Abstract
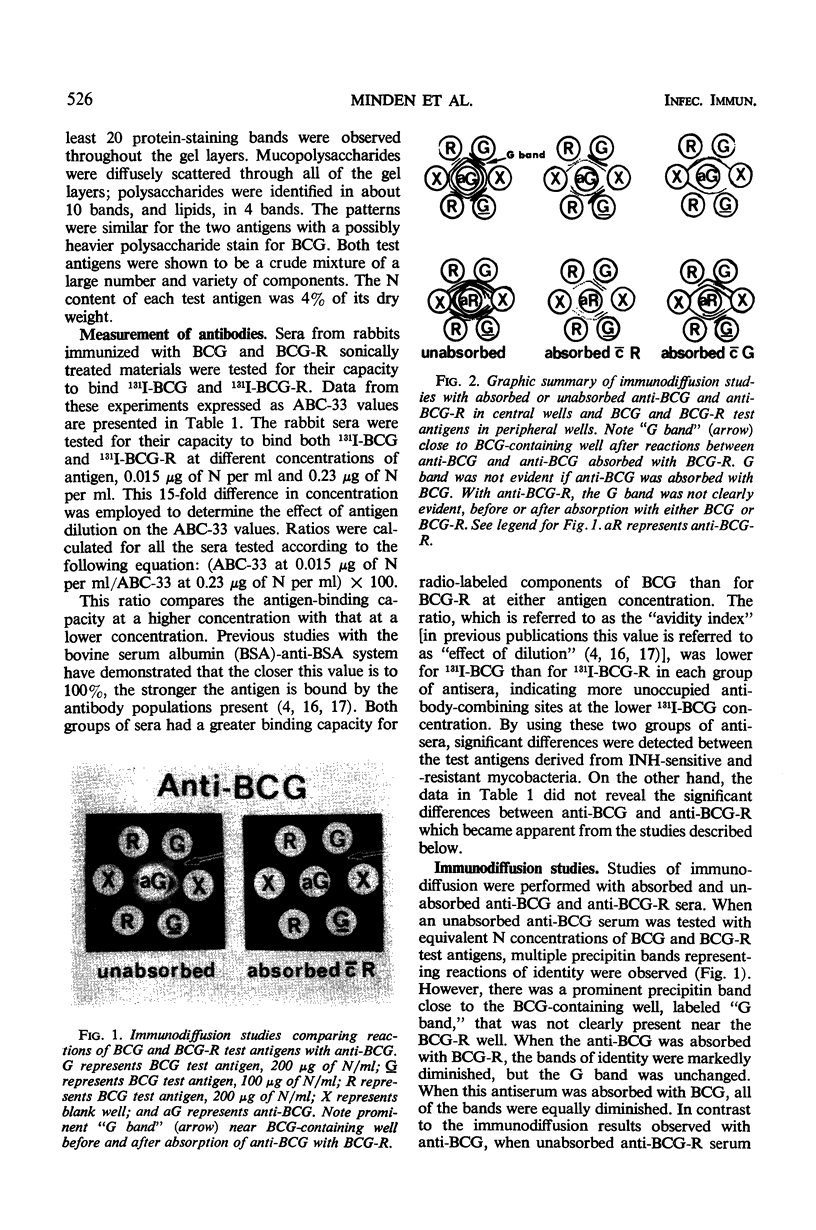
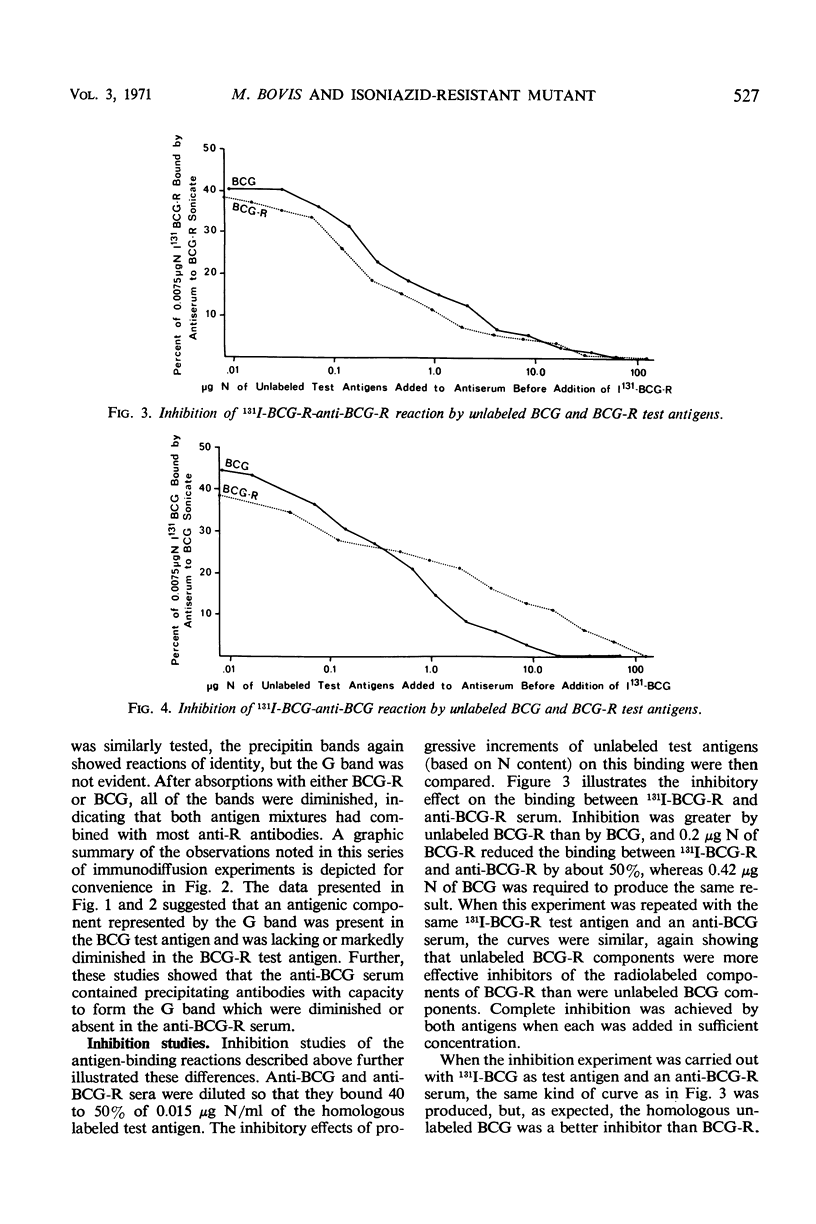
The antigenicities of Mycobacterium bovis strain BCG and an isoniazid (INH) - resistant mutant were compared. Sonic extracts of heat-killed, washed bacteria were injected into rabbits, and antisera were produced (anti-BCG and anti-BCG-R). The sonic extracts were also subjected to ultracentrifugation, and test antigens were prepared from the resulting supernatants (BCG and BCG-R). The antigens were labeled with 131I, and the antisera were tested for their capacity to bind 131I-BCG and 131I-BCG-R by precipitating 131I-antigen-antibody complexes with anti-rabbit gamma globulin. Significant differences were detected between the two test antigens. Immunodiffusion studies with unabsorbed antisera and antisera absorbed with BCG and BCG-R suggested that an antigenic component in BCG is lacking or diminished in BCG-R and that anti-BCG has antibodies not found in anti-BCG-R. These quantitative and qualitative differences were confirmed by inhibition studies. Complete inhibition was achieved in all systems by both test antigens when either was added in sufficient concentration. As expected, BCG-R inhibited the binding of 131I-BCG-R by anti-BCG-R or anti-BCG (or both) to a greater extent than BCG, and BCG was a better inhibitor in an 131I-BCG-anti-BCG-R system. However, in an 131I-BCG-anti-BCG test system, BCG was the better inhibitor only at high concentrations. It was concluded that the test antigens possess different proportions of identical or cross-reacting components and that INH-sensitive organisms possess a component(s) that is diminished or absent in the INH-resistant mutant. Whether this component(s) is responsible for INH-resistance is not known.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester L., Gaudier B. Etude immunologique du B.C.G. par la méthode d'immunodiffusion. 1. Analyse d'un sérum anti-B.C. Ann Inst Pasteur Lille. 1970;21:91–100. [PubMed] [Google Scholar]
- Bayer M. E., Anderson T. F. The surface structure of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1592–1599. doi: 10.1073/pnas.54.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. L., Waggoner R. F., McClatchy J. K. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis. 1968 Aug;98(2):295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- FERRARI A. Nitrogen determination by a continuous digestion and analysis system. Ann N Y Acad Sci. 1960 Jul 22;87:792–800. doi: 10.1111/j.1749-6632.1960.tb23236.x. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Improved surface culturing of Mycobacterium tuberculosis. J Bacteriol. 1967 Oct;94(4):1258–1259. doi: 10.1128/jb.94.4.1258-1259.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDGECOCK L. W., FAUCHER I. O. Relation of pyrogallol-peroxidative activity to isoniazid resistance in Mycobacterium tuberculosis. Am Rev Tuberc. 1957 Apr;75(4):670–674. doi: 10.1164/artpd.1957.75.4.670. [DOI] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LINSCOTT W. D. CONTAMINATION OF COMMERCIAL RABBIT ALBUMIN PREPARATIONS BY BOVINE ALBUMIN. Science. 1963 Nov 29;142(3596):1170–1172. doi: 10.1126/science.142.3596.1170. [DOI] [PubMed] [Google Scholar]
- Lidd D., Farr R. S. Some antigenic relationships between crude extracts and purified fractions of ragweed pollen. J Allergy. 1966 Jan;37(1):54–63. doi: 10.1016/0021-8707(66)90110-9. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Isoniazid-resistance and catalase activity of tubercle bacilli; a preliminary report. Am Rev Tuberc. 1954 Mar;69(3):471–472. doi: 10.1164/art.1954.69.3.471. [DOI] [PubMed] [Google Scholar]
- McClatchy J. K. Mechanism of Action of Isoniazid on Mycobacterium bovis Strain BCG. Infect Immun. 1971 Apr;3(4):530–534. doi: 10.1128/iai.3.4.530-534.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Minden P., Anthony B. F., Farr R. S. A comparison of seven procedures to detect the primary binding of antigen by antibody. J Immunol. 1969 Apr;102(4):832–841. [PubMed] [Google Scholar]
- Minden P., Farr R. S. Binding between components of the tubercle bacillus and humoral antibodies. J Exp Med. 1969 Nov 1;130(5):931–954. doi: 10.1084/jem.130.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Ramakrishnan T. Isoniazid-resistant mutants of Mycobacterium tuberculosis H37RV: uptake of isoniazid and the properties of NADase inhibitor. J Gen Microbiol. 1970 Jan;60(1):125–132. doi: 10.1099/00221287-60-1-125. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W. The uptake and fate of isoniazid in Mycobacterium tuberculosis var. bovis BCG. J Gen Microbiol. 1967 Jun;47(3):389–403. doi: 10.1099/00221287-47-3-389. [DOI] [PubMed] [Google Scholar]
- Youatt J. A review of the action of isoniazid. Am Rev Respir Dis. 1969 May;99(5):729–749. doi: 10.1164/arrd.1969.99.5.729. [DOI] [PubMed] [Google Scholar]




