Abstract
Late-stage gastric adenocarcinoma patients have a poor prognosis because of high recurrence rates. To improve long-term outcomes, perioperative chemotherapies are combined with surgery. Human epidermal growth factor receptor 2 (HER2) overexpression had been noted in gastric cancer; therefore, trastuzumab has been used occasionally in this setting. A 63-year-old male Chinese patient, who was diagnosed with adenocarcinoma in the gastric antrum, as well as lymph node metastases along the left gastric and hepatic artery, and left adrenal area, was admitted to our hospital. HER2 expression was positive, and cluster amplification was detected in a fluorescence in situ hybridization assay. The patient received three cycles of a neoadjuvant trastuzumab/oxaliplatin /capecitabine regimen. He subsequently underwent distal gastrectomy, D2+ lymphadenectomy, left adrenalectomy, cholecystectomy and Billroth II anastomosis. Treatment was continued with another five postoperative cycles of the same medication and trastuzumab application for 1 year. No recurrence has been observed 18 mo after the operation. Trastuzumab as perioperative and adjuvant medication, in combination with oxaliplatin and capecitabine for a HER2-overexpressing advanced gastric adenocarcinoma, led to recurrence-free survival of at least 18 mo after surgery.
Keywords: Gastric adenocarcinoma, Trastuzumab, Oxaliplatin, Capecitabine, Neoadjuvant medication
Core tip: Surgical resection of advanced gastric cancer is accompanied by a high frequency of recurrences and metastases. To curb cancerous cell growth, cytostatic medications are commonly used; however, tumor cell-specific drugs are being researched increasingly. Human epidermal growth factor receptor 2 (HER2) has been recognized as a target for breast cancer medications, and the application of the monoclonal antibody trastuzumab led to remissions of HER2-positive breast cancers, which comprise about 30% of all cases. HER2 overexpression was also detected in gastric cancers; therefore, trastuzumab as an adjuvant or neoadjuvant therapy has been used as a new approach to treat these tumors.
INTRODUCTION
Surgical resection of early gastric cancer is very effective; however, in advanced stages, the overall postoperative 5-year survival rate is reduced to 5%-20%, because of high frequencies of locoregional recurrences and distant metastases[1]. To improve the treatment outcomes of operable gastric cancers in later stages, perioperative chemotherapies have been combined with surgery, leading to significantly improved progression-free and overall survival rates[2]. Although perioperative chemotherapies are widely accepted as beneficial adjuncts to surgery when combined with other gastric cancer treatments, currently there is no common consensus regarding the ideal regimen[3]. In addition, human epidermal growth factor receptor 2 (HER2) overexpression had been reported in gastric cancers; therefore, trastuzumab has been introduced into chemotherapies; however, its efficacy is still under evaluation[4,5], and published data about targeting HER2 in perioperative regimens are scarce.
CASE REPORT
A 63-year-old male patient with a family history of gastric cancer was admitted to the local hospital on July 17th, 2012, with complaints of acid reflux, anorexia and upper abdominal fullness for more than 1 year. His mother died of gastric cancer, and his sister and brother were both operated on for gastric cancer. Gastroscopy images showed antral deformation and massive ulcers covered with a white smear. The surrounding mucosa was irregular, with a rigid texture, while peristalsis disappeared, and lesions involving the distal antrum, pylorus and gastric angle had led to pyloric deformation as well as stenosis. A pathological examination revealed a moderately differentiated adenocarcinoma in the gastric antrum, while HER2 expression was detected, with cluster amplification in a fluorescence in situ hybridization assay (Figure 1). Positron emission tomography-computed tomography (PET-CT) and enhanced abdominal CT showed lymph node metastasis along the left gastric and hepatic arteries. There was also a mass detected in the left adrenal area, which was suspected to comprise fused lymph nodes. The serum levels of carcino-embryonic antigen (CEA) and alpha-fetoprotein (AFP) were markedly elevated, at 137 and 1693 ng/mL, respectively. The initial diagnosis was antral carcinoma with lymph node metastasis in the perigastric and left adrenal areas. The patient received a XELOX (capecitabine plus oxaliplatin) regimen, in combination with Herceptin, as a neoadjuvant therapy. The XELOX medication comprised oxaliplatin 130 mg/m2 on day 1 and Xeloda 1000 mg/m2 po bid on days 1 to 14, repeated every 3 wk. Herceptin was given at a dose of 8 mg/kg for the first week and then 6 mg/kg every 3 wk. Efficacy evaluation by reviewing abdominal CTs showed a partial response after the patient finished 3 cycles of combination therapy (Figure 2). The serum level of CEA dramatically declined to 24 ng/mL and that of AFP to 21 ng/mL. Subsequently, the patient underwent distal gastrectomy, D2+ lymphadenectomy, left adrenalectomy, cholecystectomy, and Billroth II anastomosis by laparotomy. Intraoperative exploration showed that a 5 cm × 5 cm × 3 cm tumor located in the antrum, invading the pylorus and duodenal ampulla, with multiple lymphadenectasis along the gastric hepatoduodenal ligament and retroperitoneal area. The mass in the left adrenal area, which was 5 cm × 5 cm × 3 cm in size, was confirmed to be fused enlarged lymph nodes. There were no obvious metastatic nodules found in the liver, peritoneum and pelvic floor. The pathological diagnosis was poorly to moderately differentiated ulcerative adenocarcinoma of the gastric antrum. The tumor grew along the lesser curvature with degeneration and infiltration of the superficial muscle layer. Peritumoral fibroplasia and inflammatory cell infiltration were observed, which were consistent with post-chemotherapy changes. In addition, malignant cells were detected in the lesser curvature lymph node (LN) (6/7), pyloric LN (2/3), greater curvature LN (3/4), and posterior pancreas head LN (1/1). Other lymph nodes were negative, and no cancer cells were found in the left adrenal gland. The patient received another five cycles of XELOX and Herceptin regimen after recovery from the operation, and Herceptin treatment was continued for 1 year. The main adverse effects related to chemotherapy were grade 1 or 2 neutropenia and nausea. No obvious adverse cardiac events were detected. The patient refused postoperative radiotherapy but returned for regular follow-ups every 3-6 mo. He has remained recurrence free 18 mo after the gastrectomy.
Figure 1.
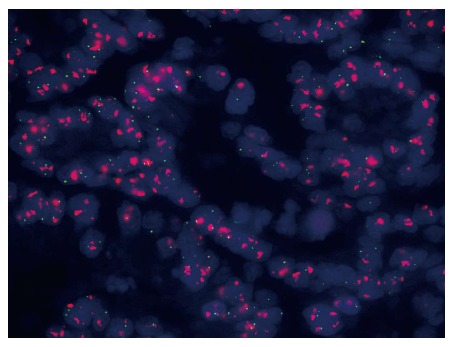
Fluorescence in situ hybridization analysis showing positive human epidermal growth factor receptor 2 expression with cluster amplification (ratio > 2.2).
Figure 2.
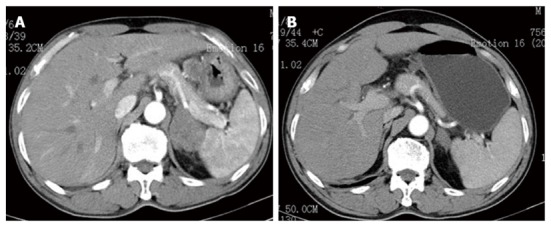
Radiological evaluation by computed tomography scan before (A) and after three cycles (B) of neoadjuvant therapy of trastuzumab combined with oxaliplatin and capecitabine. The thickness of the stomach wall and the size of the lymph nodes had decreased dramatically.
DISCUSSION
Although surgery remains the primary treatment modality for gastric cancer, as part of a comprehensive treatment regimen, the rate of local recurrence approaches 50% in advanced gastric cancer, even after radical resection, because of the biological characteristics of the tumors[6]. Therefore, integrated treatment models are used to improve the outcome in advanced gastric cancer. The aims of neoadjuvant chemotherapy are tumor down-staging, improved resection rates and decreasing the recurrence rate of metastases. A meta-analysis of nine clinical trials was presented at ASCO 2007[7], in which 2102 patients were enrolled, with a median follow-up time of 5.3 years, comparing outcomes between surgery with preoperative chemotherapy and surgery alone. The analysis revealed a statistically significant benefit in favor of adjunctive preoperative chemotherapy, with a 4% increase in 5-year survival rates (HR = 0.87, P = 0.003) and a 5% increase in R0 resection rate (67% vs 62%, P = 0.03). In the MAGIC trial[2], 503 patients were randomly assigned to receive perioperative chemotherapy or surgery alone. The results showed that the pathological staging of patients in the surgery group was significantly higher compared with those in the perioperative chemotherapy group. Among all patients undergoing resection, there was a greater proportion of stage T1 and T2 tumors in the perioperative chemotherapy group than in the surgery group (51.7% vs 36.8%, P = 0.002). The resection rate was also notably higher in the perioperative chemotherapy group than in the surgery group. Among patients treated by radical surgery, resection was considered curative by the operating surgeon in 169 of 213 patients (79.3%) in the perioperative chemotherapy group compared with 166 of 236 patients (70.3%) in the surgery group (P = 0.03).
HER2 is overexpressed in different kinds of tumors, promoting tumorigenesis, progression and metastasis[8]. High HER2 expression was first detected in gastric cancer by Fukushige et al[9] in 1986. Since then, HER2 amplification or overexpression has been reported in 7%-34% of gastric tumors, with different expression rates in different sites[10]. ToGA was the first international, multicenter, randomized phase III clinical trial to investigate the efficacy of trastuzumab for locally advanced, recurrent or metastatic inoperable gastric cancers. The trastuzumab group showed a 26% reduced mortality rate, a 2.7-mo increase in overall survival time and a 12% increase in objective response rates[11]. In our case report, the patient was diagnosed with gastric cancer with multiple lymph node metastases along the left gastric and hepatic artery, and at the left adrenal area; therefore, surgery alone was not the first option. The patient was treated with trastuzumab and XELOX for three cycles and demonstrated a partial response. After successful surgical resection, the pathology report showed fibroplasia and inflammatory cell infiltrations, indicating chemotherapy-related changes. In our case, we used a perioperative trastuzumab/oxaliplatin/capecitabine regimen in an advanced gastric cancer patient, which resulted in an improved preoperative condition and successful resection without recurrence for 1 year after treatment. Wang et al[12] and Sbitti et al[13] had reported successful neoadjuvant/perioperative trastuzumab medications for advanced gastric cancer patients. Furthermore, there is the ongoing phase II NeoHx study (NCT01130337)[14], the preliminary results of which were reported at this year’s ASCO meeting. In the NeoHx study, 36 HER2-overexpressed gastric cancer patients were enrolled to receive perioperative treatment with trastuzumab in combination with capecitabine and oxaliplatin. The R0 resection rate was 78%, and the pCR rate was 19%. These results highlight the usefulness of perioperative HER2 targeting. Trastuzumab neoadjuvant treatment for HER2-positive gastric cancers should be further investigated by randomized, controlled phase III clinical trials.
COMMENTS
Patient characteristics
A 63-year-old male with a family history of gastric cancer complaining of acid reflux, anorexia and upper abdominal fullness.
Clinical diagnosis
Adenocarcinoma in the gastric antrum, with lymph node metastases along the left gastric and hepatic artery, and left adrenal area.
Differential diagnosis
All other related diseases could be excluded.
Laboratory tests
White blood cell 5200/μL; hemoglobin 101 g/L; carcino-embryonic antigen 137 ng/mL; alpha-fetoprotein 1693 ng/mL; hepatic and renal function tests were within normal limits.
Imaging results
Gastroscopy images showed antral deformation and massive ulcers covered with a white smear. The surrounding mucosa was irregular with a rigid texture, and there were lesions involving the distal antrum, pylorus and gastric angle. Positron emission tomography-computed tomography (PET-CT) and an enhanced abdominal CT showed lymph node metastasis along the left gastric and hepatic arteries.
Pathology results
A moderately differentiated adenocarcinoma in the gastric antrum with human epidermal growth factor receptor 2 (HER2) expression was detected. Cluster amplification was demonstrated in a fluorescence in situ hybridization assay.
Treatment
Neoadjuvant capecitabine/oxaliplatin regimen in combination with herceptin, followed by surgical resection.
Related reports
It is generally accepted that successful surgical treatment of advanced gastric cancers relies on preoperative chemotherapies. Combining conventional cytostatic drugs with herceptin for HER2-positive tumors is still in the experimental phase.
Experiences and lessons
The usefulness of perioperative HER2 targeting and trastuzumab neoadjuvant treatment for HER2-positive gastric cancers should be further investigated by randomized, controlled phase III clinical trials.
Peer review
Accumulation of successful cases with HER2 positive gastric cancer treated with preoperative trastuzumab and standard chemotherapy is important for clinical practice.
Footnotes
P- Reviewer: Braat H, Ikuta S, Yokoyama Y S- Editor: Gou SX L- Editor: Stewart GJ E- Editor: Ma S
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Shah MA. Unanswered questions in the management of gastroesophageal junction adenocarcinoma: an overview from the medical oncologist’s perspective. Am Soc Clin Oncol Educ Book. 2013 doi: 10.14694/EdBook_AM.2013.33.e155. [DOI] [PubMed] [Google Scholar]
- 4.Gunturu KS, Woo Y, Beaubier N, Remotti HE, Saif MW. Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Ther Adv Med Oncol. 2013;5:143–151. doi: 10.1177/1758834012469429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner M, Laßmann S. [Update on Barrett esophagus and Barrett carcinoma] Pathologe. 2012;33 Suppl 2:253–257. doi: 10.1007/s00292-012-1662-0. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi K, Phan A, Ajani JA. Gastric cancer: advances in adjuvant and adjunct therapy. Curr Treat Options Oncol. 2003;4:413–419. doi: 10.1007/s11864-003-0042-7. [DOI] [PubMed] [Google Scholar]
- 7.Thirion P, Michiels S, Le Maitre A, Tierney J. Individual patient data-based meta-analysis assessing pre-operative chemotherapy in resectable oesophageal carcinoma. J Clin Oncol. 2007;25:4512. [Google Scholar]
- 8.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 9.Fukushige S, Matsubara K, Yoshida M, Sasaki M, Suzuki T, Semba K, Toyoshima K, Yamamoto T. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955–958. doi: 10.1128/mcb.6.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Saukel GW, Garberoglio CA, Srikureja W, Hsueh CT. Pathological complete response after neoadjuvant chemotherapy with trastuzumab-containing regimen in gastric cancer: a case report. J Hematol Oncol. 2010;3:31. doi: 10.1186/1756-8722-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sbitti Y, Essaidi I, Debbagh A, Kadiri H, Oukabli M, Moussaid Y, Slimani K, Fetohi M, Elkaoui H, Albouzidi A, et al. Is there any advantage to combined trastuzumab and chemotherapy in perioperative setting her 2neu positive localized gastric adenocarcinoma? World J Surg Oncol. 2011;9:112. doi: 10.1186/1477-7819-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernando R, Paula J, Pilar GA, Carlos L, Javier G, Maria A, Luis LG, Maica G, Esther F, Jose L, et al. NeoHx study: Perioperative treatment with trastuzumab in combination with capecitabine and oxaliplatin (XELOX-T) in patients with HER2 resectable stomach or esophagogastric junction (EGJ) adenocarcinoma—R0 resection, pCR, and toxicity analysis. J Clin Oncol. 2013;31(suppl):abstr 4098. [Google Scholar]


