Abstract
AIM: To investigate the effects of different immunosuppressive regimens and avoidance on fibrosis progression in hepatitis C virus (HCV) liver transplant (LT) recipients.
METHODS: We retrospectively compared the liver biopsies of well-matched HCV LT recipients under calcineurin inhibitors (CNI group, n = 21) and mycophenolate (MMF group, n = 15) monotherapy, with those patients who successfully withdrawn immunosuppression (IS) therapy from at least 3 years (TOL group, n = 10). To perform the well-matched analysis, all HCV transplanted patients from December 1993 were screened. Only those HCV patients who reached the following criteria were considered for the analysis: (1) at least 3 years of post-operative follow-up; (2) patients with normal liver graft function under low dose CNI monotherapy (CNI group); (3) patients with normal liver graft function under antimetabolite (Micophenolate Mofetil or coated mycophenolate sodium) monotherapy (MMF group); and (4) recipients with normal liver function without any IS. We excluded from the analysis recipients who were IS free or under monotherapy for < 36 mo, recipients with cirrhosis or with unstable liver function tests.
RESULTS: Thirty six recipients were enrolled in the study. Demographics, clinical data, time after LT and baseline liver biopsies were comparable in the three groups. After six years of follow-up, there was no worsening of hepatic fibrosis in the MMF group (2.5 ± 1.5 Ishak Units vs 2.9 ± 1.7 Ishak Units, P = 0.5) and TOL group (2.7 ± 10.7 vs 2.5 ± 1.2, P = 0.2). In contrast, a significant increase in the fibrosis score was observed in the CNI group (2.2 ± 1.7 vs 3.9 ± 1.6, P = 0.008). The yearly fibrosis progression rate was significantly worse in the CNI group (0.32 ± 0.35) vs MMF group (0.03 ± 0.31, P = 0.03), and TOL group (-0.02 ± 0.27, P = 0.02). No differences have been reported in grading scores for CNI group (2.79 ± 1.9, P = 0.7), MMF group (3.2 ± 1.5, P = 0.9) and TOL group (3.1 ± 1.4, P = 0.2). Twenty four patients were treated with low dose ribavirin (8 TOL, 7 MMF, 9 CNI). The hepatitis C titers were comparable in the three groups. No episodes of rejection have been reported despite differences of liver function test in the three groups during the observational period.
CONCLUSION: IS withdrawal and MMF monotherapy is safe and seems to be associated with the slowest fibrosis progression in HCV LT recipients.
Keywords: Liver transplantation, Hepatitis C virus recurrence, Immunosuppression withdrawal, Micofenolate mofetil, Clinical operational tolerance, Minimization of immunosuppression
Core tip: The ideal immunosuppression (IS) therapy in long term hepatitis C virus (HCV) transplant recipients is yet to be defined but over-immunosuppression should be avoided. The IS free status seems to show a favorable impact on the natural history of the disease but is only achievable in 20%-30% of liver transplant (LT) recipients. Therefore minimization of the therapy must be considered an alternative in those patients who require IS. The present study aims to compare the fibrosis progression in long-term IS-free HCV-LT recipients with those on low dose calcineurin inhibitors or on antimetabolite.
INTRODUCTION
Hepatitis C virus (HCV)-related cirrhosis is currently the most important indication for liver transplantation (LT). However, it is well known that HCV graft reinfection is universal in patients who were HCV-RNA positive at the time of transplant, and has an accelerated progression to fibrosis compared to non-transplanted patients. This leads to cirrhosis in about 30% of the recipients within 5 years of transplantation[1,2]. A worse outcome in HCV transplanted recipients in terms of patients and graft survival vs those who underwent LT for other chronic liver diseases has been well recognized. Several factors have been associated with the severity of HCV recurrence: (1) Donor-related factors: female > 65 years, prolonged cold ischemic time, macro-steatosis; (2) Recipient-related factors: > 50 years, HIV coinfection, insulin-dependent diabetes, ACE-inhibitors and azathioprine (AZA) treatment; (3) Transplant-related factors: HLA matching, IL28b genotype of the donor and recipient, >106 IU/mL HCV-viral load immediately after LT, CMV infection, treatment with OKT3 and corticosteroid boluses for acute rejection episode; and (4) Type of immunosuppression (IS)[3,4].
IS plays a key role in HCV graft reinfection, determining a fine balance between maintaining optimal host viral responses and suppressing immunity[5]. Several authors reviewed the effects of IS drugs on HCV recurrence course[6,7] but the optimal IS regimen for HCV patients has not been defined. Specific HCV LT recipients IS recommendations based on appropriate prospective clinical trials with histological findings, are still needed. In order to ensure the later and slower recurrence, the transplant community is in agreement that a high maintenance IS regimen and corticosteroid bolus to treat acute cellular rejection should be avoided[8,9].
Nowadays, due to better graft and patient survival, tacrolimus (Tac)-based regimen represents the preferred calcineurin inhibitors (CNI) despite its long-term side effects[10,11]. Mycophenolate (MMF) is commonly used as a CNI-sparing agent mainly in recipients with nephrotoxicity, but its effects on HCV recurrence are still controversial[12]. The IS regimen most commonly reported in literature for HCV recipients is based on CNI at low dose associated with a second IS agent (i.e., antimetabolites or mammalian target of rapamycin inhibitors) with or without steroid maintenance[13]. The IS free state seems to improve the natural history of the disease but up until now has been reported in only 20% of patients[14,15]. In this context, the minimization of IS may represent a valid alternative, which permits to reduce the severity of HCV graft infection and the IS long-term toxicity.
The aim of the present study was to evaluate the effect of long-term CNI and MMF monotherapy, compared to IS withdrawal on the fibrosis progression rate in LT recipients with recurrent HCV infection.
MATERIALS AND METHODS
Study design
We designed a retrospective, well-matched, three-arms study to evaluate the effects of different IS regimens and IS withdrawal on the long-term fibrosis progression rate in patients who underwent LT for HCV-related cirrhosis at our institution between December 1993 and February 2013.
Recipients were enrolled in three arms according to IS treatment: patients who completely weaned IS therapy after LT (TOL); patients who received CNI monotherapy at low dose (CNI group); patients who were treated with Antimetabolites monotherapy (MMF group).
Patients who were included in the study were: LT recipients with at least 3 years of post-operative follow-up with normal baseline liver graft function tests (i.e., aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and gamma glutamyltransferase); patients receiving an IS regimen CNI (CsA or Tac) monotherapy at low dose (defined as Co blood levels < 100 ng/mL and Tac blood levels < 5 ng/mL); MMF or coated mycophenolate sodium (ECM-PS) monotherapy or recipients who achieved IS-free state (IFS) (i.e., patients who reached a state of operational tolerance[14] for at least 48 mo without liver transplant rejection) (TOL). We excluded those recipients who were under two or more IS drugs after the first post-transplant period, or that showed basal liver function tests and/or liver biopsy-proven rejection or another transplant disorder, or the presence of significant medical complications at enrolment.
The baseline characteristics of the three groups are reported in Table 1. The study was approved by the Institutional Ethics Committee.
Table 1.
Baseline characteristics of the three groups n (%)
| Variable | Tol group | CNI group | MMF group | P-value |
| Patients (n) | 10 | 21 | 15 | NS |
| Age (yr) al TX | 52.4 ± 5.5 | 54.7 ± 10.3 | 50.4 ± 5.7 | NS |
| Gender (M/F) | 8/2 | 17/4 | 11/4 | NS |
| BMI | 26 ± 3.9 | 27 ± 3.4 | 27 ± 2.9 | NS |
| Donor age (yr) | 35 ± 13 | 44 ± 17.9 | 33 ± 18 | NS |
| Follow-up from LT (mean) | 83 ± 30.5 | 83 ± 29.1 | 89 ± 40 | NS |
| HCV genotype 1-4 | 2 (20) | 6 (28.5) | 6 (40) | NS |
| HCV genotype 2-3 | 1 (10) | 3 (14.2) | 2 (13.3) | NS |
| HCV-RNA > 500000 IU/mL | 70 | 42.8 | 46.6 | NS |
| Anti-HBc positive recipients | None | 4 (19) | 1 (6.6) | NS |
| Cholestatic hepatitis | None | None | None | NS |
| HIV positive | None | None | None | NS |
| Azathioprine treated | 1 (10) | 7 (33.3) | 9 (60) | NS |
| Mean ALT (IU/L) | 71.1 ± 54.2 | 53.9 ± 33.7 | 59.4 ± 45.6 | NS |
| ACE-inhibitors treated | 3 (30) | None | 1 (6.6) | NS |
| IDDM | 3 (30) | 7 (33.3) | 4 (26.6) | NS |
| Baseline Fibrosis score (Ishak) | 2.7 ± 0.7 | 2.2 ± 1.7 | 2.5 ± 1.5 | NS |
| Baseline grading score (Ishak) | 4.1 ± 1.8 | 3 ± 2.2 | 3.2 ± 1.6 | NS |
| Treated with ribavirin | 8 (80) | 9 (42.8) | 7 (46.6) | NS |
| Patients who achieved SVR | 2 | 8 | 5 | NS |
| Rejection episode | None | None | None | NS |
| Boluses of steorids after LT | None | None | None | NS |
| Antibodies after LT | None | None | None | NS |
| CMV infection after LT | 1 (10) | 2 (9.5) | 1 (6.7) | NS |
| Total ishemica times (min) | 502 ± 133.8 | 456 ± 86.5 | 411 ± 127.6 | NS |
| Liver donor's steatosis | None 0 (20) Mild 2 (10) Severe 0 (0) | None 17 (81) Mild 3 (14.3) Severe 1 (4.8) | None 14 (93.3) Mild 1 (6.7) Severe 0 (0) |
HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; CNI: Calcineurin inhibitors; MMF: Mycophenolate; BMI: Body mass index; CMV: Cytomegalovirus; LT: Liver transplantation; SVR: Sustained virologic response; IDDM: Insulin dependent diabetes mellitus; NS: Not significant.
Liver histology
All liver biopsies obtained during the period of observation were considered. Liver biopsies were obtained yearly during the follow-up period and additional biopsies were eventually taken whenever an acute rejection was suspected. For the TOL group, protocol biopsies were also performed at 6, 12, 36, 48 mo after IS withdrawal. Histological examinations were carried out by experienced pathologists blinded to the patients’ clinical status and the assigned therapy, except for the knowledge of a previous transplantation due to HCV-related end-stage liver disease. Biopsies were scored for the grade of necro-inflammatory activity and stage of fibrosis according to Ishak scale[16] and also examined to exclude features of acute or chronic rejection or other relevant findings. Acute rejection was defined according to standard criteria; chronic rejection was assessed according to Banff classification rejection activity index[17]. The yearly biopsy fibrosis progression rate was calculated as the difference between the staging score in the last liver biopsy and the baseline biopsy divided by the years of follow-up[18]. Liver specimens were obtained percutaneously using 1.6 mm modified Menghini needles. To minimize sampling errors, only specimens that were longer than 1.5 cm and wider than 1.4 mm were considered, including ≥ 8 portal tracts[19]. Specimens were formalin-fixed and paraffin-embedded for histological analysis. 5-ìm sections were stained for hematoxylin and eosin, as well as the Masson’s trichrome stain of collagen and cytokeratins for the assessment of ductopenia.
Virological assays
Levels of serum HCV-RNA were quantified using a competitive reverse transcription-polymerase chain reaction analysis (Amplicor, Roche Molecular System, Inc., Branchburg, NJ, United States). HCV genotypes were assessed using the Inno-Lipa HCV (Immunogenetics, Zwijnaarde, Belgium). HCV-RNA serum levels > 500 × 103 IU/L were considered as high viral replication. Recurrent hepatitis C was defined by the concomitance of detectable serum HCV-RNA and histological signs of recurrent disease[1].
Statistical analysis
Data were collected retrospectively from a prospective database (Microsoft Access 2.0, Microsoft Corporation, United States). Categorical variables were analysed using Fisher’s exact test (F test). The normal continuous data were analysed using parametric test (t test). Statistical results were expressed as mean ± SD. A P-value of < 0.05 was considered significant. The program used for statistical analysis was SPSS® 13.0 (233 South Wacker Drive, Chicago, United States) for Mac.
RESULTS
Study population
Out of 476 liver recipients, 184 (38.7%) patients received LT for HCV-related cirrhosis. Out of these 184 HCV patients, 36 (19.6%) were enrolled and divided in three groups: 10 recipients were in TOL group, 15 in MMF group, and 21 patients in CNI group. We considered the baseline as the start of IS withdrawal for the TOL group (83 ± 30.6 mo), the time of start antimetabolites monotherapy for the MMF group (89.1 ± 40.1 mo) (P = 0.79) and for the CNI, to match the group well, we took into account only those recipients who achieved the 6th year of follow-up from LT (mean follow up from LT: 83 ± 29.1) (Figure 1). All LT recipients included in the study received in the immediate post-operative period IS regimen based on CNI (CsA or Tac) ± azathioprine ± steroids. Azathioprine and steroids were withdrawal within the first 3 mo from LT. None received boluses of steroids or antibodies therapy during the entire follow-up, and no rejection episodes were recorded. After a mean follow-up of 6 years, all patients were alive, except for one patient in the CNI group.
Figure 1.
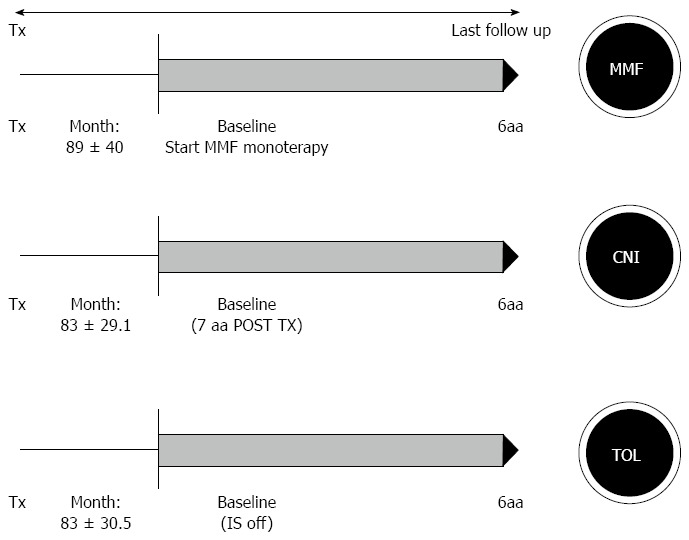
Study design. The three groups were represented by: Mycophenolate (MMF), calcineurin inhibitor (CNI) and TOL. All patients had a minimal follow up of 6 years from LT at the enrollment and were followed with yearly liver biopsy for 6 years.
Immunosuppression
Tolerant group: Of the 23 tolerant patients followed by our Institution, 10 were included in the present study (8M/2F, mean age at baseline 59 ± 5.6 years). HCV-related cirrhosis represented the indication to LT, except for one patient who presented hepatocellular carcinoma. Before treatment discontinuation, 8 recipients received CsA monotherapy at low doses, one Tac monotherapy (1.5 mg/d) and one was under MMF (1500 mg/d). Treatment was gradually withdrawn following the Tor Vergata criteria[14]; after a mean time from LT of 83 ± 30.5 mo. Tapering of treatment proceeded without any clinical and biochemical signs of rejection. Eight (80%) patients received low dose ribavirin (400 mg/d) before and during the study period. After a mean follow-up of 80 ± 15.1 mo from weaning, all recipients were alive; one patient had IS resumption after 153 mo from LT and 94 mo from IS withdrawal due to kidney transplantation.
MMF group: Fifteen adult HCV-LT recipients under Micophenolate monotherapy (11M/4F, mean age of 57.7 ± 6.12 years) achieved the inclusion criteria for the study. LT Indications were HCV-cirrhosis in 13 cases (86.7%), HCV cirrhosis associated with HCC in two cases (13.3%). After a mean follow-up of 89.1 ± 40.1 mo from LT, recipients were switched to MMF (n = 12) or ECM-PS (n = 3) from CNI monotherapy at daily doses of 1500 mg and 1440 mg respectively. Nine patients (60%) were switched to MMF for renal impairment CNI-related (defined as an estimated glomerular filtration rate < 60 mL/min per 1.73 m2) and 6 patients (40%) for hyperlipidaemia (serum cholesterol > 240 mg/dL and/or triglycerides > 150 mg/dL). Seven (46.6%) patients received low dose ribavirin (400 mg/d) before and during the study period. After 76.4 ± 36.8 mo of follow-up, all patients were alive and none experienced acute rejection. The mean daily doses were 1250 ± 398.9 mg of MMF or 1440 mg/d of ECM-PS.
CNI group: The CNI group included 21 adult HCV-LT recipients (17M/4F, mean age of 54.7 ± 10.3 years) with a mean follow-up of 83 ± 29.1 mo from LT. HCV-related cirrhosis represented the primary indication for LT for all patients; four (19%) patients had HBV co-infection also and one HCC (4.7%). According to the baseline follow-up of TOL and MMF groups, the baseline was considered as 6-years after LT. At the time of enrolment, 8 patients (38%) were on CsA at low dose (78.6 ± 41 mg/d) and 13 (61.9%) on Tac (1.96 ± 1.2 mg/d). After 5 years, all recipients were alive, except one patient who died after 126 mo from HCV recurrence. At the baseline the mean through levels of CsA was Co 86.9 ± 38.4 ng/mL and of Tac was 1.9 ± 1.1 ng/mL. Nine (42.8%) patients received low dose ribavirin (400 mg/d) before and during the study period.
Histological findings
At least six consecutive yearly biopsies were available for examination for each patient. At baseline there were no differences in the three groups, both in terms of staging (CNI group 2.2 ± 1.7; MMF group 2.5 ± 1.5; TOL group 2.7 ± 0.7) and grading (CNI group 3 ± 2.2; MMF group 3.2 ± 1.6; TOL group 4.1 ± 1.8). Comparing the baseline with 6 year biopsies, no worsening of hepatic fibrosis in MMF group (2.5 ± 1.5 vs 2.9 ± 1.7, P = 0.51) and TOL group (2.7 ± 0.7 vs 2.5 ± 1.2, P = 0.7) was reported. In contrast, a significant increase in the fibrosis score was observed in the CNI group (2.2 ± 1.7 vs 3.9 ± 1.6, P = 0.008). After six years of follow-up, no differences have been reported in grading score for CNI group (2.79 ± 1.9, P = 0.7), MMF group (3.2 ± 1.5, P = 0.9) and TOL group (3.1 ± 1.4, P = 0.2) (Table 2). The yearly fibrosis progression rate was significantly worse in CNI group than in MMF group (0.32 ± 0.35 vs 0.03 ± 0.31, respectively, P = 0.03) and TOL group (-0.02 ± 0.27, P = 0.02), but no difference of fibrosis progression rate was reported for TOL group compared to MMF group (-0.02 ± 0.27 vs 0.32 ± 0.35, respectively, P = 0.7) (Figure 2).
Table 2.
Three groups data at baseline and after 6 years of follow up
| Variables |
Tol group |
CNI group |
MMF group |
||||||
| Baseline | 6 yr | P-value | Baseline | 6 yr | P-value | Baseline | 6 yr | P-value | |
| Grading | 4.1 ± 1.8 | 3.1 ± 1.45 | NS | 3 ± 2.2 | 2.8 ± 1.9 | NS | 3.2 ± 1.6 | 3.25 ± 1.54 | NS |
| Staging | 2.7 ± 0.7 | 2.5 ± 1.2 | NS | 2.2 ± 1.7 | 3.9 ± 1.6 | 0.008 | 2.5 ± 1.5 | 2.9 ± 1.73 | NS |
CNI: Calcineurin inhibitors; MMF: Mycophenolate; NS: Not significant.
Figure 2.
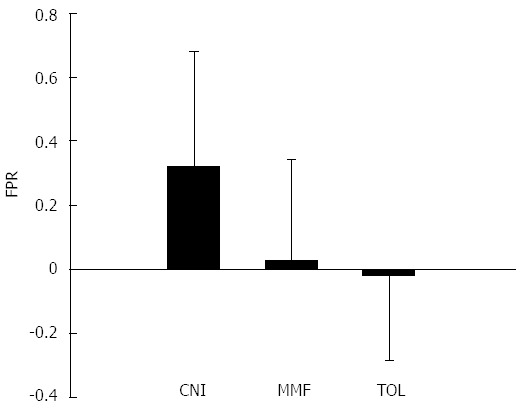
Mean fibrosis progression rate of the three groups in 6 years of follow up. FPR: Fibrosis progression rate; CNI: Calcineurin inhibitors; MMF: Mycophenolate.
Biochemical and virological findings
No significant LFT's differences or clinical evidence of rejection was observed in the three groups during the entire follow-up period. Also, after 6 years of follow-up, no difference in terms of HCV-RNA blood levels has been reported in all groups (Table 3).
Table 3.
Main clinical findings after 6 years of follow up
| Variables | Tol group | CNI group | MMF group |
P value |
|
| 6 yr | 6 yr | 6 yr | Tol vs CNII | Tol vs MMFF | |
| Patients (n) | 10 | 21 | 15 | ||
| Death (n) | 0 | 1 | 0 | ||
| ALT (IU/L) | 71.1 ± 54.2 | 53.9 ± 33.7 | 59.4 ± 45.6 | NS | NS |
| Creatinina | 1.3 ± 0.5 | 1.28 ± 0.5 | 1.7 ± 1.6 | NS | NS |
| Cholesterol | 153.6 ± 55.2 | 161.9 ± 43.2 | 155.9 ± 30 | NS | NS |
| Triglycerides | 130.6 ± 64.1 | 125.4 ± 45.6 | 121.8 ± 39.8 | NS | NS |
| HCV-RNA > 500000 IU/mL | 70 | 42.8 | 46.6 | NS | NS |
| Recurrent infections1 | 0 | 42.8 | 40 | 0.029 | 0.019 |
| Cardiovascular diseases | 50 | 23 | 6.6 | NS | NS |
| Diabetes | 30 | 33 | 27 | NS | NS |
| Dyslipidemia | 50 | 19 | 13 | NS | NS |
| FPR | -0.019 | 0.32 | 0.02 | 0.02 | NS |
Urinary and bronchopulmonary infections. FPR: Fibrosis progression rate; ALT: Alanine aminotransferase; HCV: Hepatitis C virus; CNI: Calcineurin inhibitors; MMF: Mycophenolate; NS: Not significant.
DISCUSSION
Since HCV graft infection is an universal complication in HCV-LT recipients and represents the primary cause of graft loss, the management of HCV recurrence is the most challenging issue in the scientific transplant community. Even though a variety of pre- and post-transplant factors have been associated with the severity of HCV recurrence, only a few can be modulated. Several strategies, mostly based on antiviral therapy and IS treatment modulation, have been evaluated to avoid graft injury[3-9]. Antiviral therapy could be administrated before LT to suppress the viral replication and reduce the risk of recurrence, as well as in the early post-transplantation to prevent fibrosis progression. The current antiviral standard of care consists basically of PEG-IFN/RBV treatment, but sustained viral response is achieved only in 30% of patients. Treatment is commonly used in select transplant recipients, because its efficacy may be limited by comorbidities and side effects, requiring dose reductions or discontinuation[20,21]. Recently, the new era of antiviral treatment consists of direct-acting antivirals (i.e., protease inhibitors, polymerase and other non-structural proteins inhibitors), but their benefits still need to be evaluated in relation to their poor tolerance and drug-drug interactions, particularly with IS medications[22].
Therefore, IS modulation still plays a central role because it remains one of the few features that could be modified by physicians although the ideal IS regimen for HCV recipients is yet to be defined. It is well known that IS treatment is directly correlated to the course of HCV recurrence because drugs and their doses influence the immune response against graft reinfection and the progression of liver fibrosis[1-5], but data available is limited by the lack of routine protocol liver biopsies and the heterogeneity of IS regimens reported.
The current strategy of IS treatment for HCV LT recipients, is based on few agreements. Regarding the use of steroids in literature it is well reported that: (1) repeated corticosteroid boluses for acute cellular rejection should be avoided because they are associated with increased viral replication and worse recurrence of disease[23]; (2) in maintenance therapy, steroid withdrawal in slow fashion has shown a “protective role” despite a fast interruption, this may be due to the exposure of HCV-infected cells to a suddenly-restored immune system[24]; and (3) ab initio steroid avoidance reduces the infective and metabolic complications that may aggravate the natural history of the disease[25]. Although treatment of graft rejection with anti-lymphocyte therapies has been associated with severe HCV recurrence[6], De Ruvo et al[26] retrospectively observed that 22 HCV patients who were treated with Tac and thymoglobulin did not show an increase in mortality, rejection or severity of disease vs those treated with Tac and steroids.
CNI remains the main immunosuppressant in LT recipients[3,6]. Most studies which compared Tac with CsA in HCV recipients have shown no difference in graft or patient survival and fibrosis progression[27,28]. However, a recent meta-analysis based on 16 randomized controlled trials concluded that Tac improved graft and patient survival and prevented rejection compared to CsA[10,11]. This result was confirmed by a recent study registry which concluded that Tac therapy instead of CsA increases the HCV-graft survival[29,30].
Antimetabolites are currently associated with CNI and steroids, with the aim to reduce CNI nephrotoxicity. MMF has shown a positive impact on fibrosis progression in HCV recipients either alone[31,32] or in association with CNI at low doses[33].
Data available on mammalian target of rapamicyn inhibitors is mainly on recipients with HCC and HCV infection, and histological outcomes are not reported[34,35].
Therefore, since the only clear recommendation for the HCV LT population is to avoid over-immunosuppression, we think that achievement of IS minimization, defined as the attainment of a state in which IS drugs are decreased down to levels that do not cause clinically significant side effects yet prevent rejection[36], or when the IFS may have a favorable impact on decreasing HCV recurrence.
In the present study, we reviewed the long-term fibrosis progression based on more than 200 liver biopsies in a cohort of 36 HCV LT recipients, who received IS monotherapy or no treatment. After six years, we recognized that IS withdrawal as well as MMF monotherapy favorably impacted the natural history of the disease with respect to low dose CNI. Although in 2006 Samonakis et al[37] reported a significantly lower fibrosis rate in HCV recipients who received CNI monotherapy, we discourage its long term use in HCV recipients even if minimized due to a faster progression of the disease.
Differently, this study confirms that long-term MMF monotherapy is not related with a higher risk of rejection or with fibrosis progression. In fact, as previously reported in a well-matched analysis of 15 HCV recipients under MMF monotherapy vs those treated with CNI, the MMF group showed a slowed progression of the disease[32]. The data was in agreement with Bahra et al[33], who also did not find progression of necro-inflammatory activity and fibrosis in 40 recipients who were treated with MMF and low dose of CNI. Thus, in literature, limited data is available about the clinical impact of MMF on its antifibrotic proprieties in HCV recipients[38,39]. We are convinced that MMF can be safely administered not only in association with CNI at low dose, in order to reduce CNI side effects[40,41], but also as a monotherapy in order to reduce severity of HCV recurrence. This is also supported by the fact that specific IS agents may elicit negative effects on the disease with an indirect mechanism. In particular, it has been reported that insulin resistance and diabetes, common side effects of long-term CNI administration, are associated with higher fibrosis progression after LT[42,43]. Therefore, the use of a IS regimen with reduced or absent metabolic side effects, as MMF, could be a possible strategy that may slow post-transplant disease progression[8].
Moreover, the absence of fibrosis progression in the TOL group confirms our hypothesis that the less potent the drug, the slower the natural course of hepatitis C. Literature reports 21 cases of IFS in HCV LT recipients[5]. Unfortunately, in most studies, protocol liver biopsies were not performed thus histological data is not available. Only the Tor Vergata group reported the pathological findings over a period of 10 years[44]. Of the 34 HCV patients originally enrolled to the weaning study, 6 were off IS for over 10 years. These recipients have been followed and compared to those who could not achieve IS withdrawal. When baseline biopsies were compared with 10-year biopsies, the Tolerant-HCV patients showed an improvement in grading and no difference in staging, meanwhile in the non-Tolerant-HCV group, staging increased. In terms of the fibrosis progression rate at 10 years, the Tolerant-HCV patients showed a slower progression of tissue damage than the non-Tolerant patients. At the last biopsy taken, 63% of non-Tolerant-HCV patients showed features of advanced fibrosis (defined as a fibrosis > 4 according to the Ishak score[45] and 40% frank cirrhosis). On the contrary, none of the patients in the Tolerant-HCV group presented with either advanced fibrosis or cirrhosis, and no evidence of early or late chronic rejection was ever observed during the follow-up.
Even if it may not be possible to draw a definitive conclusion on the basis of this data, the major strength of this study derives from the availability of yearly protocol liver biopsies in all patients, which allowed us to minimize potential biases attributable to sampling errors by different specialists, and to closely monitor the histological evolution of recurrent HCV-related disease. We are convinced that even though the ideal IS in the HCV transplant setting is yet to be defined, HCV recipients could benefit from a “tailored IS regimen”, with the final goal of achieving a permanent and complete IS withdrawal. The recipients who could be safety weaned off IS could be identified immediately after LT by searching the biomarkers that are able to predict the state of tolerance. This could allow for the reduction of risk for IS withdrawal failure and also attempt the weaning process before fibrosis occurs[46]. Therefore, IS minimization may represent a valid alternative in those recipients who are not able to achieve the IFS. To this regard, MMF or EC-MPS monotherapy represents a alternative to CNI, either alone or in association with a low CNI dose to contrast the histological liver damage.
In conclusion, although our finding must be confirmed by a large prospective trial, the long term LT recipient should always be considered for withdrawal. In those who require IS, the use of antimetabolites as MMF or EC-MPS should be considered in spite of a low dose of CNI.
COMMENTS
Background
Hepatitis C virus (HCV) graft reinfection is universal after liver transplantation in patients who were HCV-RNA positive at the time of transplant and has an accelerated progression to fibrosis compared to non-transplanted patients. This leads to cirrhosis in about 30% of the recipients within 5 years of transplantation. Immunosuppression (IS) is one of the main variables responsible for the degree of progression of HCV disease recurrence. The transplant community is in agreement that a high maintenance IS regimen and corticosteroid bolus should be avoided in HCV recipients.
Research frontiers
After liver transplantation for HCV-end stage liver disease, the IS treatment most commonly reported in literature is based on calcineurin inhibitor (CNI) at low dose associated with a second IS agent with or without steroid maintenance. The IS-free state seems to improve the natural history of the disease but, right now, only a very few patients could achieve a tolerant status. The research hotspot is to identify the optimal IS regimen for HCV recipients after liver transplant (LT) in order to reduce the severity of HCV graft infection and the IS long-term toxicity.
Innovations and breakthroughs
Few studies proposed that IS minimization, and when possible, IS withdrawal might reduce HCV fibrosis progression allowing higher immune response against HCV re-infection. Drugs most commonly used in monotherapy are CNIs (cyclosporine or tacrolimus), but were associated with long-term side effects. Therefore, mycophenolate (MMF) monotherapy was introduced to reduce CNI-long term toxicity (in particular nefrotoxicity and hiperlipidaemia). Nowadays, in the era of immune tolerance, also the impact of completely withdrawal of IS has been investigated on the severity of HCV recurrence after LT. It has been reported that IS weaning determinates a lower HCV fibrosis progression rate and improves IS side effects. Therefore, authors evaluate the effect of long-term CNI and MMF monotherapy, compared to IS withdrawal on the fibrosis progression rate in HCV recipients.
Applications
The study results that IS withdrawal and MMF monotherapy is safe and seems to be associated with the slowest fibrosis progression in HCV recipients. Therefore, IS minimization and, when achievable, IS withdrawal might represent the new goal for HCV recipients after liver transplantation.
Terminology
HCV recurrence: defined by the presence of HCV-RNA in serum and/or liver alone, while diagnosis of disease recurrence requires histological confirmation. Clinical operational tolerance: was defined as the complete discontinuation of IS with normal graft function and without features of acute and chronic rejection. IS minimization: defined as the attainment of a state in which IS drugs are decreased down to levels that do not cause clinically significant side effect and yet prevent rejection.
Peer review
The present study reviewed the long-term fibrosis progression in HCV LT recipients, who received IS monotherapy or were off any drugs. This study confirms also that long-term MMF monotherapy is not related with higher risk of rejection episode. The results are interesting and suggest that IS minimization may represent a valid alternative in those recipients who are not able to achieve the IFS.
Footnotes
P- Reviewer: Cucchetti A, Zapata RL S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Berenguer M, López-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35:666–678. doi: 10.1016/s0168-8278(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer M, Prieto M, Rayón JM, Mora J, Pastor M, Ortiz V, Carrasco D, San Juan F, Burgueño MD, Mir J, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–858. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 3.Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009;9:1707–1713. doi: 10.1111/j.1600-6143.2009.02702.x. [DOI] [PubMed] [Google Scholar]
- 4.Roche B, Samuel D. Risk factors for hepatitis C recurrence after liver transplantation. J Viral Hepat. 2007;14 Suppl 1:89–96. doi: 10.1111/j.1365-2893.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 5.Manzia TM, Angelico R, Toti L, Lai Q, Ciano P, Angelico M, Tisone G. Hepatitis C virus recurrence and immunosuppression-free state after liver transplantation. Expert Rev Clin Immunol. 2012;8:635–644. doi: 10.1586/eci.12.66. [DOI] [PubMed] [Google Scholar]
- 6.Samonakis DN, Germani G, Burroughs AK. Immunosuppression and HCV recurrence after liver transplantation. J Hepatol. 2012;56:973–983. doi: 10.1016/j.jhep.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Akamatsu N, Sugawara Y. Liver transplantation and hepatitis C. Int J Hepatol. 2012;2012:686135. doi: 10.1155/2012/686135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trotter JF. Hot-topic debate on hepatitis C virus: the type of immunosuppression matters. Liver Transpl. 2011;17 Suppl 3:S20–S23. doi: 10.1002/lt.22414. [DOI] [PubMed] [Google Scholar]
- 9.Berenguer M. Hot topic in hepatitis C virus research: the type of immunosuppression does not matter. Liver Transpl. 2011;17 Suppl 3:S24–S28. doi: 10.1002/lt.22347. [DOI] [PubMed] [Google Scholar]
- 10.McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578–1585. doi: 10.1111/j.1600-6143.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 11.Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;(4):CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gedaly R, Clifford TM, McHugh PP, Jeon H, Johnston TD, Ranjan D. Prevalent immunosuppressive strategies in liver transplantation for hepatitis C: results of a multi-center international survey. Transpl Int. 2008;21:867–872. doi: 10.1111/j.1432-2277.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 13.Wiesner RH, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Lake JR. Mycophenolate mofetil combination therapy improves long-term outcomes after liver transplantation in patients with and without hepatitis C. Liver Transpl. 2005;11:750–759. doi: 10.1002/lt.20453. [DOI] [PubMed] [Google Scholar]
- 14.Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, Lionetti R, Anselmo A, Toti L, Angelico M. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006;44:702–709. doi: 10.1016/j.jhep.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Orlando G, Manzia T, Baiocchi L, Sanchez-Fueyo A, Angelico M, Tisone G. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transpl Immunol. 2008;20:43–47. doi: 10.1016/j.trim.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 17.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 18.Snover DC, Freese DK, Sharp HL, Bloomer JR, Najarian JS, Ascher NL. Liver allograft rejection. An analysis of the use of biopsy in determining outcome of rejection. Am J Surg Pathol. 1987;11:1–10. doi: 10.1097/00000478-198701000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 20.Roche B, Samuel D. Hepatitis C virus treatment pre- and post-liver transplantation. Liver Int. 2012;32 Suppl 1:120–128. doi: 10.1111/j.1478-3231.2011.02714.x. [DOI] [PubMed] [Google Scholar]
- 21.Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl. 2006;12:1192–1204. doi: 10.1002/lt.20865. [DOI] [PubMed] [Google Scholar]
- 22.Coilly A, Roche B, Samuel D. Current management and perspectives for HCV recurrence after liver transplantation. Liver Int. 2013;33 Suppl 1:56–62. doi: 10.1111/liv.12062. [DOI] [PubMed] [Google Scholar]
- 23.Neumann UP, Berg T, Bahra M, Puhl G, Guckelberger O, Langrehr JM, Neuhaus P. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77:226–231. doi: 10.1097/01.TP.0000101738.27552.9D. [DOI] [PubMed] [Google Scholar]
- 24.Berenguer M, Aguilera V, Prieto M, San Juan F, Rayón JM, Benlloch S, Berenguer J. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J Hepatol. 2006;44:717–722. doi: 10.1016/j.jhep.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, Montgomery RA, Cameron AM, Maley WR. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 26.De Ruvo N, Cucchetti A, Lauro A, Masetti M, Cautero N, Di Benedetto F, Dazzi A, Del Gaudio M, Ravaioli M, Di Francesco F, et al. Preliminary results of a “prope” tolerogenic regimen with thymoglobulin pretreatment and hepatitis C virus recurrence in liver transplantation. Transplantation. 2005;80:8–12. doi: 10.1097/01.tp.0000164349.54297.95. [DOI] [PubMed] [Google Scholar]
- 27.Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21–29. doi: 10.1002/lt.21035. [DOI] [PubMed] [Google Scholar]
- 28.Berenguer M, Aguilera V, San Juan F, Benlloch S, Rubin A, López-Andujar R, Moya A, Pareja E, Montalva E, Yago M, et al. Effect of calcineurin inhibitors in the outcome of liver transplantation in hepatitis C virus-positive recipients. Transplantation. 2010;90:1204–1209. doi: 10.1097/TP.0b013e3181fa93fa. [DOI] [PubMed] [Google Scholar]
- 29.Irish WD, Arcona S, Bowers D, Trotter JF. Cyclosporine versus tacrolimus treated liver transplant recipients with chronic hepatitis C: outcomes analysis of the UNOS/OPTN database. Am J Transplant. 2011;11:1676–1685. doi: 10.1111/j.1600-6143.2011.03508.x. [DOI] [PubMed] [Google Scholar]
- 30.Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- 31.Germani G, Pleguezuelo M, Villamil F, Vaghjiani S, Tsochatzis E, Andreana L, Burroughs AK. Azathioprine in liver transplantation: a reevaluation of its use and a comparison with mycophenolate mofetil. Am J Transplant. 2009;9:1725–1731. doi: 10.1111/j.1600-6143.2009.02705.x. [DOI] [PubMed] [Google Scholar]
- 32.Manzia TM, Angelico R, Toti L, Bellini MI, Sforza D, Palmieri G, Orlando G, Tariciotti L, Angelico M, Tisone G. Long-term, maintenance MMF monotherapy improves the fibrosis progression in liver transplant recipients with recurrent hepatitis C. Transpl Int. 2011;24:461–468. doi: 10.1111/j.1432-2277.2011.01228.x. [DOI] [PubMed] [Google Scholar]
- 33.Bahra M, Neumann UI, Jacob D, Puhl G, Klupp J, Langrehr JM, Berg T, Neuhaus P. MMF and calcineurin taper in recurrent hepatitis C after liver transplantation: impact on histological course. Am J Transplant. 2005;5:406–411. doi: 10.1111/j.1600-6143.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- 34.Wagner D, Kniepeiss D, Schaffellner S, Jakoby E, Mueller H, Fahrleitner-Pammer A, Stiegler P, Tscheliessnigg KH, Iberer F. Sirolimus has a potential to influent viral recurrence in HCV positive liver transplant candidates. Int Immunopharmacol. 2010;10:990–993. doi: 10.1016/j.intimp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 35.McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O’Leary JG, Davis GL, Levy MF, et al. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant. 2011;11:2379–2387. doi: 10.1111/j.1600-6143.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 36.Londoño MC, Rimola A, O’Grady J, Sanchez-Fueyo A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J Hepatol. 2013;59:872–879. doi: 10.1016/j.jhep.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Samonakis DN, Mela M, Quaglia A, Triantos CK, Thalheimer U, Leandro G, Pesci A, Raimondo ML, Dhillon AP, Rolles K, et al. Rejection rates in a randomised trial of tacrolimus monotherapy versus triple therapy in liver transplant recipients with hepatitis C virus cirrhosis. Transpl Infect Dis. 2006;8:3–12. doi: 10.1111/j.1399-3062.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 38.Roos N, Poulalhon N, Farge D, Madelaine I, Mauviel A, Verrecchia F. In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther. 2007;321:583–589. doi: 10.1124/jpet.106.117051. [DOI] [PubMed] [Google Scholar]
- 39.Morath C, Schwenger V, Beimler J, Mehrabi A, Schmidt J, Zeier M, Muranyi W. Antifibrotic actions of mycophenolic acid. Clin Transplant. 2006;20 Suppl 17:25–29. doi: 10.1111/j.1399-0012.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 40.Pageaux GP, Rostaing L, Calmus Y, Duvoux C, Vanlemmens C, Hardgwissen J, Bernard PH, Barbotte E, Vercambre L, Bismuth M, et al. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transpl. 2006;12:1755–1760. doi: 10.1002/lt.20903. [DOI] [PubMed] [Google Scholar]
- 41.Orlando G, Baiocchi L, Cardillo A, Iaria G, De Liguori Carino N, De Luca L, Ielpo B, Tariciotti L, Angelico M, Tisone G. Switch to 1.5 grams MMF monotherapy for CNI-related toxicity in liver transplantation is safe and improves renal function, dyslipidemia, and hypertension. Liver Transpl. 2007;13:46–54. doi: 10.1002/lt.20926. [DOI] [PubMed] [Google Scholar]
- 42.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Rosen CB, Heimbach JK, Janssen HL, Charlton MR. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9:1406–1413. doi: 10.1111/j.1600-6143.2009.02642.x. [DOI] [PubMed] [Google Scholar]
- 43.Foxton MR, Quaglia A, Muiesan P, Heneghan MA, Portmann B, Norris S, Heaton ND, O’Grady JG. The impact of diabetes mellitus on fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2006;6:1922–1929. doi: 10.1111/j.1600-6143.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 44.Manzia TM, Angelico R, Baiocchi L, Toti L, Ciano P, Palmieri G, Angelico M, Orlando G, Tisone G. The Tor Vergata weaning of immunosuppression protocols in stable hepatitis C virus liver transplant patients: the 10-year follow-up. Transpl Int. 2013;26:259–266. doi: 10.1111/tri.12023. [DOI] [PubMed] [Google Scholar]
- 45.Hui AY, Liew CT, Go MY, Chim AM, Chan HL, Leung NW, Sung JJ. Quantitative assessment of fibrosis in liver biopsies from patients with chronic hepatitis B. Liver Int. 2004;24:611–618. doi: 10.1111/j.1478-3231.2004.0957.x. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-Fueyo A. Identification of tolerant recipients following liver transplantation. Int Immunopharmacol. 2010;10:1501–1504. doi: 10.1016/j.intimp.2010.06.011. [DOI] [PubMed] [Google Scholar]


