Abstract
AIM: To investigate the effect of nonsteroidal anti-inflammatory drugs (NSAIDs) on the incidence of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP).
METHODS: Two independent reviewers searched PubMed (1966 to October 2013), Embase (1984 to October 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 4, 2013) for relevant randomized controlled trials (RCTs) studying the effectiveness of prophylactic NSAID administration in the prevention of PEP. Using the Cochrane Collaboration Handbook, meta-analyses were conducted to evaluate the overall effect of NSAIDs in preventing the incidences of PEP and moderate to severe pancreatitis.
RESULTS: Eight RCTs were identified from the literature search and included 1883 patients that underwent ERCP, with 971 patients in the NSAID group and 912 patients in the placebo group. Sixty-nine out of 971 (7.11%) patients developed PEP in the NSAID group in comparison to 143 out of 912 (15.68%) patients in the placebo group. The pooled RR of PEP incidence with prophylactic NSAID administration was 0.43 (95%CI: 0.33-0.56), which demonstrates that NSAID administration after ERCP significantly reduced the incidence of PEP when compared to the placebo group (P < 0.0001). Subgroup analysis was performed and revealed that the presence (NSAID group) or absence (placebo group) of NSAIDs had no significant effect on the development of moderate to severe pancreatitis (RR = 0.79, 95%CI: 0.52-1.18). Moreover, the administration of NSAIDs as a rectal suppository (RR = 0.35, 95%CI: 0.26-0.48; P < 0.0001) was more effective than oral administration (RR = 0.97, 95%CI: 0.53-1.80) or through infusion (RR = 0.43, 95%CI: 0.12-1.54).
CONCLUSION: NSAIDs effectively reduce the incidence of PEP but not of moderate to severe pancreatitis.
Keywords: Nonsteroidal anti-inflammatory drugs, Post-endoscopic retrograde cholangiopancreatography pancreatitis, Randomized controlled trial, Meta-analysis
Core tip: This meta-analysis was designed to compare the incidence of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) in the presence or absence of prophylactic nonsteroidal anti-inflammatory drug (NSAID) administration after ERCP. A total of eight studies were included in the pooled analysis and contained 1883 patients that underwent ERCP with 971 patients in the NSAID group and 912 patients in the control group. Patients receiving NSAIDs after ERCP had a reduced incidence of PEP when compared with the placebo group, though NSAID administration did not reduce the incidence of moderate to severe pancreatitis.
INTRODUCTION
Acute pancreatitis, a common adverse event occurring after endoscopic retrograde cholangiopancreatography (ERCP), has puzzled endoscopic experts for several years. The incidence of post-ERCP pancreatitis (PEP) ranges from 2.1% to 39%[1]. A majority of PEP episodes are associated with mild pancreatitis. In a small percentage of cases, however, patients develop moderate to severe pancreatitis, which is associated with systemic inflammatory responses and multiple organ failure that ultimately increase the risk for morbidity and mortality. Currently, the pathogenesis of PEP is poorly understood. It is hypothesized that mechanical, thermal, chemical and hydrostatic injuries induce a cascade reaction that leads to intracellular pancreatic enzyme self-activation, ultimately causing autodigestion of the pancreas and inflammation due to the release of bioactive substances[2,3]. Several factors have been associated with an increased risk for PEP such as sphincter of Oddi dysfunction, female sex, precut sphincterotomy, and injection of pancreatic contrast agents[2]. Patients harboring these risk factors are more likely to suffer from PEP[4].
The incidence of PEP accompanied by substantial morbidity or occasional mortality has become an obstacle for clinicians treating patients after ERCP, and effective strategies to prevent PEP still are lacking[5]. Thus, considerable effort has been devoted to developing strategies to reduce or even eliminate the incidence of PEP[6]. Studies and clinical trials have investigated the effects of pancreatic stents, pancreatic enzyme inhibitors, and somatostatin analogues on PEP, but the results are still controversial[7,8].
Previous studies have reported that nonsteroidal anti-inflammatory drug (NSAID) administration may prevent PEP through the inhibition of prostaglandins, phospholipase A2 and neutrophil-endothelial interactions[7,9,10]. Although several systematic reviews have been performed to explore the efficacy of NSAIDs in the prevention of PEP[11-13], the benefit of prophylactic NSAID administration in reduction of PEP incidence is still controversial. To this end, we performed an updated meta-analysis to evaluate the effectiveness of NSAIDs in preventing PEP.
MATERIALS AND METHODS
Search strategy
Clinical research evaluating the effects of prophylactic NSAID administration on PEP incidence was searched from PubMed (1966 to October 2013), Embase (1984 to October 2013) and Cochrane Library biomedical literature databases (CENTRAL; Cochrane Controlled trials Register: Issue 4, 2013) by two independent reviewers (Li X and Tao LP; educated through a series of evidence-based medicine classes) using the following key words: nonsteroidal anti-inflammatory drugs, NSAID, diclofenac, indomethacin, ERCP, endoscopic retrograde cholangiopancreatography, pancreatitis, PEP and post-endoscopic retrograde cholangiopancreatography pancreatitis. To ensure all relevant citations were included in this study, the reference lists from relevant articles were manually screened. This meta-analysis was limited to clinical and human studies.
Inclusion criteria
The following inclusion criteria were applied to select the studies for this meta-analysis: (1) randomized controlled trials (RCTs) involving NSAIDs vs placebo groups in PEP prevention; (2) human studies; (3) participants older than 14 years; (4) patients who had undergone ERCP; and (5) published outcomes assessing the NSAID effectiveness in PEP prevention. The following exclusion criteria were applied: (1) incomplete RCTs; (2) repetitive reports; and (3) different co-interventions between the intervention arms. Two researchers independently reviewed the titles and abstracts of relevant articles based on the aforementioned inclusion and exclusion criteria. Discrepancies encountered by the reviewers were discussed and resolved through consultation with endoscopic experts to reach a consensus.
Data extraction
Relevant data, including number of patients, incidence of PEP, NSAID dose and route of administration, were independently extracted from the selected trials by the two reviewers (Li X and Tao LP). Disagreements or uncertainties were discussed until consensus was achieved.
Subgroup and sensitivity analyses
To evaluate potential clinical heterogeneity, sensitivity and subgroup analyses were performed to identify differences in treatment protocols. The following subgroup and sensitivity analyses were performed: (1) NSAID administration route; (2) PEP definition; (3) research setting; and (4) NSAID dosage.
Publication bias
To determine if publication bias was present, a funnel plot of effect size against sample size was generated for the studies included in the meta-analysis, allowing the log standard error to be mapped against the log odds ratio for each individual study.
Statistical analysis
RevMan 5.0 software (Cochrane Collaboration, Oxford, United States) was used in this meta-analysis to generate fixed-effects and random-effects models according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). Pooled risk ratios (RRs) were calculated using a general inverse variance fixed-effects model. χ2 tests with a P value less than 0.05 and a Higgins I2 value of less than 50% classified the included trials as homogenous. If the chi-square test revealed study heterogeneity (P < 0.05, I2 > 50%), a random-effects model was applied. Pooled RRs were presented as standard plots with 95%CIs. To avoid the possibility of clinical heterogeneity with respect to study population and therapeutic modality, pooling was not implemented and the results were instead assessed by subgroup analyses or descriptive statistics.
RESULTS
Study characteristics and assessment
The initial search of PubMed, Embase and CENTRAL identified 205 relevant articles. After applying the inclusion and exclusion criteria, eight studies were selected for this meta-analysis. The details of study selection are summarized in Figure 1. All eight articles were RCTs that investigated the effect of NSAIDs on PEP prevention[5,9,14-19]. The main characteristics of the eligible studies are presented in Table 1. Quality assessment was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and the details are shown in Table 2. None of the included studies had significant flaws in methodology.
Figure 1.
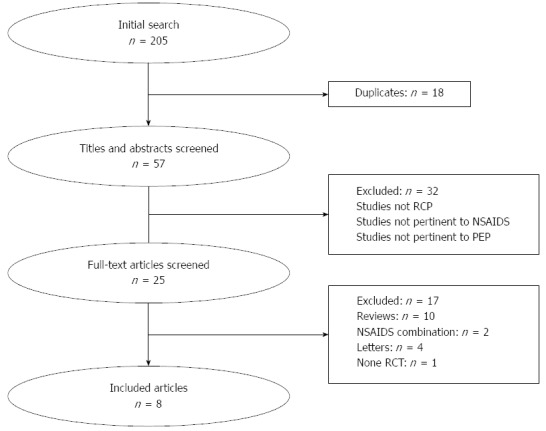
Schematic representation of the article screening process. RCP: Rretrograde cholangiopancreatography; NSAID: Nonsteroidal anti-inflammatory drug; PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Table 1.
Characteristics of the included articles n (%)
| Ref. | Year | Country | Setting | Number of patients | NSAID dose and duration | PEP in NSAID group | PEP in placebo group |
| Murray et al[18] | 2003 | Scotland | Single center | 220 | Suppository, 100 mg after ERCP | 7 (6.4) | 17 (15.5) |
| Cheon et al[17] | 2007 | United States | Single center | 207 | Oral, 50 mg before and after ERCP | 17 (16.2) | 17 (16.7) |
| Sotoudehmanesh et al[9] | 2007 | Iran | Single center | 480 | Suppository, 100 mg before ERCP | 7 (2.8) | 15 (6.1) |
| Montaño Loza et al[19] | 2007 | Mexico | Single center | 150 | Suppository, 100 mg before ERCP | 4 (5.3) | - |
| Khoshbaten et al[16] | 2008 | Iran | Single center | 100 | Suppository, 100 mg after ERCP | 2 (4.0) | 13 (26.0) |
| Senol et al[15] | 2009 | Turkey | Single center | 80 | Infusion, 75 mg after ERCP | 3 (7.5) | 7 (17.5) |
| Elmunzer et al[14] | 2012 | United States | Multi-center | 602 | Suppository, 50 mg after ERCP | 27 (9.1) | 52 (25.1) |
| Otsuka et al[5] | 2012 | Japan | Single center | 104 | Suppository, 50 mg before ERCP | 2 (3.9) | 10 (18.9) |
ERCP: Endoscopic retrograde cholangiopancreatography; NSAID: Nonsteroidal anti-inflammatory drug; PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Table 2.
Quality assessments of the included studies
| Ref. | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome | Incomplete outcome data | Selective reporting |
| Murray et al[18] | Mentioned, not described | Described | Mentioned, not described | Not mentioned | Completed | Not mentioned |
| Cheon et al[17] | Described | Described | Mentioned, not described | Mentioned, not described | Completed | Not mentioned |
| Sotoudehmanesh et al[9] | Mentioned, not described | Mentioned, not described | Mentioned, not described | Described | Completed | Not mentioned |
| Montaño Loza et al[19] | Mentioned, not described | Not mentioned | Not mentioned | Not mentioned | Completed | Not mentioned |
| Khoshbaten et al[16] | Mentioned, not described | Not mentioned | Mentioned, not described | Not mentioned | Completed | Not mentioned |
| Senol et al[15] | Mentioned, not described | Not mentioned | Not mentioned | Not, mentioned | Completed | Not mentioned |
| Elmunzer et al[14] | Mentioned, not described | Not mentioned | Described | Described | Completed | Not mentioned |
| Otsuka et al[5] | Mentioned, not described | Mentioned, not described | Not mentioned | Not mentioned | Completed | Not mentioned |
Meta-analysis of NSAID effectiveness in PEP prevention
The eight included studies had a total of 1883 participants, with 212 suffering from PEP. Of the 212 PEP patients, 69 were in the NSAID group and 143 were in the placebo group. Results of a χ2 test indicated that there was no heterogeneity among the studies (χ2 = 10.68; df = 7, I2 = 34%). Thus, a fixed-effects model was applied and demonstrated that NSAIDs significantly reduced the incidence of PEP when compared to the placebo group (pooled RR = 0.43, 95%CI: 0.33-0.56; P < 0.001) (Figure 2).
Figure 2.
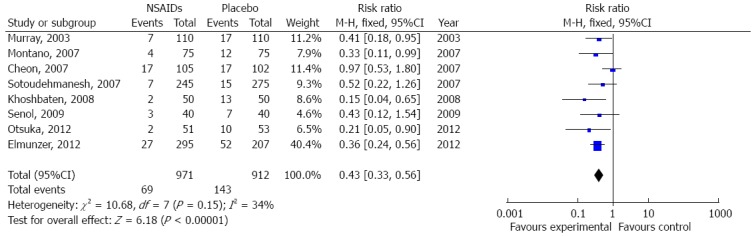
Meta-analysis of the effect of prophylactic nonsteroidal anti-inflammatory drug administration on post-endoscopic retrograde cholangiopancreatography pancreatitis incidence. A fixed-effect model was applied to this pooled meta-analysis, which included eight articles, to analyze the effect of prophylactic nonsteroidal anti-inflammatory drug (NSAID) administration on post-endoscopic retrograde cholangiopancreatography pancreatitis incidence.
Meta-analysis of PEP severity
Six of the eight articles explored the severity of PEP and provided details as to whether it was mild, moderate or severe[5,9,14,17-19]. Two meta-analyses were conducted to evaluate the effect of NSAID administration on PEP severity. The first meta-analysis revealed that prophylactic NSAID administration did not prevent mild PEP when compared with the placebo group (OR = 1.14, 95%CI: 0.91-1.42) (Figure 3). Evaluation of the incidence of moderate to severe PEP in the presence or absence of NSAIDs showed that there was no significant effect on the PEP incidence (pooled OR = 0.79, 95%CI: 0.52-1.18) (Figure 4). There was no significant heterogeneity in the mild PEP cases (df = 5, I2 = 0%) or moderate to severe PEP cases (df = 4, I2 = 0%).
Figure 3.
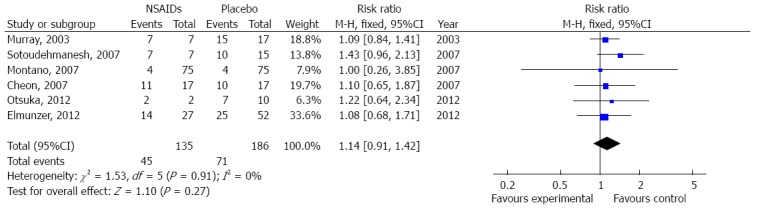
Meta-analysis of the effect of nonsteroidal anti-inflammatory drug administration on mild pancreatitis post endoscopic retrograde cholangiopancreatography. Subgroup-analysis, which included six articles with a fixed-effect model, was performed to analyze the effect of prophylactic nonsteroidal anti-inflammatory drug (NSAID) administration on the incidence of mild pancreatitis.
Figure 4.
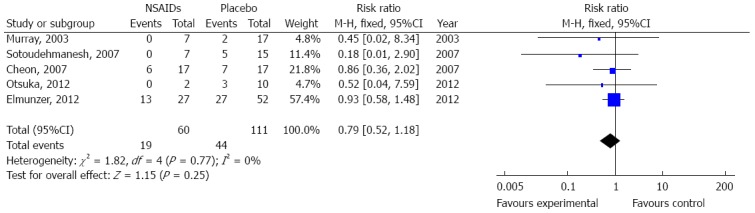
Meta-analysis of the effect of nonsteroidal anti-inflammatory drug administration on moderate to severe pancreatitis post endoscopic retrograde cholangiopancreatography. Subgroup-analysis, which included five articles with a fixed-effect model, was performed to analyze the effect of prophylactic nonsteroidal anti-inflammatory drug (NSAID) administration on the incidence of moderate to severe pancreatitis.
Subgroup and sensitivity analyses
To take into account differences between the included studies, we performed sensitivity and subgroup analyses (Table 3). Different NSAID administration routes were used between the studies. A total of six studies[5,9,14,16,18,19] administered NSAIDs as a rectal suppository and a subgroup analysis revealed that this route of administration significantly reduced PEP incidence (RR = 0.35, 95%CI: 0.25-0.49; P < 0.0001). No significant heterogeneity was identified among these six trials (df = 5, I2 = 0%). Only two articles, however, evaluated oral administration[17] or intramuscular infusion[15] of NSAIDs and these studies contained less than 300 participants. After including the article by Cheon et al[17], a sensitivity analysis was performed and identified significant heterogeneity. The P value for the chi-square test changed from 0.74 to 0.11 and the I2 value changed from 0% to 42%, which demonstrated that heterogeneity existed for this article.
Table 3.
Subgroup and sensitivity analyses to evaluate the effect of nonsteroidal anti-inflammatory drug administration on post-endoscopic retrograde cholangiopancreatography pancreatitis prevention
| Trials | Subgroup (n) | RR (95%CI) | Z | P value |
Heterogeneity |
||
| χ2 | P | I2 (%) | |||||
| The overall effect of NSAIDs on PEP | |||||||
| All forms | 8 studies (1883) | 0.43 (0.33-0.56) | 6.18 | < 0.0001 | 10.68 | 0.15 | 34 |
| Different administration routes | |||||||
| Suppository | 6 studies (1596) | 0.35 (0.26-0.48) | 6.47 | < 0.0001 | 2.72 | 0.74 | 0 |
| Oral | 1 study (207) | 0.97 (0.53-1.80) | 0.09 | 0.93 | - | - | - |
| Infusion | 1 study (80) | 0.43 (0.12-1.54) | 1.30 | 0.19 | - | - | - |
| Different definitions of PEP | |||||||
| The same criteria | 6 studies (1563) | 0.46 (0.34-0.61) | 5.24 | < 0.0001 | 8.38 | 0.14 | 40 |
| Others | 2 studies (320) | 0.30 (0.15-0.61) | 3.31 | 0.001 | 1.38 | 0.24 | 27 |
| Different research settings | |||||||
| Single center | 7 studies (1381) | 0.47 (0.33-0.66) | 4.27 | < 0.0001 | 9.45 | 0.15 | 37 |
| Multi-center | 1 study (502) | 0.36 (0.24-0.56) | 4.61 | < 0.0001 | - | - | - |
| Different dosages | |||||||
| 100 mg | 4 studies (990) | 0.36 (0.22-0.59) | 4.05 | < 0.0001 | 2.15 | 0.54 | 0 |
| 75 mg | 1 study (80) | 0.43 (0.12-1.54) | 1.30 | 0.19 | - | - | - |
| 50 mg | 3 studies (813) | 0.47 (0.33-0.65) | 4.49 | < 0.0001 | 7.91 | 0.02 | 75 |
NSAID: Nonsteroidal anti-inflammatory drug; PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Varying definitions of PEP may affect the pooled effects of included articles. Thus, a sensitivity analysis was performed to evaluate if the definition of PEP affected its incidence with or without NSAID administration. Six of the eight studies defined PEP in accordance with the consensus established by Cotton et al[10]. With this analysis, a significant reduction was found in the incidence of PEP in the NSAID group vs the placebo group (OR = 0.46, 95%CI: 0.34-0.61; P < 0.0001).
Adverse effects of NSAIDs
NSAID-related adverse effects were only reported by Elmunzer et al[14]. Eleven bleeding events were noted in four patients in the NSAID group while seven bleeding events were reported in the placebo group. The risk of adverse effects of NSAID administration with a standard dosage was not significantly increased. Two cases of renal failure without death occurred in the placebo group. Because of the small sample size, related statistical analyses could not be performed to estimate the incidence of adverse effects of NSAIDs on PEP. All enrolled patients in the eight RCTs were discharged in good health.
Publication bias
Publication bias was assessed for all pooled RRs with CIs using a Begg and Mazumdar’s rank correlation test. As shown in Figure 5, there was a low likelihood of publication bias (Egger’s test).
Figure 5.
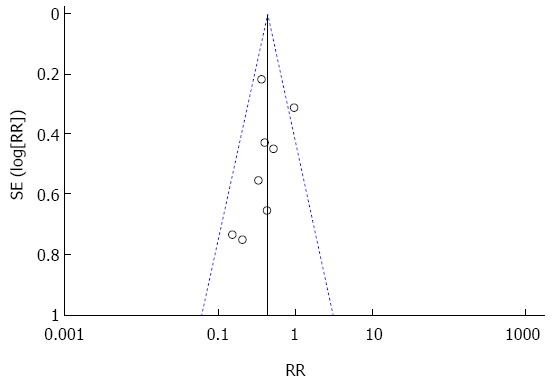
Funnel plot to evaluate the effect of nonsteroidal anti-inflammatory drug administration on post-endoscopic retrograde cholangiopancreatography pancreatitis.
DISCUSSION
The meta-analysis presented here revealed that prophylactic administration of NSAIDs post ERCP reduced the incidence of PEP, though NSAID administration was not associated with the level of PEP severity. These findings are consistent with previously published meta-analyses[12,13,20]. NSAID-associated reduction of PEP incidence was consistent in a majority of the included articles, with the exception of the study by Cheon et al[17]. The findings from this analysis lend strong support to prophylactic NSAID administration to reduce the risk for PEP. Analysis of all included studies revealed no significant difference between the NSAID and placebo groups in PEP severity. We believe that prophylactic NSAID administration for PEP prevention is a feasible, cost-effective and efficient treatment option, especially in poorly equipped hospitals.
Sensitivity analyses to evaluate the effect of NSAID administration route on PEP incidence revealed that administration as a rectal suppository reduced the risk for PEP when compared with oral or intramuscular administration. The heterogeneity resulting from inclusion of the Cheon et al[17] study may be due in part to the administration route used, indicating that oral administration of NSAIDs may differ from other routes. There are several reasons why their study did not find a positive correlation between NSAID administration and reduced PEP incidence. First, the NSAIDs may have been destroyed by the gastric duct acidity when administrated orally. Second, there may have been low NSAID bioavailability due to extensive first-pass metabolism. Finally, the approximate time to serum peak concentration and elimination half-time may have affected NSAID activity. These factors may cause a decrease and inactivation of effective or available NSAIDs, which could ultimately lead to the lack of effect observed on PEP reduction. In the case of Senol et al[15], NSAID effectiveness may be due in part to the small number of patients assayed. Although Cheon et al[17] suggested that differences in administration routes or time to reach peak concentration were not clinically relevant, our sensitivity analyses identified significant heterogeneity due specifically to their results. However, as only eight trials were included, this deduction may be underpowered. Thus, future RCTs should examine NSAID administration route in relation to PEP incidence.
The inhibition of inflammatory signaling by NSAIDs predominantly serves to prevent an inflammatory reaction. Results from this study indicate that prophylactic administration of NSAIDs at conventional dosages did not increase the frequency of adverse effects, consistent with previously published studies[21]. A few previous studies reported that NSAID-related acute pancreatitis and common adverse effects occurred occasionally in the prevention of PEP[22,23]. As ERCP is an invasive procedure, it may induce bleeding, which needs to be discriminated from NSAID-related bleeding. This meta-analysis revealed few adverse effects in patients that prophylactically received NSAIDs, with the exception of four bleeding cases from one study. This low incidence of adverse effects may be due in part to the short-term NSAID administration used for PEP prevention compared with conventional long-term administration of NSAIDs for other issues. We suggest that NSAID administration, specifically diclofenac and indomethacin, in ERCP patients is safe and effective. Although a subgroup analysis was not performed to evaluate differences between diclofenac and indomethacin, a difference would not contribute to significant heterogeneity as these are equivalent in different inflammatory stages[12].
PEP development is the result of iatrogenic injury and activation of pancreatic enzymes, a breakthrough finding for PEP prevention[24]. Several studies have assessed the risk factors for PEP development, including patient-related, procedure-related and operator-related factors[25]. Murray et al[18] and Khoshbaten et al[16] demonstrated a statistically significant benefit in patients at high risk for PEP who received NSAIDs. Alternatively, Sotoudehmanesh et al[9] found that only patients receiving a pancreatic duct injection obtained significant benefits from NSAID administration, whereas Otsuka et al[5] only observed benefits in sphincterotomized patients. Therefore, a collective analysis of the results suggests that prophylactic NSAID administration yields significant benefits for high-risk patients.
Significant efforts have been devoted to reducing the incidence of PEP. Sphincter spasms, trypsin activation, pancreatic secretion, inflammation and cytokine cascades have received significant attention[26]. Recent studies have suggested that pancreatic stent placement is the most effective measure in preventing PEP[27]. However, stent placement requires extensive equipment and experienced endoscopists, difficult features to obtain in comparison to drug administration[28]. Animal models and human studies have been developed to identify new forms of pharmacotherapy, though effective drugs have yet to be confirmed. According to RCTs and meta-analyses, a majority of the drugs that were initially promoted as effective were later found to be ineffective (e.g., ulinastatin and corticosteroids). The effects of other drugs, such as nitroglycerine and gabexate mesylate, remain controversial[26]. Moreover, the combination of therapeutic agents with stent placement may reduce the risk for complications after ERCP.
In comparison to previous meta-analyses on this subject, the present meta-analysis includes more recent, high quality RCTs to enhance the evaluation of the effect of NSAIDs on PEP incidence. Moreover, comprehensive subgroup analyses were performed to detect potential differences among the studies. NSAID administration route was consequently identified as a factor that affects PEP incidence, with suppository administration being beneficial in preventing PEP. However, this meta-analysis also had several limitations, such as the inclusion of only eight studies, which is considered lower quality according to the Cochrane Handbook for Systematic Reviews of Interventions. From these studies, only one article[17] described the generation of random sequences and three articles[16-18] described allocation concealment. Guided by our pre-established criteria, the majority of these eight articles could not be classified as low risk due to missing details in their methods sections. Thus, more attention should be paid to the quality of the methodology in future studies. Another limitation is the varying definition of pancreatitis applied by each study, which has led to inclusion of patients that were diagnosed with pancreatitis on the basis of hyperamylasemia and abdominal pain alone. To some extent, this likely influenced the incidence of pancreatitis among the NSAID and placebo groups. Therefore, standardized diagnostic methods should be applied (e.g., B-ultrasonography and CT) to ensure a proper and consistent diagnosis of pancreatitis.
CONCLUSION
In summary, the comprehensive meta-analysis and subgroup analyses presented here provide updated pooled evidence on the benefits of NSAID administration in the prevention of PEP. We recommend administering NSAIDs before or post ERCP to prevent PEP. Prophylactic NSAID administration in preventing PEP is effective, safe and economical. Future research should involve larger, multi-center RCTs to confirm the effect of prophylactic NSAIDs on the incidence of PEP.
COMMENTS
Background
Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) is a common complication in patients who have undergone ERCP. Although randomized controlled trials have been implemented to study the effects of prophylactic nonsteroidal anti-inflammatory drug (NSAID) administration on PEP incidence, clinical application remains controversial.
Research frontiers
Administration of NSAIDs as a prophylactic measure to prevent PEP in patients that have undergone ERCP is still controversial. Some studies have indicated an increased risk for bleeding, though whether this bleeding is due to surgical trauma rather than NSAID administration has not been thoroughly evaluated. Additionally, the administration route and dosage of prophylactic NSAIDs still remain unevaluated. Thus, a meta-analysis of the existing randomized controlled trials on NSAID administration and PEP incidence is beneficial.
Innovations and breakthroughs
This study examined eight randomized controlled trials and used meta-analysis to demonstrate that prophylactic NSAID administration decreases the incidence of PEP. Subgroup analyses demonstrated that NSAID administration as a rectal suppository is more beneficial in reducing PEP incidence than oral or intramuscular administration routes. NSAID administration did not affect the proportion of moderate to severe PEP cases when compared to the placebo group.
Applications
This meta-analysis provides stronger evidence on the positive effects of prophylactic NSAID administration on the incidence of PEP and indicates that rectal suppository administration of NSAIDs was most beneficial.
Terminology
PEP is a common complication of ERCP and can lead to significant increases in morbidity and mortality when pancreatitis is moderate to severe. Meta-analysis is a statistical tool that pools quantitative data from separate but similar studies to examine strong overall effects of interest. Sensitivity analysis quantitatively analyzes the stability of these overall effects. Subgroup analysis explores the overall effects in subsets of the study data to analyze heterogeneity among the studies.
Peer review
This meta-analysis was designed to assess the effect of prophylactic NSAID administration on the incidence of PEP after ERCP. This study found that PEP incidence is reduced when NSAIDs were administered and, through subgroup analysis, rectal suppository administration of NSAIDs was identified as the most beneficial administration route. This study is well designed and of great use to clinicians.
Footnotes
P- Reviewer: De Silva AP, Lorenzo-Zuniga V S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Lazaraki G, Katsinelos P. Prevention of post ERCP pancreatitis: An overview. Ann Gastroenterol. 2008;21:27–38. [Google Scholar]
- 2.Donnellan F, Byrne MF. Prevention of Post-ERCP Pancreatitis. Gastroenterol Res Pract. 2012;2012:796751. doi: 10.1155/2012/796751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhasin DK, Rana SS, Nadkarni N. Protocol-based management strategy for post-endoscopic retrograde cholangiopancreatography pancreatitis: can it make a difference? J Gastroenterol Hepatol. 2008;23:344–347. doi: 10.1111/j.1440-1746.2008.05349.x. [DOI] [PubMed] [Google Scholar]
- 4.Testoni PA. Pharmacological prevention of post-ERCP pancreatitis: the facts and the fiction. JOP. 2004;5:171–178. [PubMed] [Google Scholar]
- 5.Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, et al. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47:912–917. doi: 10.1007/s00535-012-0554-7. [DOI] [PubMed] [Google Scholar]
- 6.Badalov N, Tenner S, Baillie J. The Prevention, recognition and treatment of post-ERCP pancreatitis. JOP. 2009;10:88–97. [PubMed] [Google Scholar]
- 7.Dumonceau JM. How to prevent post-ERCP pancreatitis? Acta Gastroenterol Belg. 2011;74:543–547. [PubMed] [Google Scholar]
- 8.Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119–1124. doi: 10.1097/MEG.0b013e3282f16206. [DOI] [PubMed] [Google Scholar]
- 9.Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978–983. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 10.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 11.Pezzilli R, Cariani G, Santini D, Calculli L, Casadei R, Morselli-Labate AM, Corinaldesi R. Therapeutic management and clinical outcome of autoimmune pancreatitis. Scand J Gastroenterol. 2011;46:1029–1038. doi: 10.3109/00365521.2011.584896. [DOI] [PubMed] [Google Scholar]
- 12.Elmunzer BJ, Waljee AK, Elta GH, Taylor JR, Fehmi SM, Higgins PD. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut. 2008;57:1262–1267. doi: 10.1136/gut.2007.140756. [DOI] [PubMed] [Google Scholar]
- 13.Dai HF, Wang XW, Zhao K. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2009;8:11–16. [PubMed] [Google Scholar]
- 14.Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senol A, Saritas U, Demirkan H. Efficacy of intramuscular diclofenac and fluid replacement in prevention of post-ERCP pancreatitis. World J Gastroenterol. 2009;15:3999–4004. doi: 10.3748/wjg.15.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoshbaten M, Khorram H, Madad L, Ehsani Ardakani MJ, Farzin H, Zali MR. Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol. 2008;23:e11–e16. doi: 10.1111/j.1440-1746.2007.05096.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, Schmidt S, Lazzell-Pannell L, Lehman GA. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc. 2007;66:1126–1132. doi: 10.1016/j.gie.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Murray B, Carter R, Imrie C, Evans S, O’Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–1791. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 19.Montaño Loza A, Rodríguez Lomelí X, García Correa JE, Dávalos Cobián C, Cervantes Guevara G, Medrano Muñoz F, Fuentes Orozco C, González Ojeda A. [Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes] Rev Esp Enferm Dig. 2007;99:330–336. doi: 10.4321/s1130-01082007000600005. [DOI] [PubMed] [Google Scholar]
- 20.Zheng MH, Meng MB, Gu DN, Zhang L, Wu AM, Jiang Q, Chen YP. Effectiveness and tolerability of NSAIDs in the prophylaxis of pancreatitis after endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis. Curr Ther Res Clin Exp. 2009;70:323–334. doi: 10.1016/j.curtheres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 22.SUSSMAN S. Severe salicylism and acute pancreatitis. Calif Med. 1963;99:29–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra M. Toxicity of indomethacin. Report of a case of acute pancreatitis. JAMA. 1967;200:552–553. [PubMed] [Google Scholar]
- 24.Wagh MS, Sherman S. Indomethacin for post-ERCP pancreatitis prophylaxis: another attempt at the Holy Grail. Am J Gastroenterol. 2007;102:984–986. doi: 10.1111/j.1572-0241.2007.01163.x. [DOI] [PubMed] [Google Scholar]
- 25.Spanier BW, Tuynman HA, van der Hulst RW, Dijkgraaf MG, Bruno MJ. Acute pancreatitis and concomitant use of pancreatitis-associated drugs. Am J Gastroenterol. 2011;106:2183–2188. doi: 10.1038/ajg.2011.303. [DOI] [PubMed] [Google Scholar]
- 26.Manes G. Prevention of ERCP-induced pancreatitis. Int Ther Gastrointest Endosc. 2010;27:311–318. [Google Scholar]
- 27.Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354–1365. doi: 10.1016/j.cgh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Dumonceau JM, Rigaux J, Kahaleh M, Gomez CM, Vandermeeren A, Devière J. Prophylaxis of post-ERCP pancreatitis: a practice survey. Gastrointest Endosc. 2010;71:934–939, 939.e1-2. doi: 10.1016/j.gie.2009.10.055. [DOI] [PubMed] [Google Scholar]


