Abstract
AIM: To elucidate the endoscopic features that predict the cancer following endoscopic submucosal dissection (ESD) in patients with high-grade neoplasia (HGN).
METHODS: We retrospectively analyzed the medical records of patients who underwent ESD of gastric neoplasms from January 2007 to September 2010. ESD was performed in 555 cases involving 550 patients. A total of 112 lesions from 110 consecutive patients were initially diagnosed as HGN without cancer by forceps biopsy, and later underwent ESD. We classified lesions into two groups according to histologic discrepancies between the biopsy and ESD diagnosis. Gastric adenoma in the final diagnosis by ESD specimens were defined as adenoma group. Lesions with coexisting cancer after ESD were defined as cancer group.
RESULTS: The mean age was 65.3 years, and 81 patients were male. There was no significant difference in the age or gender distribution between the adenoma (n = 52) and cancer (n = 60) groups. Thirty-six of these lesions (32.1%) showed histologic concordance between the forceps biopsy and ESD specimens, 16 (14.3%) showed a downgraded histology (low-grade neoplasia), and 60 (53.6%) showed an upgraded histology (cancer). A red color change of the mucosal surface on endoscopy was found in 27/52 (51.9%) of cases in the adenoma group and in 46/60 (76.7%) of cases in the cancer group (P = 0.006). Ulceration of the mucosal surface on endoscopy was found in 5 (9.6%) of 52 lesions in the adenoma group and in 17 (28.3%) of 60 lesions in the cancer group (P = 0.013). In the multivariate analysis, a reddish surface color change and mucosal ulceration were significant predictive factors correlated with cancer after ESD of the HGN by forceps biopsy.
CONCLUSION: HGN with a red color change or mucosal ulceration correlated with the presence of gastric cancer. These finding may help to guide the diagnosis and treatment.
Keywords: Stomach, Neoplasms, Carcinoma, Adenoma, Endoscopy, Dissection, Risk factors
Core tip: A discrepancy may exist between the diagnosis using endoscopic forceps biopsies (EFB) samples and totally resected specimens because only a small portion is sampled. Considering the risk of missing a cancer diagnosis, it is necessary to identify morphological characteristics on endoscopy that suggest coexisting cancer in patients diagnosed with high-grade neoplasia (HGN) on initial EFB. HGN with a red color change or mucosal ulceration correlated with the presence of gastric cancer. Characteristic endoscopic findings suggesting gastric cancer may help to determine the diagnosis and guide treatment in patients with HGN.
INTRODUCTION
The terms adenoma/dysplasia refer to the architecture of an abnormal proliferative lesion formed by dysplastic epithelium[1]. Currently, each of these terms are classified into two categories using the revised Vienna classification, encompassing categories 3 and 4 of this classification [category 3-which includes low grade adenoma/dysplasia (low-grade neoplasia, LGN) and category 4-which includes high grade adenoma/dysplasia (high-grade neoplasia, HGN), carcinoma in situ, suspicious for invasive carcinoma, and intramucosal carcinoma)[2-4]. The revised Vienna classification has helped to guide treatment strategies[4]. Based on this classification, it is strongly recommended that category 4 lesions be completely resected endoscopically or by local surgical resection, unlike category 3 lesions.
Endoscopic forceps biopsies (EFB) are commonly used to provide a histologic diagnosis for gastric neoplasms. If gastric cancer is confirmed by EFB, pre-resection staging is needed to decide whether or not endoscopic resection can be considered, even for early gastric cancer (EGC). If EFB specimens were diagnosed with HGN, it is generally considered an absolute indication for local resection[4], because the reported progression to cancer for HGN cases is 10%-81%[5-7]. However, a discrepancy may exist between the diagnosis using EFB samples and totally resected specimens because only a small portion is sampled[8]. Therefore, HGN from EFB samples cannot exclude the presence of cancer foci in the remaining lesion[9,10]. If these patients undergo endoscopic resection, clinicians should strive for a more accurate diagnosis.
It is not been shown whether HGN cases diagnosed with initial EFB should have a repeat endoscopy with more biopsies, or if further evaluation for gastric cancer should be conducted because cancer in the remaining mass cannot be excluded. Considering the risk of missing a cancer diagnosis, it is necessary to identify morphological characteristics on endoscopy that suggest coexisting cancer in patients diagnosed with HGN on initial EFB.
To our knowledge, there have been no reports of predictive factors of coexisting cancer foci in cases diagnosed with HGN by initial EFB. The aim of this study was to identify endoscopic features predicting cancer after endoscopic submucosal dissection (ESD) in patients with an initial diagnosis of gastric adenoma with HGN.
MATERIALS AND METHODS
Study population
We retrospectively analyzed the medical records of patients who underwent ESD for gastric neoplasms at Gachon University Gil Medical Center (Incheon, Korea). From January 2007 to September 2010, ESD for gastric neoplasia was performed in 555 cases involving 550 patients. ESD was performed using standard ESD techniques, and was indicated if the following criteria were met: lesions with HGN regardless of size, well-to-moderately differentiated adenocarcinoma confined to the mucosa < 2 cm by endoscopic measurements without evidence of lymph node involvement or distant metastases on computed tomography (CT) and endoscopic ultrasonography (EUS). A total of 112 lesions from 110 consecutive patients were initially diagnosed with HGN without cancer using a forceps biopsy, and later had ESD performed. All gastric adenomas with HGN were diagnosed using a forceps biopsy, and were confirmed as either a gastric adenoma or gastric cancer after ESD. All ESDs were performed within 2 mo following the initial HGN diagnosis by forceps biopsy.
We classified lesions into two groups according to histologic discrepancies between the biopsy and ESD diagnosis. LGN and HGN in the final diagnosis by ESD specimens were clustered into one category termed “adenoma group”. Lesions with coexisting cancer after ESD were defined as “cancer group”. That is, the “adenoma group” had lesions without coexisting cancer and the “cancer group” included lesions with coexisting foci of cancer.
Informed consent, with adequate explanation of the procedure, was obtained from each patient. This study was approved by the Institutional Review Board of the Gachon University Gil Medical Center (IRB No. GIRBD0022-2012).
Forceps biopsy
The gastric lesion size was measured using endoscopic forceps [open size = 6 mm in diameter (Olympus FB-24K-1; Olympus, Tokyo, Japan)]. If dysplasia or cancer was suspected, we typically sampled two to four biopsy specimens from each lesion. The specimens were fixed in formaldehyde, and submitted to a pathologist for histologic evaluation.
Endoscopic procedures
Gastric lesions were first identified and demarcated using white-light endoscopy and chromoendoscopy that sprayed 0.1%-0.5% indigo carmine was routinely performed to determine the tumor shape and margin (GIF-Q 240, 260, and H260; Olympus). After gastric lesion visualization, lesion margins were marked using electrocautery (VIO 300D; ERBE, Tübingen, Germany) using argon plasma coagulation (VIO APC2; ERBE), fixed flexible snares (Kachu Technology, Seoul, Korea) or a dual knife (KD-650L; Olympus). Submucosal injection of hypertonic saline mixed with epinephrine was used to produce a mucosal bleb. A circumferential incision (precut) was performed using the fixed flexible snare, insulated-tipped2 (IT2) knife (KD-611L; Olympus), or a dual knife along the outer borders of the lesion. The lesions were dissected from the deep layers of stomach wall with an IT2 knife, dual knife, or fixed flexible snare with electrosurgical units (VIO 300D; ERBE). The resected specimens were oriented using small pins, and fixed in an 8% formaldehyde solution. The specimens were embedded in paraffin, and cut into 2-mm sections for pathologic diagnosis.
Endoscopic reports
Endoscopic reports and photographs were reviewed by two experienced endoscopists for endoscopic lesion features. These reports and photographs were blinded and reviewed. If there was a discrepancy between the two endoscopists, a final consensus was reached after further discussion. Parameters included the lesion size (maximal diameter), location, macroscopic type and surface configuration (red color change, nodular change, and mucosal ulceration).
The Paris classification was used to classify the macroscopic lesion type: elevated, flat, or depressed[11]. Elevated lesions were subdivided as follows: type 0-I, type 0-IIa, or a combination of these types, such as type 0-I + IIa, 0-IIa + IIb, 0-IIa + IIc. Depressed lesions were subdivided as follows: type 0-IIc, 0-III, and a combination of these types, such as type 0-IIc + IIa, 0-III + IIa. A type 0-IIb lesion was classified as flat.
A red color change was defined as a red discoloration on the mucosal surface of the lesion compared to the surrounding mucosa. Nodular change was defined as the presence of irregularly raised nodular mucosa in the main lesion. Mucosal ulceration was defined as a lesion with a mucosal defect. The longitudinal and horizontal location of lesions was described using the Japanese Classification of Gastric Cancer[12]. In this system, the stomach is anatomically divided into three sections, the upper, mid, and lower third. The cross-sectional parts of the stomach are divided into four equal sections: anterior wall, lesser curvature, posterior wall, and greater curvature.
Histologic procedures
Pathology slides of EFB samples and ESD specimens were reviewed by single experienced pathologist who had no information on the initial pathologic diagnosis and clinical information. The pathologic diagnosis of gastric dysplasia and cancer were determined based on recently published criteria[13,14]. LGN was diagnosed if the following criteria were met: the glands had a similar shape and were slightly crowded with a regular arrangement, and the nuclei were basally oriented, spindle-shaped, and mildly hyperchromatic. If the lesions had more architectural abnormalities, including gland branching and budding, they were diagnosed as HGN. A cancer diagnosis required a higher nuclear grade, and either a prominent back-to-back or syncytial growth pattern, abortive microglands, or small clusters of epithelial cells within the lamina propria between glands. Lesion with combined findings of adenoma and cancer were classified as cancer.
Statistical analysis
Statistical analysis was performed using SPSS 12.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) for MS Windows®. In the univariate analysis, continuous data were analyzed using the independent t-test, and other categorical data were analyzed using the χ2 or Fisher’s exact test. Multivariate analysis by logistic regression was performed using the statistically significant variables found in the univariate analysis. Two-tailed P values of 0.05 or less were considered to indicate significance.
RESULTS
Patients’ characteristics
Baseline patient characteristics are summarized in Table 1. The mean patient age was 65.3 years (range: 40-82), and 81 (72.3%) were males. Post-ESD specimen pathology classified 52 cases in the adenoma group and 60 cases in the cancer group (Figures 1 and 2). There was no significant difference in the age or gender distribution between the two groups. After the histology review of the 112 ESD specimens, 36 of these lesions (32.1%) showed histologic concordance of the diagnosis between the forceps biopsy and ESD specimens, 16 (14.3%) showed a downgraded histology (LGN), and 60 (53.6%) showed an upgraded histology (cancer).
Table 1.
Baseline characteristics and endoscopic features in the adenoma and carcinoma groups n (%)
| Adenoma group | Cancer group | Univariate analysis |
Multivariate analysis |
||
| (n = 52) | (n = 60) | P value | OR (95%CI) | P value | |
| Age (yr) | 65.2 ± 10.3 | 65.5 ± 10.2 | 0.879 | ||
| Gender | 0.270 | ||||
| Male | 35 (67.3) | 46 (76.7) | |||
| Female | 17 (32.7) | 14 (23.3) | |||
| Lesion size | 16.1 ± 10.7 | 18.1 ± 11.3 | 0.347 | ||
| Tumor location (longitudinal) | 0.327 | ||||
| Upper third | 1 (1.9) | 4 (6.7) | |||
| Middle third | 5 (9.6) | 3 (5.0) | |||
| Lower third | 46 (88.5) | 53 (88.3) | |||
| Tumor location (horizontal) | 0.308 | ||||
| Anterior wall | 11 (21.2) | 12 (20.0) | |||
| Lesser curvature | 16 (30.8) | 26 (43.3) | |||
| Posterior wall | 7 (13.5) | 10 (16.7) | |||
| Greater curvature | 18 (34.6) | 12 (20.0) | |||
| Size (cm) | 0.542 | ||||
| < 1 | 10 (19.2) | 11 (18.3) | |||
| 1-1.9 | 28 (53.8) | 26 (43.3) | |||
| 2-2.9 | 10 (19.2) | 14 (23.3) | |||
| ≥ 3 | 4 (7.7) | 9 (15.0) | |||
| Macroscopic type | 0.121 | ||||
| Elevated | 24 (46.2) | 18 (30.0) | |||
| Flat | 17 (32.7) | 20 (33.3) | |||
| Depressed | 11 (21.2) | 22 (36.7) | |||
| Surface configuration | |||||
| Nodular change | 26 (50.0) | 38 (63.3) | 0.155 | ||
| Red color change | 27 (51.9) | 46 (76.7) | 0.006 | 2.682 (1.061-6.784) | 0.037 |
| Mucosal ulceration | 5 (9.6) | 17 (28.3) | 0.013 | 3.562 (1.056-12.021) | 0.041 |
| Interval between biopsy and ESD (d) | 29.5 ± 17.1 | 29.0 ± 15.1 | 0.886 | ||
ESD: Endoscopic submucosal dissection.
Figure 1.
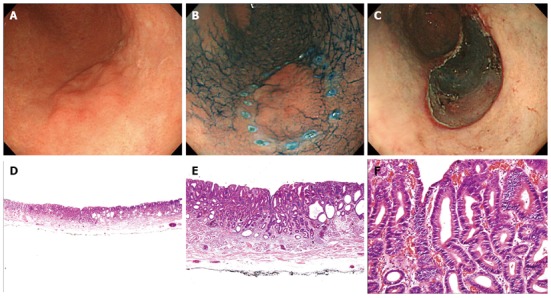
Endoscopic and pathologic findings in a 66-year-old female patient with low-grade neoplasia. In this case, forceps biopsy specimens showed high-grade neoplasia, but endoscopic submucosal dissection (ESD) revealed low-grade neoplasia. A, B: A 22-mm, type 0-IIb lesion was observed in the greater curvature aspect of the antrum. This lesion had a nodular change; C: There was artificial ulceration after successful ESD; D-F: The pathologic findings in the ESD specimen showed a tubular adenoma with low-grade neoplasia. The glands had a similar shape and were slightly crowded with a regular arrangement, and the nuclei were basally oriented, spindle-shaped, and mildly hyperchromatic (D: HE stain, × 12.5; E: HE stain, × 40; F: HE stain, × 200).
Figure 2.

Endoscopic and pathologic findings in a 51-year-old male patient with mucosal cancer. In this case, forceps biopsy specimens showed high-grade neoplasia, but endoscopic submucosal dissection (ESD) revealed adenocarcinoma. A, B: An 8-mm, type 0-IIc lesion was observed in the greater curvature aspect of the antrum. This lesion had a smooth surface, red color change, and mucosal ulceration; C: There was artificial ulceration after successful ESD; D-F: The pathologic findings in the ESD specimen showed a tubular adenocarcinoma with focal areas of invasion into the lamina propria. The carcinoma cells have hyperchromatic nuclei with irregular nuclear membranes and prominent nuclei (D: HE stain, × 12.5; E: HE stain, × 40; F: HE stain, × 200).
Endoscopic findings
The mean lesion diameter was 16.1 ± 10.7 mm in the adenoma group and 18.1 ± 11.3 mm in the cancer group. The prevalence of cancer increased as the size of the adenoma increased, but no significant difference was noted between the groups.
The most common longitudinal and horizontal location was the lower third (88.5% vs 88.3%) and lesser curvature (30.8% vs 43.3%) in both groups. The elevated type was the most common in the adenoma group, and a depressed type was the most common in the cancer group. However, no significant difference was noted between the two groups.
Nodular change was seen in 26 cases in the adenoma group and in 38 cases in the cancer group, but these differences were not significant (Table 1). A red color change and surface ulceration on endoscopy were found significantly more frequently in the cancer group (Figure 2). A red color change of the mucosal surface on endoscopy was found in 27/52 (51.9%) of cases in the adenoma group and in 46/60 (76.7%) of cases in the cancer group (P = 0.006). Ulceration of the mucosal surface on endoscopy was found in 5 (9.6%) of 52 lesions in the adenoma group and in 17 (28.3%) of 60 lesions in the cancer group (P = 0.013).
In the multivariate analysis, a red surface color change (OR = 2.682; 95%CI: 1.061-6.784, P = 0.037) and mucosal ulceration (OR = 3.562; 95%CI: 1.056-12.021, P = 0.041) were significant predictive factors of cancer after ESD of HGD by forceps biopsy.
DISCUSSION
Generally, when a diagnosis of HGN is made from EFB, the possibility remains that the lesion may have been under-diagnosed or the presence of cancer foci may have been missed. This possibility has not been strongly considered, because HGN and cancer require the same treatment and clinical management[4]. According to the revised Vienna classification[4], it is recommended that follow-up or endoscopic resection be performed for LGN, and endoscopic or local surgical resection be conducted for HGN or cancer. Previous studies have been conducted to identify predictive factors in adenomas for HGN or carcinoma because these require different therapeutic plans. No previous studies have identified a predictive factor for cancer upon the diagnosis of HGN from EFB. This may result in an inaccurate diagnosis and unsuccessful treatment of lesions in their early stage. Therefore, the revised Vienna classification is deemed incomplete in terms of the possibility of missing a cancer diagnosis.
In the present study, 53.6% (60/112) of patients with gastric HGN were shown to have gastric cancer after ESD. We suggested that cases of possible gastric cancer should be carefully reviewed to determine the need for re-biopsy, differing therapeutic methods, and further evaluation for the presence of metastasis prior to resection.
A histologic diagnosis from EFB samples provides the most reliable information for the diagnosis of gastric neoplasia prior to complete resection. However, EFB does not fully evaluate the entire lesion, and may lead to an inaccurate diagnosis. EFB is a simple and rapid technique, but this method has some of weaknesses[15]. Differentiation between adenomas and well-differentiated cancer can be difficult in small biopsy specimens. In addition, cancer can sometimes be present focally in the lesion. Histologic modifications such as regeneration, which is induced by inflammation, may increase the difficulty of diagnosing atypia. To reduce these errors, we can increase the number of biopsy specimens. If sufficient EFB specimens are sampled or additional endoscopic sessions for repeated EFB are been performed, a more accurate histological diagnosis is possible. However, this strategy will increase costs and create submucosal lesion fibrosis from previous biopsies[9]. In lesions with fibrosis, endoscopic resection may be more difficult, increasing the risk of complications such as bleeding and perforation[9]. Therefore, additional biopsies should be performed carefully if endoscopic resection is anticipated.
Several reports of conventional endoscopic findings that predict malignancy in gastric adenomas have been published[13,16-20]. Previous reports show that LGN greater than 1.0 cm in size was an independent risk factor for coexisting HGN foci[13], and gastric adenoma with HGN were significant factors associated with cancer[19]. A lesion greater than 1 or 2 cm, depressive morphology, and a red surface color change, and mucosal ulceration are predictive factors for the presence of cancer in adenoma with LGN and HGN[16,17,19,20]. A lesion size greater than 1 cm, a depressed lesion, and mucosal erythema are predictive factors for HGN or cancer in LGN[18]. In case of LGN diagnosis by EFB, depressed lesion larger than 15 mm in size and protruding lesion larger than 20 mm in size was reported risk factors suggesting cancer[20].
The present study has shown that the size, nodularity, and macroscopic type are not predictive factors for cancer in HGN lesions. The multivariate analysis showed that only a red color change and mucosal ulceration were independent risk factors for cancer in HGN. Based on the previous literature and our results, we hypothesize that morphological changes occur in a sequential order while LGN progresses to HGN and then cancer. If the size of a depressed lesion increases, and surface erythema or surface ulceration develops with LGN, this indicates a high potential for malignant transformation into HGN or cancer. We suggested that red color change and mucosal ulceration are the two most reliable predictive factors for cancer in adenoma with HGN.
There is no standard method of resection for HGN. ESD provides a higher en bloc resection rate with tumor-free margins regardless of size, thus allowing for more accurate and detailed pathological evaluation and minimizing recurrence risk compared to conventional endoscopic mucosal resection[21,22]. Also, it is less invasive than surgical resection, including laparoscopic wedge resection and function-preserving gastrectomies, conserving the whole stomach, and improving postoperative quality of life[22,23]. If the HGN by EFB is predicted to be EGC, sufficient resection margins by ESD are necessary for curative resection.
Proper evaluation for the presence of cancer foci, depth of invasion, and the presence of metastasis is important in potential candidates for endoscopic resection. It was recently reported that metastasis is found not only in submucosal but also in intramucosal cancers. Patients with EGC have a low risk of lymph node metastasis: 2.2% in mucosal and 17.9% in submucosal cancer[24]. In our study, all patients in the cancer group had EGC: 51/60 (85.0%) were mucosal and 9/60 (15.0%) were submucosal. In patients with EGC, the method of resection is determined depending on whether or not lymph node metastasis is present. When lymph node metastasis is found, radical surgery with complete removal of the perigastric lymph nodes provides a greater likelihood of a cure compared to endoscopic resection[19]. We suggested that the need to evaluate potential lymph node metastasis should be considered before endoscopic resection for predicted EGC. In patients with HGN by EFB, there is no standard pre-resection diagnostic, and pre-resection evaluation for invasion and metastasis in patients with HGN is not recommended.
A recent study showed that many imaging modalities, including CT, abdominal ultrasonography (AUS), EUS, and positron emission tomography, exhibited no significant differences in their mean sensitivities and specificities. The sensitivities and specificities of AUS, EUS, and magnetic resonance imaging (MRI) varied from poor (< 60%) to high (≥ 80%)[25]. To date, no imaging modality consistently achieves both high sensitivity and specificity in the evaluation of T and N staging, and enables decision making for the treatment of EGC. In general, lymph node metastasis is significantly associated with submucosal cancer invasion[26]. In previous studies, the overall accuracy rate for EGC invasion depth staging was 63%-73.7% with conventional endoscopy and 67.4%-71% for EUS[27,28]. EUS was not superior in overall diagnostic accuracy to conventional endoscopy using morphological characteristics. A recent large-scale study showed that the overall accuracy of T staging of EGC using conventional endoscopy was 78%[29]. As a result, the accuracy of conventional endoscopy compared to other methods is not inferior and provides reliable accuracy in evaluating invasion depth. A detailed endoscopic morphological evaluation could provide useful information for selection of the optimal subsequent evaluation methods and treatment strategy prior to endoscopic resection. However, conventional endoscopic findings should be applied complementary with other techniques.
In the present study, we identified two independent predictive risk factors. If these two risk factors were used with biopsy of proven HGN lesions, the sensitivity and specificity for cancer detection would have been 76.7% and 92.3%, respectively. This sensitivity was rather unsatisfactory. Therefore, methods other than conventional endoscopic findings are required to improve sensitivity. Recently, newly developed endoscopic imaging techniques, such as narrow-band imaging endoscopy with or without magnifying endoscopy, I-scan, autofluorescence imaging, and flexible spectral imaging color enhancement were reported to be capable of predicting the histologic characteristics and defining the margins of gastric tumors[30]. However, not all institutions will have newly developed imaging equipment because of its cost.
Our study has several limitations. First, the possibility of selection biases may be exist because this study had a retrospective design. Second, pathologic diagnosis was examined by a single pathologist. Third, the concordance rate of endoscopic features between observers was not investigated. Fourth, sampling error also must be involved because under-diagnosis or over-diagnosis of adenoma could occur only on the basis on small biopsy samples before resection.
In conclusion, HGN in lesions with red color change and mucosal ulceration is correlated with the presence of gastric cancer. Careful diagnosis is necessary before endoscopic resection, but is difficult given current diagnostic modalities. Characteristic endoscopic findings suggesting gastric cancer may help to determine the diagnosis and guide treatment in patients with HGN.
COMMENTS
Background
A discrepancy may exist between the diagnosis using endoscopic forceps biopsies (EFB) samples and totally resected specimens because only a small portion is sampled. Considering the risk of missing a cancer diagnosis, it is necessary to identify morphological characteristics on endoscopy that suggest coexisting cancer in patients diagnosed with high-grade neoplasia (HGN) on initial EFB.
Research frontiers
There have been no reports of predictive factors of coexisting cancer foci in cases diagnosed with HGN by initial EFB. The aim of this study was to identify endoscopic features predicting cancer after endoscopic submucosal dissection (ESD) in patients with an initial diagnosis of gastric adenoma with HGN.
Innovations and breakthroughs
Based on the previous literature and our results, we hypothesize that morphological changes occur in a sequential order while low-grade neoplasia (LGN) progresses to HGN and then cancer. If the size of a depressed lesion increases, and surface erythema or surface ulceration develops with LGN, this indicates a high potential for malignant transformation into HGN or cancer. The authors suggested that red color change and mucosal ulceration are the two most reliable predictive factors for cancer in adenoma with HGN. A detailed endoscopic morphological evaluation could provide useful information for selection of the optimal subsequent evaluation methods and treatment strategy prior to endoscopic resection. However, conventional endoscopic findings should be applied complementary with other techniques. Also, a histologic diagnosis from EFB samples provides the most reliable information for the diagnosis of gastric neoplasia prior to complete resection. Endoscopic findings should be used as additional information.
Applications
In patients with early gastric cancer, the method of resection is determined depending on whether or not lymph node metastasis is present. In the present study, 53.6% of patients with gastric HGN were shown to have gastric cancer after ESD. Therefore, the authors suggested that the need to evaluate potential lymph node metastasis should be considered before endoscopic resection for predicted EGC. In patients with HGN by EFB, there is no standard pre-resection diagnostic, and pre-resection evaluation for invasion and metastasis in patients with HGN is not recommended. In present study, HGN in lesions with red color change and mucosal ulceration is correlated with the presence of gastric cancer. Therefore, these findings suggesting gastric cancer may help to determine the diagnosis and guide treatment in patients with HGN.
Terminology
The authors classified lesions into two groups according to histologic discrepancies between the biopsy and ESD diagnosis. LGN and HGN in the final diagnosis by ESD specimens were clustered into one category termed “adenoma group”. Lesions with coexisting cancer after ESD were defined as “cancer group”. That is, the “adenoma group” had lesions without coexisting cancer and the “cancer group” included lesions with coexisting foci of cancer.
Peer review
This is a retrospective study of patients with gastric lesions where biopsies showed gastric adenoma with HGN. Half of these patients turned out to have cancer and endoscopic features in this group were compared with those who didn’t have cancer.
Footnotes
Supported by Grants of the Gachon University Gil Medical Center, No. 2013-35 and 37
P- Reviewer: Kasuga A, Tham TCK S- Editor: Nan J L- Editor: A E- Editor: Ma S
References
- 1.Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol. 1997;28:127–133. doi: 10.1016/s0046-8177(97)90095-2. [DOI] [PubMed] [Google Scholar]
- 2.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001;36:445–456. doi: 10.1007/s005350170067. [DOI] [PubMed] [Google Scholar]
- 4.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fertitta AM, Comin U, Terruzzi V, Minoli G, Zambelli A, Cannatelli G, Bodini P, Bertoli G, Negri R, Brunati S. Clinical significance of gastric dysplasia: a multicenter follow-up study. Gastrointestinal Endoscopic Pathology Study Group. Endoscopy. 1993;25:265–268. doi: 10.1055/s-2007-1010311. [DOI] [PubMed] [Google Scholar]
- 6.Rugge M, Farinati F, Baffa R, Sonego F, Di Mario F, Leandro G, Valiante F. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology. 1994;107:1288–1296. doi: 10.1016/0016-5085(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 7.Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396. doi: 10.1055/s-2004-814330. [DOI] [PubMed] [Google Scholar]
- 8.Yoon WJ, Lee DH, Jung YJ, Jeong JB, Kim JW, Kim BG, Lee KL, Lee KH, Park YS, Hwang JH, et al. Histologic characteristics of gastric polyps in Korea: emphasis on discrepancy between endoscopic forceps biopsy and endoscopic mucosal resection specimen. World J Gastroenterol. 2006;12:4029–4032. doi: 10.3748/wjg.v12.i25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CG. Tissue acquisition in gastric epithelial tumor prior to endoscopic resection. Clin Endosc. 2013;46:436–440. doi: 10.5946/ce.2013.46.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YJ, Park JC, Kim JH, Shin SK, Lee SK, Lee YC, Chung JB. Histologic diagnosis based on forceps biopsy is not adequate for determining endoscopic treatment of gastric adenomatous lesions. Endoscopy. 2010;42:620–626. doi: 10.1055/s-0030-1255524. [DOI] [PubMed] [Google Scholar]
- 11.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 13.Min BH, Kim KM, Kim ER, Park CK, Kim JJ, Lee H, Lee JH, Chang DK, Kim YH, Rhee PL, et al. Endoscopic and histopathological characteristics suggesting the presence of gastric mucosal high grade neoplasia foci in cases initially diagnosed as gastric mucosal low grade neoplasia by forceps biopsy in Korea. J Gastroenterol. 2011;46:17–24. doi: 10.1007/s00535-010-0289-2. [DOI] [PubMed] [Google Scholar]
- 14.Misdraji J, Lauwers GY. Gastric epithelial dysplasia. Semin Diagn Pathol. 2002;19:20–30. [PubMed] [Google Scholar]
- 15.Kato M, Nishida T, Takehara T. Reply to the letter by L. Czako et al. regarding “Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group”. J Gastroenterol. 2012;47:349–349. doi: 10.1007/s00535-010-0350-1. [DOI] [PubMed] [Google Scholar]
- 16.Park DI, Rhee PL, Kim JE, Hyun JG, Kim YH, Son HJ, Kim JJ, Paik SW, Rhee JC, Choi KW, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501–506. doi: 10.1055/s-2001-15089. [DOI] [PubMed] [Google Scholar]
- 17.Jung SH, Chung WC, Lee KM, Paik CN, Jung JH, Lee MK, Lee YK, Chung IS. Risk factors in malignant transformation of gastric epithelial neoplasia categorized by the revised Vienna classification: endoscopic, pathological, and immunophenotypic features. Gastric Cancer. 2010;13:123–130. doi: 10.1007/s10120-010-0550-7. [DOI] [PubMed] [Google Scholar]
- 18.Cho SJ, Choi IJ, Kim CG, Lee JY, Kook MC, Park S, Ryu KW, Lee JH, Kim YW. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011;43:465–471. doi: 10.1055/s-0030-1256236. [DOI] [PubMed] [Google Scholar]
- 19.Jung MK, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705–2711. doi: 10.1007/s00464-008-9875-2. [DOI] [PubMed] [Google Scholar]
- 20.Kasuga A, Yamamoto Y, Fujisaki J, Okada K, Omae M, Ishiyama A, Hirasawa T, Chino A, Tsuchida T, Igarashi M, et al. Clinical characterization of gastric lesions initially diagnosed as low-grade adenomas on forceps biopsy. Dig Endosc. 2012;24:331–338. doi: 10.1111/j.1443-1661.2012.01238.x. [DOI] [PubMed] [Google Scholar]
- 21.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 22.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 23.Chung JW, Jung HY, Choi KD, Song HJ, Lee GH, Jang SJ, Park YS, Yook JH, Oh ST, Kim BS, et al. Extended indication of endoscopic resection for mucosal early gastric cancer: analysis of a single center experience. J Gastroenterol Hepatol. 2011;26:884–887. doi: 10.1111/j.1440-1746.2010.06611.x. [DOI] [PubMed] [Google Scholar]
- 24.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 25.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 26.Son HJ, Song SY, Kim S, Noh JH, Sohn TS, Kim DS, Rhee JC. Characteristics of submucosal gastric carcinoma with lymph node metastatic disease. Histopathology. 2005;46:158–165. doi: 10.1111/j.1365-2559.2005.02049.x. [DOI] [PubMed] [Google Scholar]
- 27.Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, Okita K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361–365. doi: 10.1136/gut.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705–713. doi: 10.1055/s-0030-1255617. [DOI] [PubMed] [Google Scholar]
- 29.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917–927. doi: 10.1016/j.gie.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 30.Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video) Endoscopy. 2004;36:1080–1084. doi: 10.1055/s-2004-825961. [DOI] [PubMed] [Google Scholar]


