algorithm, if the patient has a high probability for advanced fibrosis (> 0.676) based on the NAFLD Fibrosis Score, then a liver biopsy is indicated unless there exists clinical evidence of cirrhosis[84]. In the case of an intermediate score (-1.455 to 0.676), an alternative non-invasive method such as a transient ultrasonography (Fibroscan®) to determine the presence of advanced fibrosis, in which case a liver biopsy would be indicated[84]. If the NAFLD Fibrosis Score suggested a low probability for advanced fibrosis (< -1.455), a liver biopsy would most likely be unnecessary as the NAFLD Fibrosis score has a NPV of 93%, but could potentially be confirmed by an alternative method such as an assessment of CK18 fragments[84].
NATURAL HISTORY
The natural history of patients with NAFLD has a mixed picture. In a large cohort study, it was demonstrated that liver related illness was the third leading cause of death in liver patients, and the hazard ratio for general mortality and liver related mortality was 1.038 and 9.32, respectively[129]. As in the general population, the leading cause of death in patients with NAFLD is cardiovascular disease[129]. This highlights the need for patients with NAFLD to have extensive risk management therapy for the prevention of cardiovascular disease, however to date no concrete guidelines have been made for the prevention of adverse cardiac events in these patients[130].
Data regarding the progression of NAFLD from simple steatosis to steatohepatitis is conflicting. One monitoring the outcomes for 40 patients over a median time of 11 years showed no progression to NASH or cirrhosis, and only 12 of the patients had abnormal liver tests at the conclusion of the study[131]. Another more recent study in 2010 followed 52 NAFLD patients for 3 years and found that at the conclusion, of the 13 that began with simple steatosis 15% had normal livers, 23% remained at baseline, 39% developed borderline NASH and 23% developed NASH. Among the 22 subject who began with borderline NASH, 18% had simple steatosis, 59% remained at borderline NASH and 23% had NASH[132]. While the data is inconsistent, it is clear that NAFLD is a slowly progressing disease, and may regress or stay at baseline for years.
Studies have estimated the overall risk of a patient with simple steatosis advancing to clinically significant cirrhosis at approximately 1%-2%[133]. However, patients who have progressed to or presented with NASH are at increased risk of developing hepatic decompensation and liver failure[134]. The risk of cirrhosis in patients with NASH varies from 0% at five years to 12% at 8 years[135,136]. While another study, which followed 129 patients for a mean of 13.7 years, reported 5.4% of the patients developing end-stage liver disease, including hepatocellular carcinoma (HCC)[137]. The development of subsequent development of HCC represents another significant concern for clinicians managing patients with especially NASH. In a study of 46 patients with bridging fibrosis and 43 patients with cirrhosis, 20% had developed HCC after 5 years[138].
PREVENTION AND MANAGEMENT
Prevention
With the increasing prevalence and incidence of NAFLD in the world, the role of preventing the disease has become a hot topic. Due to the lack of understanding of the pathogenesis of the disease, the prevention of NAFLD remains a difficult problem. General prevention of NAFLD involves a modification of the risk factors for the disease. The largest risk controllable risk factors for NAFLD are weight, insulin resistance and metabolic syndrome[139]. Therefore, general education on lifestyle modifications, such as diet and exercise, which can reduce the risk for the development of insulin resistance, weight gain and metabolic syndrome can be seen as the mainstay for prevention of NAFLD.
While other interventions, such as alcohol consumption and dietary supplementation, have been suggested for disease prevention, at this time none are recommended by the AGA[83]. Similarly, other interventions have been shown to prevent the progression of steatosis to NASH, these are described in detail below. It is important to note, that NAFLD without steatohepatitis is not an indication for treatment, however management options are directed towards either those with steatohepatitis or managing the associated comorbidities present in those with steatosis alone. Furthermore, it is important to note that the use of preventative vaccines, such as the hepatitis A and B vaccines, is recommended in patients suffering from NAFLD and NASH, in order to prevent further insult to the already damaged liver.
Lifestyle modifications
The cornerstone to the management of steatosis and NASH to date has been lifestyle changes, with the two most central components being diet and exercise. Diet and exercise have been proposed as a treatment option for steatosis and NASH, however study data is limited to date. Studies have been held back by the lack of consensus in regards to the methodology used to determine whether the disease progressed or improved. While histologic comparison (the NAFLD Activity Score is a frequently used histologic grade for studies) would be the ideal measure, the difficulty and risks in obtaining biopsies remain a hurdle. For this reason, studies vary in their monitoring of steatohepatitis in the study subjects, other measures used include insulin resistance and AST/ALT.
A 2010 study by Promrat et al[140] showed an improvement in NAFLD Activity Scores (NAS) as well as ALT in patients who lost 7%-10% of their body weight vs control. Another randomized study[141] showed an improvement in insulin sensitivity and NAS in patients using orlistat who lost > 5% of their body weight, and there was a more significant improvement in subjects who lost > 9% of their weight. Additional prospective and case control studies have shown that diet, including one study focusing on the Mediterranean Diet, combined with weight loss can help reduce disease burden measured by varying measures[142-144].
Other studies have shown an improvement in the disease through exercise alone[143]. Finally, studies have shown that diet and exercise have been successful in preventing steatosis progression to NASH[145]. Currently the American Gastroenterology Association recommends both exercise and healthy weight loss, either separately or in combination therapy, as interventions for NASH[83]. While both interventions are indicated from a general health perspective and from numerous studies, it is important to note that presently the Cochrane Review is unable to make a recommendation regarding weight loss in NAFLD due to the lack of quality data at this time, highlighting the need for further research in the area[146].
Alcohol use is another life style modification that has been investigated. While the deleterious effects of heavy alcohol use on the liver are well established, the moderate use of alcohol has been investigated in relation to NAFLD. A 2014 retrospective study demonstrated patients who were moderate alcohol users had a decrease in severity of disease histologically[147]. Other studies have showed modest wine drinking[148] was correlated with a decreased prevalence of NAFLD. Additionally comparison between nondrinkers and moderate drinkers with NAFLD demonstrated lower rates of steatohepatitis in the moderate drinkers[149]. While some benefit may exist for moderate alcohol consumption, based on the data no recommendation can be made regarding alcohol consumption, other than heavy drinking should be avoided.
Dietary supplementation
Antioxidant supplementation has been widely hypothesized to have benefits in patients with NASH, as oxidative stress is a central component to liver injury and damage. Vitamin E is perhaps the most well researched of the proposed antioxidants. Following up on early work done as proof of concept in animal models, several studies have demonstrated consistent decreases in ALT and improvement in liver histology[150,151]. The most recent major randomized control trial (RCT) in 2010, the PIVENS trial, showed a decrease in inflammation and ALT, however did not show a decrease in liver fibrosis scores[152], sufficient evidence, therefore, exists for nondiabetic patients to receive 800 IU daily of Vitamin E as an early treatment for NASH. It is important to note that studies have suggested that Vitamin E supplementation has been linked to an increase in all-cause mortality[153] (although conflicting data has since been published)[154], as well as prostate cancer[155]. While these risks are low, it is important for clinicians consider these risks and discuss them with their patients before recommending Vitamin E.
Caffeine and coffee use has also been linked to decreased fibrosis, decreased progression to steatohepatitis as well as decreased incidence of the disease amongst its users[156-159]. On the other hand, a recent study demonstrated the protective effects of coffee in women with NAFLD, however the same effect was not observed in patients who used espresso[160]. While this may suggest that coffee itself may have protective effect vs caffeine alone, further studies have found an increased activity of the autophagy-lysosomal pathways in mice fed caffeine, inferring a biochemical explanation for the protective role of caffeine[161].
Several other supplements have also been suggested as treatment options for NAFLD, none currently embrace enough evidence to be recommended for widespread therapeutic use at this time. In a recent meta-analysis probiotics have been shown to lower aminotransferases, total cholesterol, TNFα and improve insulin resistance in NAFLD[162]. Given the relatively benign nature of probiotics and their availability over the counter, clinicians should be aware of their potential benefit in NAFLD. Ursodeoxycholic acid has been demonstrated in numerous small studies to have a benefit on liver enzymes and other measurable outcomes, however larger studies did not demonstrate significant histologic improvement[163-165]. Omega-3 fatty acids have also shown promise for treating NASH, several studies have shown measurable decreases in ALT and hepatic fat content in patients[166-168]. However despite these trials, the efficacious dose has not been established, histopathologic data is sparse and high quality randomized controlled trials have not been done, although one is in process[169]. High dose niacin therapy has also been shown to prevent steatohepatitis and liver deposition in rats, showing promise as a potential future therapeutic treatment modality with further research[170].
Metformin
Due to the link between insulin resistance and NAFLD, several studies have investigated the use of oral insulin sensitizing agents for the treatment of the disease. One of the most studied insulin sensitizing drugs is metformin. While several studies have demonstrated a decrease in ALT and increases in insulin sensitivity in patients with fatty liver[171-173], few have established histologic improvement[174]. Another study established that metformin is less effective at decreasing liver enzymes and hepatic content than exercise alone[175]; furthermore in a 2010 study, metformin was observed to have only a weak improvement over diet alone[176]. In a study looking specifically at patients with insulin resistances, without diabetes, metformin was found to have little to no histopathologic improvement when compared to controls, however it should be noted that this study included small number of patients and had a significant dropout rate[177]. Another study comparing metformin, vitamin E and diet similarly showed no histologic difference, despite ALT improvement[178]. While there is one small study showing histologic improvement in NASH patients following a 48 wk treatment period[179], the inconclusive and conflicting evidence lead to the conclusion that further investigations are necessary before recommending the use of metformin for NASH without coexisting diabetes.
TZDs
Another insulin sensitizing agent widely investigated for the treatment of NASH are the TZDs, specifically pioglitazone and rosiglitazone. As described earlier, the TZDs are activators of PPARγ, which has been observed to be down regulated in models of NAFLD. One of the earliest RCTs on rosiglitazone, the FLIRT trial done in 2008, demonstrated a 31% improvement in steatosis and transaminase levels respectively when compared to placebo[180]; a follow up FLIRT 2 trial in 2010, solidified these results[181]. Another study demonstrated a significant decrease in transaminase levels and histopathologic damage in nondiabetic patients treated with pioglitazone vs placebo[182]. Follow up studies showed that pioglitazone, when compared to metformin, decreased hepatic steatosis in diabetics with NASH[183], and rosiglitazone alone performed similarly when compared with the addition of metformin or losartan[184].
A meta-analysis demonstrated similar improvements in histopathologic outcomes in patients with NASH, and showed statistical significance to pioglitazone improving fibrosis in NASH[185]. It is important to note that all studies noted the side effect of weight gain in patients taking TZDs. There have been numerous studies investigating TZDs and their risk of significant cardiovascular events. A recent meta-analysis showed rosiglitazone had significantly higher odds of congestive heart failure (CHF), myocardial infarction, and death when compared to pioglitazone[186]. Pioglitazone itself has also been shown to have a 0.5% higher rate of CHF when compared to control groups in another study[187]. For this reason rosiglitazone is largely unavailable in most markets. Further research is needed to assess the long-term risks and benefits of pioglitazone in diabetic patients before further recommendations can be made, as most of the data to date is in the nondiabetic population.
Fibrates and other PPAR agonists
The fibrates are agonists of PPARα and are used clinically for the management of dyslipidemias. They have also been shown to decrease hepatic triacylglycerol content in rats, via the upregulation of triglyceride lipase[188]. A small (27 patient) study in 2010, demonstrated a minimal and statistically insignificant decrease in intrahepatic triglyceride content in obese patients taking fenofibrate when compared to placebo[189]. Another small study demonstrated a significant decrease in ALT in patients taking fenofibrate; additionally histologic improvement was observed in the amount of hepatocellular ballooning degeneration but not in the amount of steatosis or fibrosis[190]. At this time, fibrates need further investigation with RCTs, however the lack of data on histopathologic improvement is a cause for pause at the moment.
New data published in 2013 regarding a recently developed dual PPARα/δ agonist (GFT505), has been shown to decease hepatic and peripheral insulin sensitivity in obese patients[191]. Data from this study also saw a 20.5% decrease in ALT levels over the study period. Another 2013 study has demonstrated GFT505’s efficacy in improving steatosis, lowering liver enzymes and decreasing inflammation in rodent models of NAFLD and NASH[192]. While human data on GFT505’s effects on NAFLD does not currently exist, early results are promising.
Statins
The cardiovascular manifestations of liver diseases, including NAFLD are commonly seen and contribute significantly to the morbidity and mortality of liver disease patients[193-196]. Statins are a commonly prescribed preventative treatment for patients with cardiovascular risk factors and disease. The use of statins in patients with liver disease including NAFLD has been established as safe and can be used in the treatment of cardiovascular disease in these patients[83,197,198]. The use of statins as a therapy targeted specifically at NASH has shown promise in recent studies. The results have been mixed, with one early study showing no improvement in serologic markers[199], and other studies showing benefit[200-202].
The prospective GREACE study in 2010 included a large patient population (1600) with abnormal liver enzymes and coronary artery disease, and compared several outcomes between groups treated with statins[203]. Significant reductions in liver enzymes were found in the statin group, as well as a 68% relative risk reduction in the chance of having an adverse cardiovascular event. Data in the St Francis Heart Study showed a decrease in hepatic steatosis (visualized via CT) in 455 patients receiving combination therapy of atorvastatin and vitamins C and E when compared to placebo[204]. Despite this data, the use of combination therapy in the St Francis Heart Study makes it difficult to determine the significance of the statin on the results. Additionally, histopathologic data on the contribution of statins to the treatment of NASH alone is scarce. The use of statins specifically for NASH is cannot be recommended at this time and requires further research. Patients who need statins due to coexisting cardiac risk factors, should not, however, have a statin withheld from their treatment due to NASH[205].
Surgery
Weight loss has been established as a viable therapy for patients with NAFLD[140,146]. It is therefore reasonable to infer the benefit for bariatric surgery for patients with NASH. Studies have demonstrated that the presence of NASH does not increase complications of bariatric surgery[206] and have observed a significant decrease in steatohepatitis in patients following bariatric surgery[207,208]. Two recent meta-analyses have likewise concluded that by nearly all measurable outcomes, NASH improves following bariatric surgery[209,210]. Despite this data, there remains a lack of controlled evidence as well as long term data to indicate bariatric surgery exclusively for NASH[211]. Other, less invasive options for bariatric therapy, such as intragastric balloons, have shown benefits in improving liver function, insulin resistance, and histopathologic measures in obese patients with and without NASH[212-214].
Patients with NAFLD can progress to end-stage liver disease, with the only remaining option is liver transplantation. Transplants for cirrhosis due to NAFLD have had results comparable to patients without NAFLD[215]. NAFLD can recur after transplant or develop de novo. Steroid therapy, obesity, hyperlipidemia and diabetes are the best predictors for the development of NAFLD post-transplant. Unfortunately weight gain is a known side effect of immunosuppressant agents. The use of Vitamin E and insulin sensitizers has not been studied in post-transplant patients, and therefore diet and exercise remain the only recommendations at the time being.
Miscellaneous potential treatment modalities
Several novel therapies for NAFLD have emerged over the past several years given the increasing prevalence of the disease. While none are ready for use for widespread use, they represent potential future therapeutic options for a growing population of those suffering from NAFLD. A mixture of high molecular weight beeswax alcohols, D-002, has been shown to improve ultrasonographic findings, insulin resistance and well as symptoms in patients with NAFLD[216]. In a phase 2 trial (NCT00501592) obeticholic acid, a farnesoid X receptor agonist, has been shown to increase insulin sensitivity and reduce markers of liver inflammation and fibrosis in patients with diabetes and NAFLD[217]. The FLINT trial of obeticholic acid was ended early, due to earlier than expected improvements in the treatment group (NCT01265498).
The use of pentoxifylline as anti-TNF therapy in NAFLD has also been investigated. Early small studies have demonstrated a benefit in aminotransferases and histopathologically[218]. The use of therapeutic phlebotomy is also in phase 2 clinical trials (NCT00641524) and has shown moderate improvement in liver histology in NAFLD[219]. In another study, a plant supplement Chlorella vulgaris was seen to decrease levels of transaminases and increase insulin sensitivity[220]. While all of these therapies are still in their infancy, with future investigations they may become another tool used in the management of fatty liver.
SPECIAL CONSIDERATIONS FOR CHILDREN AND ADOLESCENTS
With increasing rates of diabetes and obesity among children and adolescents in the United States and worldwide, NAFLD has also been seen more frequently in younger age groups. Children as young as 2 years old have been reported to have NAFLD and associated steatohepatitis has been seen at age 8[221]. In a study of 742 children undergoing autopsy, fatty liver was found in 13% of the subjects[221]. For this reason it is important for clinicians to keep fatty liver in mind when working up a patient with incidental findings of elevated transaminases.
There are several special considerations to consider when diagnosing and treating children with suspected fatty liver disease. First, when diagnosing steatosis and NASH in very young children, it is especially important to rule out inheritable metabolic disorders such as hemochromatosis and lysosomal storage disease. It is important to keep in mind that children can have significant histologic abnormalities despite normal to moderately elevated ALT levels[222]. Additionally, when considering the use of imaging, it is important to consider the added lifetime risk of radiation, as well as the tendency for children to have phobias regarding the confined enclosure used in CT and magnetic resonance imaging. If the diagnosis of NAFLD is in doubt, the use of liver biopsy is still indicated. Consideration should be made for the potential risk of complications, one studies have shown a 2.4%-4.5% risk of major complications in children undergoing percutaneous liver biopsy[223,224]. However most of these complications were seen in patients with other comorbidities. The histopathologic appearance of NAFLD in children can also differ from adults. Macrovesicular hepatocellular steatosis with portal inflammation and fibrosis is often seen, with the absence of ballooning degeneration[225].
Treatments for NASH in children do not differ significantly from those recommended for adults. Data has shown lifestyle modification is the mainstay of treatment[22]. The largest study of pharmacotherapy for NASH in children to date was the TONIC trial in 2011[226]. It demonstrated no difference in children between metformin or vitamin E and placebo, however the primary outcome of the study was reduction in ALT. When stratified for reduction in steatohepatitis in children with NASH, a 58% improvement was seen in the vitamin E treatment group (P = 0.006). The TZDs have not been investigated in children sufficiently for recommendation.
CONCLUSION
Finally, NAFLD is a growing problem worldwide and with the increasing prevalence and incidence of obesity NAFLD could soon become an epidemic. Despite the positive strides made over the last several years, much remains to be understood regarding the mechanism of the disease. Our incomplete understanding of the pathogenesis is a major hurdle in the path towards developing methods for preventing and treating the disease. Additionally, the lack of awareness of the disorder and absence of reliable noninvasive diagnostic tools, has likely left many patients undiagnosed. For these reasons, there is a pressing need for further research into developing additional diagnostic tools and therapeutic modalities. Until then, education regarding lifestyle modifications for at risk populations is imperative. In NAFLD, as is the case in many diseases, an ounce of prevention is worth a pound of cure.
Footnotes
P- Reviewer: Enjoji M, Miura K, Scheimann AO S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530.e1; quiz e60. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. [PubMed] [Google Scholar]
- 3.Suzuki A, Angulo P, Lymp J, St Sauver J, Muto A, Okada T, Lindor K. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41:64–71. doi: 10.1002/hep.20543. [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Whalley S, Puvanachandra P, Desai A, Kennedy H. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med. 2007;7:119–124. doi: 10.7861/clinmedicine.7-2-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378–390. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 8.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Gastroenterology Organisation Global Guidelines. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis 2012. Available from: http://www.worldgastroenterology.org/assets/export/userfiles/2012_NASH and NAFLD_Final_long.pdf. [DOI] [PubMed]
- 10.Neuschwander-Tetri BA. Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci. 2005;330:326–335. doi: 10.1097/00000441-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 15.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, Hobbs HH. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA. 2010;107:7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 18.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Hooper AJ, Adams LA, Burnett JR. Genetic determinants of hepatic steatosis in man. J Lipid Res. 2011;52:593–617. doi: 10.1194/jlr.R008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandsma RH, Prinsen BH, van Der Velden Mde S, Rake JP, Boer T, Smit GP, Reijngoud DJ, Kuipers F. Increased de novo lipogenesis and delayed conversion of large VLDL into intermediate density lipoprotein particles contribute to hyperlipidemia in glycogen storage disease type 1a. Pediatr Res. 2008;63:702–707. doi: 10.1203/PDR.0b013e31816c9013. [DOI] [PubMed] [Google Scholar]
- 21.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hesham A-Kader H. Nonalcoholic fatty liver disease in children living in the obeseogenic society. World J Pediatr. 2009;5:245–254. doi: 10.1007/s12519-009-0048-8. [DOI] [PubMed] [Google Scholar]
- 23.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 24.Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, Bass N. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904–912. doi: 10.1002/hep.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zain SM, Mohamed R, Mahadeva S, Cheah PL, Rampal S, Basu RC, Mohamed Z. A multi-ethnic study of a PNPLA3 gene variant and its association with disease severity in non-alcoholic fatty liver disease. Hum Genet. 2012;131:1145–1152. doi: 10.1007/s00439-012-1141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubuquoy C, Robichon C, Lasnier F, Langlois C, Dugail I, Foufelle F, Girard J, Burnol AF, Postic C, Moldes M. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J Hepatol. 2011;55:145–153. doi: 10.1016/j.jhep.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Chamoun Z, Vacca F, Parton RG, Gruenberg J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol Cell. 2013;105:219–233. doi: 10.1111/boc.201200036. [DOI] [PubMed] [Google Scholar]
- 30.Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB; Genetics of Obesity-Related Liver Disease (GOLD) Consortium, Nguyen T, Kamel IR, Bonekamp S, Eberhardt MS, Clark JM, Kao WH, Speliotes EK. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2013;11:1183–1190.e2. doi: 10.1016/j.cgh.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogue A, Renaud MP, Claude N, Guillouzo A, Spire C. Comparative gene expression profiles induced by PPARγ and PPARα/γ agonists in rat hepatocytes. Toxicol Appl Pharmacol. 2011;254:18–31. doi: 10.1016/j.taap.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Costet P, Legendre C, Moré J, Edgar A, Galtier P, Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 33.Domenici FA, Brochado MJ, Martinelli Ade L, Zucoloto S, da Cunha SF, Vannucchi H. Peroxisome proliferator-activated receptors alpha and gamma2 polymorphisms in nonalcoholic fatty liver disease: a study in Brazilian patients. Gene. 2013;529:326–331. doi: 10.1016/j.gene.2013.06.091. [DOI] [PubMed] [Google Scholar]
- 34.Dongiovanni P, Valenti L. Peroxisome proliferator-activated receptor genetic polymorphisms and nonalcoholic Fatty liver disease: any role in disease susceptibility? PPAR Res. 2013;2013:452061. doi: 10.1155/2013/452061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao L, Marcus-Samuels B, Mason MM, Moitra J, Vinson C, Arioglu E, Gavrilova O, Reitman ML. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest. 2000;106:1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Guo X, Wu P, Song J, Ye C, Yu S, Zhang J, Dong W. Association between the Pro12Ala polymorphism of PPAR-γ gene and the non-alcoholic fatty liver disease: a meta-analysis. Gene. 2013;528:328–334. doi: 10.1016/j.gene.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. CDC’s Diabetes Program - Data & Trends - Prevalence of Diabetes - Number (in Millions) of Civilian, Noninstitutionalized Persons with Diagnosed Diabetes, United States, 1980-2011. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm.
- 38.Fryar C, Carroll M, Ogden C. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960-1962. Through 2009-2010. Available from: http://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.htm.
- 39.Takamura T, Misu H, Ota T, Kaneko S. Fatty liver as a consequence and cause of insulin resistance: lessons from type 2 diabetic liver. Endocr J. 2012;59:745–763. doi: 10.1507/endocrj.ej12-0228. [DOI] [PubMed] [Google Scholar]
- 40.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50 Suppl:S74–S79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Greenberg AS, Kraemer FB, Soni KG, Jedrychowski MP, Yan QW, Graham CE, Bowman TA, Mansur A. Lipid droplet meets a mitochondrial protein to regulate adipocyte lipolysis. EMBO J. 2011;30:4337–4339. doi: 10.1038/emboj.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima-Cabello E, García-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillón J, Lozano-Rodríguez T, Fernández-Bermejo M, Olcoz JL, González-Gallego J, García-Monzón C, Sánchez-Campos S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond) 2011;120:239–250. doi: 10.1042/CS20100387. [DOI] [PubMed] [Google Scholar]
- 50.Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c) Exp Biol Med (Maywood) 2007;232:614–621. [PubMed] [Google Scholar]
- 51.Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 52.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 53.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 55.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 56.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 57.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 58.Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis. 2010;30:391–401. doi: 10.1055/s-0030-1267539. [DOI] [PubMed] [Google Scholar]
- 59.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 60.Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43:474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 61.Pitsavos C, Panagiotakos D, Weinem M, Stefanadis C. Diet, exercise and the metabolic syndrome. Rev Diabet Stud. 2006;3:118–126. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721–728. doi: 10.3949/ccjm.75.10.721. [DOI] [PubMed] [Google Scholar]
- 63.Cortez-Pinto H, Jesus L, Barros H, Lopes C, Moura MC, Camilo ME. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr. 2006;25:816–823. doi: 10.1016/j.clnu.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 64.Westerbacka J, Lammi K, Häkkinen AM, Rissanen A, Salminen I, Aro A, Yki-Järvinen H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804–2809. doi: 10.1210/jc.2004-1983. [DOI] [PubMed] [Google Scholar]
- 65.Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46–52. doi: 10.1016/j.nut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004;49:1578–1583. doi: 10.1023/b:ddas.0000043367.69470.b7. [DOI] [PubMed] [Google Scholar]
- 67.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol. 2009;51:918–924. doi: 10.1016/j.jhep.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 70.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 71.Krishna Mohan I, Das UN. Prevention of chemically induced diabetes mellitus in experimental animals by polyunsaturated fatty acids. Nutrition. 2001;17:126–151. doi: 10.1016/s0899-9007(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 72.Nobili V, Giorgio V, Liccardo D, Bedogni G, Morino G, Alisi A, Cianfarani S. Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur J Endocrinol. 2014;170:547–553. doi: 10.1530/EJE-13-0609. [DOI] [PubMed] [Google Scholar]
- 73.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 75.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 76.Musso G, Olivetti C, Cassader M, Gambino R. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis. 2012;32:49–64. doi: 10.1055/s-0032-1306426. [DOI] [PubMed] [Google Scholar]
- 77.Nobili V, Cutrera R, Liccardo D, Pavone M, Devito R, Giorgio V, Verrillo E, Baviera G, Musso G. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189:66–76. doi: 10.1164/rccm.201307-1339OC. [DOI] [PubMed] [Google Scholar]
- 78.Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration. 2009;78:141–146. doi: 10.1159/000170785. [DOI] [PubMed] [Google Scholar]
- 79.Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–2020. doi: 10.2337/db06-0097. [DOI] [PubMed] [Google Scholar]
- 80.Rushmore TH, Ghazarian DM, Subrahmanyan V, Farber E, Ghoshal AK. Probable free radical effects on rat liver nuclei during early hepatocarcinogenesis with a choline-devoid low methionine diet. Cancer Res. 1987;47:6731–6740. [PubMed] [Google Scholar]
- 81.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28:159–165. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007–1019. doi: 10.1016/j.jhep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 85.Wilkins T, Tadkod A, Hepburn I, Schade RR. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician. 2013;88:35–42. [PubMed] [Google Scholar]
- 86.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272–282. doi: 10.1016/j.dld.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 88.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 89.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 90.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev. 2009;10:412–419. doi: 10.1111/j.1467-789X.2009.00594.x. [DOI] [PubMed] [Google Scholar]
- 92.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 93.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–2961. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 94.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 95.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lassailly G, Caiazzo R, Hollebecque A, Buob D, Leteurtre E, Arnalsteen L, Louvet A, Pigeyre M, Raverdy V, Verkindt H, et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol. 2011;23:499–506. doi: 10.1097/MEG.0b013e3283464111. [DOI] [PubMed] [Google Scholar]
- 97.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 98.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 99.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 100.Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M, Amor IB, Staccini-Myx A, Saint-Paul MC, Berthier F, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther. 2010;32:1315–1322. doi: 10.1111/j.1365-2036.2010.04480.x. [DOI] [PubMed] [Google Scholar]
- 101.Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, Chan HL, Wong VW. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 103.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 104.Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 105.Copaci I, Micu L, Voiculescu M. The role of cytokines in non-alcoholic steatohepatitis. A review. J Gastrointestin Liver Dis. 2006;15:363–373. [PubMed] [Google Scholar]
- 106.Wong VW, Hui AY, Tsang SW, Chan JL, Tse AM, Chan KF, So WY, Cheng AY, Ng WF, Wong GL, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:1154–1161. doi: 10.1016/j.cgh.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 107.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 108.Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, Tsutsumi M, Takase S. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol. 2007;102:1931–1938. doi: 10.1111/j.1572-0241.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 109.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirovski G, Dorn C, Huber H, Moleda L, Niessen C, Wobser H, Schacherer D, Buechler C, Wiest R, Hellerbrand C. Elevated systemic monocyte chemoattractrant protein-1 in hepatic steatosis without significant hepatic inflammation. Exp Mol Pathol. 2011;91:780–783. doi: 10.1016/j.yexmp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 111.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 112.Hui JM, Farrell GC, Kench JG, George J. High sensitivity C-reactive protein values do not reliably predict the severity of histological changes in NAFLD. Hepatology. 2004;39:1458–1459. doi: 10.1002/hep.20223. [DOI] [PubMed] [Google Scholar]
- 113.Yoneda M, Uchiyama T, Kato S, Endo H, Fujita K, Yoneda K, Mawatari H, Iida H, Takahashi H, Kirikoshi H, et al. Plasma Pentraxin3 is a novel marker for nonalcoholic steatohepatitis (NASH) BMC Gastroenterol. 2008;8:53. doi: 10.1186/1471-230X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kayadibi H, Gültepe M, Yasar B, Ince AT, Ozcan O, Ipcioglu OM, Kurdas OO, Bolat B, Benek YZ, Guveli H, et al. Diagnostic value of serum prolidase enzyme activity to predict the liver histological lesions in non-alcoholic fatty liver disease: a surrogate marker to distinguish steatohepatitis from simple steatosis. Dig Dis Sci. 2009;54:1764–1771. doi: 10.1007/s10620-008-0535-0. [DOI] [PubMed] [Google Scholar]
- 115.Yilmaz Y, Ulukaya E, Gul OO, Arabul M, Gul CB, Atug O, Oral AY, Aker S, Dolar E. Decreased plasma levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with nonalcoholic fatty liver disease. Clin Biochem. 2009;42:802–807. doi: 10.1016/j.clinbiochem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 116.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 117.Bonnefont-Rousselot D, Ratziu V, Giral P, Charlotte F, Beucler I, Poynard T. Blood oxidative stress markers are unreliable markers of hepatic steatosis. Aliment Pharmacol Ther. 2006;23:91–98. doi: 10.1111/j.1365-2036.2006.02719.x. [DOI] [PubMed] [Google Scholar]
- 118.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 119.Janica J, Ustymowicz A, Lukasiewicz A, Panasiuk A, Niemcunowicz-Janica A, Turecka-Kulesza E, Lebkowska U. Comparison of contrast-enhanced ultrasonography with grey-scale ultrasonography and contrast-enhanced computed tomography in diagnosing focal fatty liver infiltrations and focal fatty sparing. Adv Med Sci. 2013;58:408–418. doi: 10.2478/ams-2013-0027. [DOI] [PubMed] [Google Scholar]
- 120.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 121.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 122.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 123.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 125.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 126.Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis. 2011;12:10–16. doi: 10.1111/j.1751-2980.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 127.Janiec DJ, Jacobson ER, Freeth A, Spaulding L, Blaszyk H. Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver biopsies. Obes Surg. 2005;15:497–501. doi: 10.1381/0960892053723268. [DOI] [PubMed] [Google Scholar]
- 128.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 129.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 130.Monsour HP, Frenette CT, Wyne K. Fatty liver: a link to cardiovascular disease--its natural history, pathogenesis, and treatment. Methodist Debakey Cardiovasc J. 2012;8:21–25. doi: 10.14797/mdcj-8-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 132.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 133.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–378. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 134.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48 Suppl 1:S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 136.Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 137.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 138.Hashimoto E, Yatsuji S, Kaneda H, Yoshioka Y, Taniai M, Tokushige K, Shiratori K. The characteristics and natural history of Japanese patients with nonalcoholic fatty liver disease. Hepatol Res. 2005;33:72–76. doi: 10.1016/j.hepres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, Nitzan Kaluski D, Halpern Z, Oren R. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145–1151. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 140.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 142.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 143.Bhat G, Baba CS, Pandey A, Kumari N, Choudhuri G. Life style modification improves insulin resistance and liver histology in patients with non-alcoholic fatty liver disease. World J Hepatol. 2012;4:209–217. doi: 10.4254/wjh.v4.i7.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, Fragopoulou E, Zafiropoulou R, Manios Y, Papatheodoridis G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. 2014;33:678–683. doi: 10.1016/j.clnu.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 145.Caporaso N, Morisco F, Camera S, Graziani G, Donnarumma L, Ritieni A. Dietary approach in the prevention and treatment of NAFLD. Front Biosci (Landmark Ed) 2012;17:2259–2268. doi: 10.2741/4049. [DOI] [PubMed] [Google Scholar]
- 146.Peng L, Wang J, Li F. Weight reduction for non-alcoholic fatty liver disease. Cochrane Database Syst Rev. 2011;(6):CD003619. doi: 10.1002/14651858.CD003619.pub3. [DOI] [PubMed] [Google Scholar]
- 147.Kwon HK, Greenson JK, Conjeevaram HS. Effect of lifetime alcohol consumption on the histological severity of non-alcoholic fatty liver disease. Liver Int. 2014;34:129–135. doi: 10.1111/liv.12230. [DOI] [PubMed] [Google Scholar]
- 148.Dunn W, Xu R, Schwimmer JB. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology. 2008;47:1947–1954. doi: 10.1002/hep.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dunn W, Sanyal AJ, Brunt EM, Unalp-Arida A, Donohue M, McCullough AJ, Schwimmer JB. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57:384–391. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bell LN, Wang J, Muralidharan S, Chalasani S, Fullenkamp AM, Wilson LA, Sanyal AJ, Kowdley KV, Neuschwander-Tetri BA, Brunt EM, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56:1311–1318. doi: 10.1002/hep.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 154.Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen S, Teoh NC, Chitturi S, Farrell GC. Coffee and non-alcoholic fatty liver disease: brewing evidence for hepatoprotection? J Gastroenterol Hepatol. 2014;29:435–441. doi: 10.1111/jgh.12422. [DOI] [PubMed] [Google Scholar]
- 157.Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55:429–436. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 158.Birerdinc A, Stepanova M, Pawloski L, Younossi ZM. Caffeine is protective in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:76–82. doi: 10.1111/j.1365-2036.2011.04916.x. [DOI] [PubMed] [Google Scholar]
- 159.Catalano D, Martines GF, Tonzuso A, Pirri C, Trovato FM, Trovato GM. Protective role of coffee in non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2010;55:3200–3206. doi: 10.1007/s10620-010-1143-3. [DOI] [PubMed] [Google Scholar]
- 160.Anty R, Marjoux S, Iannelli A, Patouraux S, Schneck AS, Bonnafous S, Gire C, Amzolini A, Ben-Amor I, Saint-Paul MC, et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol. 2012;57:1090–1096. doi: 10.1016/j.jhep.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 161.Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Ilkayeva OR, Gooding J, Ching J, Zhou J, et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59:1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- 162.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu SD, Li L, Wang JY. Ursodeoxycholic acid for nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2012;24:1247–1253. doi: 10.1097/MEG.0b013e3283572ec0. [DOI] [PubMed] [Google Scholar]
- 164.Troisi G, Crisciotti F, Gianturco V, D’Ottavio E, Lo Iacono C, Formosa V, Bernardini S, Bellomo A, Marigliano B, Marigliano V. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203–207. doi: 10.7417/CT.2013.1550. [DOI] [PubMed] [Google Scholar]
- 165.Xiang Z, Chen YP, Ma KF, Ye YF, Zheng L, Yang YD, Li YM, Jin X. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: a systematic review. BMC Gastroenterol. 2013;13:140. doi: 10.1186/1471-230X-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 167.Rossmeisl M, Medrikova D, van Schothorst EM, Pavlisova J, Kuda O, Hensler M, Bardova K, Flachs P, Stankova B, Vecka M, et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim Biophys Acta. 2014;1841:267–278. doi: 10.1016/j.bbalip.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 168.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 169.Janczyk W, Socha P, Lebensztejn D, Wierzbicka A, Mazur A, Neuhoff-Murawska J, Matusik P. Omega-3 fatty acids for treatment of non-alcoholic fatty liver disease: design and rationale of randomized controlled trial. BMC Pediatr. 2013;13:85. doi: 10.1186/1471-2431-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ganji SH, Kukes GD, Lambrecht N, Kashyap ML, Kamanna VS. Therapeutic role of niacin in the prevention and regression of hepatic steatosis in rat model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2014;306:G320–G327. doi: 10.1152/ajpgi.00181.2013. [DOI] [PubMed] [Google Scholar]
- 171.Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012;12:e6099. doi: 10.5812/hepatmon.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E. The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic Fatty liver disease: a randomized double blinded clinical trial. Hepat Mon. 2013;13:e9270. doi: 10.5812/hepatmon.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism. 2011;60:1278–1284. doi: 10.1016/j.metabol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 174.Shyangdan D, Clar C, Ghouri N, Henderson R, Gurung T, Preiss D, Sattar N, Fraser A, Waugh N. Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review. Health Technol Assess. 2011;15:1–110. doi: 10.3310/hta15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sánchez-Muñoz V, Salas-Romero R, Del Villar-Morales A, Martínez-Coria E, Pegueros-Pérez A, Franco-Sánchez JG. [Decrease of liver fat content by aerobic exercise or metformin therapy in overweight or obese women] Rev Invest Clin. 2013;65:307–317. [PubMed] [Google Scholar]
- 176.Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Ventura V, Greco M, Abenavoli L, Belfiore A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond) 2010;34:1255–1264. doi: 10.1038/ijo.2010.40. [DOI] [PubMed] [Google Scholar]
- 177.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 179.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 181.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 182.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 183.Gupta AK, Bray GA, Greenway FL, Martin CK, Johnson WD, Smith SR. Pioglitazone, but not metformin, reduces liver fat in Type-2 diabetes mellitus independent of weight changes. J Diabetes Complications. 2010;24:289–296. doi: 10.1016/j.jdiacomp.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54:1631–1639. doi: 10.1002/hep.24558. [DOI] [PubMed] [Google Scholar]
- 185.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. doi: 10.1136/bmj.d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 188.Karahashi M, Hoshina M, Yamazaki T, Sakamoto T, Mitsumoto A, Kawashima Y, Kudo N. Fibrates reduce triacylglycerol content by upregulating adipose triglyceride lipase in the liver of rats. J Pharmacol Sci. 2013;123:356–370. doi: 10.1254/jphs.13149fp. [DOI] [PubMed] [Google Scholar]
- 189.Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fernández-Miranda C, Pérez-Carreras M, Colina F, López-Alonso G, Vargas C, Solís-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 191.Cariou B, Hanf R, Lambert-Porcheron S, Zaïr Y, Sauvinet V, Noël B, Flet L, Vidal H, Staels B, Laville M. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 193.Gökçe S, Atbinici Z, Aycan Z, Cınar HG, Zorlu P. The relationship between pediatric nonalcoholic fatty liver disease and cardiovascular risk factors and increased risk of atherosclerosis in obese children. Pediatr Cardiol. 2013;34:308–315. doi: 10.1007/s00246-012-0447-9. [DOI] [PubMed] [Google Scholar]
- 194.Domanski JP, Park SJ, Harrison SA. Cardiovascular disease and nonalcoholic fatty liver disease: does histologic severity matter? J Clin Gastroenterol. 2012;46:427–430. doi: 10.1097/MCG.0b013e31822fb3f7. [DOI] [PubMed] [Google Scholar]
- 195.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 196.Perazzo H, Poynard T, Dufour JF. The interactions of nonalcoholic fatty liver disease and cardiovascular diseases. Clin Liver Dis. 2014;18:233–248. doi: 10.1016/j.cld.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 197.Nseir W, Mahamid M. Statins in nonalcoholic fatty liver disease and steatohepatitis: updated review. Curr Atheroscler Rep. 2013;15:305. doi: 10.1007/s11883-012-0305-5. [DOI] [PubMed] [Google Scholar]
- 198.Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, Gwon HC, Bae JH, Hong BK, Choi DH, et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage) J Clin Lipidol. 2012;6:340–351. doi: 10.1016/j.jacl.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 199.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990–994. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 200.Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184:233–234. doi: 10.1016/j.atherosclerosis.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 201.Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135–141. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 202.Gómez-Domínguez E, Gisbert JP, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643–1647. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 203.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 204.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 205.Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623. doi: 10.1002/14651858.CD008623.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Weingarten TN, Swain JM, Kendrick ML, Charlton MR, Schroeder BJ, Lee RE, Narr BJ, Ribeiro TC, Schroeder DR, Sprung J. Nonalcoholic steatohepatitis (NASH) does not increase complications after laparoscopic bariatric surgery. Obes Surg. 2011;21:1714–1720. doi: 10.1007/s11695-011-0521-z. [DOI] [PubMed] [Google Scholar]
- 207.Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 208.Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22:1044–1049. doi: 10.1007/s11695-011-0559-y. [DOI] [PubMed] [Google Scholar]
- 209.De Ridder RJ, Schoon EJ, Smulders JF, van Hout GC, Stockbrügger RW, Koek GH. Review article: Non-alcoholic fatty liver disease in morbidly obese patients and the effect of bariatric surgery. Aliment Pharmacol Ther. 2007;26 Suppl 2:195–201. doi: 10.1111/j.1365-2036.2007.03483.x. [DOI] [PubMed] [Google Scholar]
- 210.Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 211.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;2010:CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, Wee A, Lim SG, Ho KY. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76:756–760. doi: 10.1016/j.gie.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 213.Forlano R, Ippolito AM, Iacobellis A, Merla A, Valvano MR, Niro G, Annese V, Andriulli A. Effect of the BioEnterics intragastric balloon on weight, insulin resistance, and liver steatosis in obese patients. Gastrointest Endosc. 2010;71:927–933. doi: 10.1016/j.gie.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 214.Ricci G, Bersani G, Rossi A, Pigò F, De Fabritiis G, Alvisi V. Bariatric therapy with intragastric balloon improves liver dysfunction and insulin resistance in obese patients. Obes Surg. 2008;18:1438–1442. doi: 10.1007/s11695-008-9487-x. [DOI] [PubMed] [Google Scholar]
- 215.Said A. Non-alcoholic fatty liver disease and liver transplantation: outcomes and advances. World J Gastroenterol. 2013;19:9146–9155. doi: 10.3748/wjg.v19.i48.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Illnait J, Rodríguez I, Mendoza S, Fernández Y, Mas R, Miranda M, Piñera J, Fernández JC, Mesa M, Fernández L, et al. Effects of D-002, a mixture of high molecular weight beeswax alcohols, on patients with nonalcoholic fatty liver disease. Korean J Intern Med. 2013;28:439–448. doi: 10.3904/kjim.2013.28.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 218.Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49. doi: 10.1186/1476-511X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Beaton MD, Chakrabarti S, Levstik M, Speechley M, Marotta P, Adams P. Phase II clinical trial of phlebotomy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:720–729. doi: 10.1111/apt.12255. [DOI] [PubMed] [Google Scholar]
- 220.Panahi Y, Ghamarchehreh ME, Beiraghdar F, Zare R, Jalalian HR, Sahebkar A. Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Hepatogastroenterology. 2012;59:2099–2103. doi: 10.5754/hge10860. [DOI] [PubMed] [Google Scholar]
- 221.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 222.Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, Brunt EM, Scheimann AO, Unalp-Arida A. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. 2014;164:707–713.e3. doi: 10.1016/j.jpeds.2013.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cohen MB, A-Kader HH, Lambers D, Heubi JE. Complications of percutaneous liver biopsy in children. Gastroenterology. 1992;102:629–632. doi: 10.1016/0016-5085(92)90112-c. [DOI] [PubMed] [Google Scholar]
- 224.Scheimann AO, Barrios JM, Al-Tawil YS, Gray KM, Gilger MA. Percutaneous liver biopsy in children: impact of ultrasonography and spring-loaded biopsy needles. J Pediatr Gastroenterol Nutr. 2000;31:536–539. doi: 10.1097/00005176-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 225.Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, Xanthakos SA, Whitington PF, Charatcharoenwitthaya P, Yap J, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50:1113–1120. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shoulders CC, Jones EL, Naoumova RP. Genetics of familial combined hyperlipidemia and risk of coronary heart disease. Hum Mol Genet. 2004;13 Spec No 1:R149–R160. doi: 10.1093/hmg/ddh069. [DOI] [PubMed] [Google Scholar]
- 228.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 229.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 230.Grønning-Wang LM, Bindesbøll C, Nebb HI. The Role of Liver X Receptor in Hepatic de novo Lipogenesis and Cross-Talk with Insulin and Glucose Signaling. IN: Baez RV, editor. Lipid Metabolism. Published in print edition; 2013. Available from: http: //www.intechopen.com/books/lipid-metabolism/the-role-of-liver-x-receptor-in-hepatic-de-novo-lipogenesis-and-cross-talk-with-insulin-and-glucose- [Google Scholar]
- 231.Blasiole DA, Davis RA, Attie AD. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol Biosyst. 2007;3:608–619. doi: 10.1039/b700706j. [DOI] [PubMed] [Google Scholar]
- 232.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 234.Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Rüegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]


